Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Early Lipid Metabolic Effects of the Anti-Psychotic Drug Olanzapine on Weight Gain and the Associated Gene Expression
Authors Chen CC , Nakano T , Hsu LW, Chu CY, Huang KT
Received 21 October 2021
Accepted for publication 9 March 2022
Published 23 March 2022 Volume 2022:18 Pages 645—657
DOI https://doi.org/10.2147/NDT.S345046
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Chien-Chih Chen,1,2 Toshiaki Nakano,3,4 Li-Wen Hsu,3,4 Chia Yi Chu,1 Kuang-Tzu Huang4,5
1Department of Psychiatry, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan; 2School of Medicine, Chang Gung University, Taoyuan, Taiwan; 3Graduate Institute of Clinical Medical Sciences, Chang Gung University College of Medicine, Kaohsiung, Taiwan; 4Liver Transplantation Center, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan; 5Institute for Translational Research in Biomedicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
Correspondence: Kuang-Tzu Huang, Institute for Translational Research in Biomedicine, Kaohsiung Chang Gung Memorial Hospital, No. 123, DAPI Road Niaosong Dist, Kaohsiung, 833, Taiwan, Tel +886-7-731-7123 (Ext.8193), Email [email protected]
Background: Atypical antipsychotics such as olanzapine often cause metabolic side effects such as obesity and diabetes, leading to an increased risk of nonalcoholic fatty liver disease. The aim of the present study was to investigate the effects of olanzapine treatment on hepatic lipid metabolism and its possible relationship with adipose tissue status.
Methods: Using a female rat model, we investigated the effects of chronic olanzapine administration on the regulation of carbohydrate and lipid metabolism including lipid biosynthesis, oxidation, efflux, and lipolysis in liver and adipose tissue.
Results: The body weight, liver mass and visceral adiposity after olanzapine treatment (2 mg/kg) for five weeks were not significantly different compared with vehicle controls. The serum level of triglycerides was higher in the vehicle controls than in olanzapine-treated rats. Unexpectedly, olanzapine treatment did not reduce glucose tolerance in our model. The expression of functional thermogenic protein uncoupling protein 1 (UCP1) was increased in brown adipose tissue (BAT) of the olanzapine group. Additionally, olanzapine treatment also reduced adipose inflammation in white adipose tissue (WAT). The transcription factor sterol regulatory element-binding protein (SREBP)-1c, a key early regulator of lipogenesis, was downregulated following olanzapine treatment. The expression of genes related to the triglycerides synthesis apparatus in the liver was upregulated in the olanzapine group. Olanzapine treatment induced genes involved in PPAR-α signaling and mitochondrial fatty acid oxidation in response to increased ATGL-mediated lipolysis in the liver.
Conclusion: Together, our findings suggest a complicated link between olanzapine therapy and metabolic disturbance and may garner interest in assessing the action of antipsychotic-induced metabolic disturbances.
Keywords: atypical antipsychotics, olanzapine, metabolic disturbances, weight gain, lipogenesis, lipolysis
Introduction
While atypical antipsychotics are a mainstay of psychiatric pharmacotherapy for the treatment of schizophrenia and psychiatric illnesses, severe metabolic adverse effects, including insulin resistance, diabetes, and obesity, especially from clozapine and olanzapine, have attracted increasing concern.1,2 These adverse effects could lead to increased morbidity and mortality and impair drug adherence and quality of life for patients.1 In recent years, Researchers have proposed several potential mechanisms for atypical antipsychotic-induced weight gain, including modulation of neurotransmitter action at the central level and involvement of body weight regulatory hormones such as leptin and ghrelin, resulting in increased appetite and reduced ability to perceive the feeling of satiety.3–6 Moreover, altered adipose tissue endocrine function and a low-grade inflammatory state in adipose tissue by atypical antipsychotic treatment may be associated with an increased risk of metabolic abnormalities and cardiovascular diseases of atypical antipsychotics.7,8 However, the underlying mechanisms of atypical antipsychotic-associated metabolic responses have yet to be completely understood.
Peripheral tissues, such as the liver and adipose tissue, play important roles in the lipid metabolic response and are the principal targets for atypical antipsychotics. Both clinical and animal studies have reported that olanzapine and clozapine induce systemic and hepatic insulin resistance, hypertriglyceridemia and nonalcoholic fatty liver disease1,9–12 with reports of hepatic lipolysis reduction, reduced oxidation of fatty acids, and increases in lipid accumulation and sterol regulatory element-binding protein (SREBP) activation.11,13,14 We previously demonstrated that atypical antipsychotics increased adipogenic differentiation in adipose-derived stem cells (ASCs) through enhanced expression of SREBP-1 and its downstream lipid biosynthesis genes.15 Similarly, other studies also found that chronic atypical antipsychotic treatment elevated the expression of lipogenic genes and proinflammatory mediators during human adipocyte differentiation.16
Adipose triglyceride lipase (ATGL), a major triglyceride hydrolase, involved in the initial step in intracellular triglyceride catabolism.17 ATGL-stimulated release of fatty acids (FAs) may regulate peroxisome proliferator-activated receptor-α (PPAR-α) target genes in the liver,18–20 presumably through FAs released as PPAR-α ligands. Recent studies have shown that deficiency of liver ATGL causes marked hepatic steatosis in mice.20,21 In contrast, overexpression of ATGL in the liver contributes to increased FA oxidation and amelioration of hepatic steatosis.22 This raises the possibility that ATGL plays a compelling role in hepatic lipid homeostasis. The aim of the present study was to investigate the effects of chronic olanzapine treatment on adipose tissue and the liver, with a specific focus on hepatic lipolysis and its possible relationship with adipose tissue status. In this study, we examined the direct effect of olanzapine in inducing lipogenic and lipolytic transcriptional responses in female rats after chronic treatment.
Materials and Methods
Animal Experiments
Female Sprague-Dawley rats, 6 weeks of age, were obtained from BioLASCO Taiwan Co., Ltd (Taipei, Taiwan). All animals were maintained in specific pathogen-free animal facilities with water and commercial rat food provided ad libitum.
According to our previous results and other reports, doses corresponding to 50% of the maximal physiological human dose were used.32 All animals were housed under the same conditions for a period of 2 weeks prior to drug treatment. Rats were trained to self-administer a cookie dough mixture weighed 0.3 g (cookie dough mixed with the commercial diet: 25.5% cornstarch, 20.9% sucrose, 14.1% fat, 10.6% protein, 1.4% fiber, 2.5% crude ash and 25% moisture) without the drugs for one week. After acclimatization, rats were randomly assigned to two groups, the control group (n=5) and olanzapine group (n=5). Olanzapine was mixed with the dough mixture and offered twice daily for 5 weeks. Physiological parameters and food and water intake were monitored.
Ethics Statement
All animal experimentation was approved by the Institutional Animal Care and Use Committee in Kaohsiung Chang Gung Memorial Hospital. The Committee recognized that the proposed animal experiment followed the Animal Protection Law by the Council of Agriculture, Executive Yuan, R.O.C. and the Guide for the Care and Use of Laboratory Animals by the Institute of Laboratory Animal Resources, National Research Council, USA.
Liver Histology and Lipid Droplet Staining
Hepatic tissues from all animals were fixed in 10% formalin, embedded in paraffin, cut into 5 μm sections and subjected to histological examination. Paraffinized sections were stained with hematoxylin-eosin (H&E). For detection of lipid accumulation, lipid droplets were assessed in frozen liver specimens embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek, Torrance, CA, USA) by Oil Red O staining and the nuclei were counterstained with hematoxylin. Images were captured using an AXIO Imager M2 microscope (ZEISS, Oberkochen, Germany).
Glucose Tolerance Tests
For glucose tolerance tests, glucose (2 g/kg) was injected intraperitoneally after starvation for 16 h, and blood glucose was measured at indicated time points using a glucometer (OneTouch Ultra 2 meter; LifeScan Inc., PA, USA).
Hepatic Triglyceride Quantification Assay
Liver tissues were washed with cold phosphate-buffered saline (PBS) and then homogenized in 5% NP-40 solution using a Dounce homogenizer. The concentration of cellular triglycerides was determined using an EnzyChrom™ triglyceride assay kit (Kampenhout, Belgium) and normalized with the protein concentration according to the protocol provided by the manufacturer.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA from perigonadal fat (WAT), brown fat (BAT) and liver was extracted using NucleoSpin-RNA kits (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany), and cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster, CA, USA) according to the manufacturer’s instructions. Quantitative RT-PCR was performed using SYBR Green PCR Master Mix. 36B4 was the reference gene used for gene expression in WAT and BAT, while GAPDH was used for genes expressed in the liver. The change in the expression of the target genes was calculated relative to the mean critical threshold (CT) values of the reference gene. The 2−∆CT value, which Changes the expression of the target genes compared to the reference gene, and the 2−∆∆CT value, which corresponds to the expression ratio of each gene compared with the vehicle-treated control group, were calculated.
Western Blot Analysis
The proteins were separated by 10% SDS-PAGE and immunoblotted according to standard protocols. The membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 hr and then incubated overnight at 4 °C with specific primary antibodies against ATGL (Cell Signaling; Danvers, MA, USA), PPAR-α (Santa Cruz Biotechnology), and β-actin (Millipore Corporation, Bedford, MA, USA), followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch; West Grove, PA, USA). Protein bands were visualized using an enhanced chemiluminescence (ECL) reagent (Millipore), and the relevant bands were quantified by densitometry using GeneTools (SYNGENE, Cambridge, UK).
Statistical Analysis
SPSS (version 22, IBM Corporation, Armonk, New York) was used to perform all statistical analyses. Independent sample t-tests were used to evaluate the statistical significance between two groups. Data are shown as the mean ± SD, and a probability (p)-value < 0.05 was considered statistically significant.
Results
Effects of Olanzapine on Weight Gain, Food Intake and Physiological Parameters
Both body weight and dietary intake were recorded during the five-week olanzapine treatment. Weight gain and food intake were higher in olanzapine-treated rats than in control rats in the first two weeks (Figure 1A). This weight gain was coupled with increased food intake (Figure 1B), suggesting that the increased body weight was attributed to food intake. However, there were no significant differences in either body weight or dietary intake between the groups from two weeks to the end of treatment. This may be due to the effect of olanzapine on weight gain plateauing. Consistent with the body weight, visceral fat mass and liver mass also showed no difference between the two groups at the end of treatment (Figure 1C). Although there was a lack of changes in weight gain and tissue mass between the groups, the serum levels of triglycerides were lower in the olanzapine treatment group than in the vehicle control group (Figure 1D). Surprisingly, the olanzapine-treated rats did not display higher levels of glucose in the intraperitoneal glucose tolerance test (IGTT) (Figure 1E) even though elevated glucose levels were expected.
Effects of Olanzapine on Thermogenesis and Inflammation in Adipose Tissue
As the adipose tissue is fundamentally involved in the pathogenesis of obesity-related diseases, we further examined the key genes that regulate inflammation and thermogenesis in brown and white adipose tissue. We observed that olanzapine strongly increased UCP-1 expression in the BAT (Figure 2A) but had no effect on proinflammatory cytokines such as IL-1β, TNF-α and MCP-1 (Figure 2B). Olanzapine treatment exerted no effect on the UCP-1 gene expression in the WAT (Figure 2C). TNF-α, as well as genes involved in macrophage recruitment, such as MCP-1, were downregulated by olanzapine treatment in the WAT (Figure 2D). The difference in ATGL expression between the two groups was not reaching statistical significance (Figure 2D).
Effects of Olanzapine on Lipid Accumulation
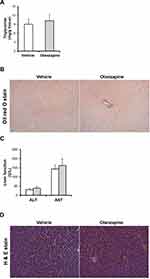
To investigate the effect of olanzapine on hepatic steatosis, we further evaluated hepatic lipid accumulation and triglyceride content by Oil Red O staining and H&E staining. We found no significant effect of olanzapine on hepatic triglyceride content (Figure 3A). In line with this, olanzapine had no significant effects on lipid droplet accumulation in the liver (Figure 3B). Olanzapine treatment had no significant effects on serum ALT and AST levels or hepatic injury following five weeks of treatment (Figure 3C and D).
Effects of Olanzapine on Genes Regulating Lipid Metabolism in the Liver
To determine alterations in the lipid metabolism pathways by olanzapine treatment, the mRNA levels of critical genes were confirmed. In the olanzapine-treated group, the lipogenic transcription factor SREBP-1c and its downstream genes, such as acetyl-CoA carboxylase (ACC), were downregulated in the liver but did not Change the level of fatty acid synthase (FAS). Stearoyl-CoA desaturase 1 (SCD1), a key enzyme in FA metabolism, was upregulated in olanzapine treatment (Figure 4A). The levels of mRNAs in the triglyceride synthesis apparatus, such as CD36, FATP5, ACSL3, ACSL5 and DGAT1, were upregulated in the olanzapine group (Figure 4B). We next evaluated the genes involved in lipolysis and fatty acid oxidation. Olanzapine increased ATGL and PPAR-α mRNA expression (Figure 4C), also corresponding to the protein levels (Figure 4D-E and Supplementary Figure 1). Consistent with this observation, several genes involved in fatty acid oxidation, such as CPT-1α, CACT, SIRT1, SIRT2 and ACOX1 were also increased following olanzapine treatment (Figure 4F). In addition, the genes involved in gluconeogenesis, including glucose 6-phosphate dehydrogenase (G6PD), FOXO1 and LacCerSyn, were increased by olanzapine (Figure 4G). Taken together, these data indicated that olanzapine exerted corresponding effects on hepatic lipid biosynthesis and lipolysis. Figure 4 Continued. Figure 4 Continued.

Discussion
Incidence of the metabolic syndrome has received increased attention in patients with schizophrenia as metabolic abnormalities are major risk factors for cardiovascular disease and mortality.1,23 Although the causes of metabolic abnormalities among patients with schizophrenia are complicated, the risk factors are attributed to lifestyle habits, long duration of disease and treatment, and negative symptoms.1,24,25 Additionally, metabolic syndrome is highly prevalent in patients with schizophrenia who are taking atypical antipsychotics.1,2 However, the effects of atypical antipsychotic-induced metabolic dysfunctions remain incompletely understood. Previous studies have reported that induction of weight loss was observed in adolescent patients, obese mice, and male rats,26–28 which, however, was inconsistent with previous studies reporting that olanzapine-induced weight gain is prevalent in a proportion of human patients and rodents over longer experimental periods.29–32 Albaugh et al demonstrated that the drug effects plateaued within 7 to 10 days, and weight gain eventually plateaued under the same daily dose of olanzapine.3 Similarly, our results showed that animals receiving olanzapine had an increased daily food intake within two weeks, accompanied by significant weight gain only observed in the first two weeks, which may be due to the olanzapine concentration reaching a plateau. Chronic olanzapine treatment was associated with neither worsened weight gain or hyperglycemia nor food intake at the end of the present study. Our data agree with Albaugh et al, who found that a same dose of olanzapine did not result in an extended period of weight gain.3 Another reason for the discrepancies between our study and previous studies may be the difference in dosages per day, times of treatment per day, delivery provided methods (such as oral gavage or intraperitoneal injection) or the length of the experimental period. A previous study has showed that oral gavage (2mg/kg) of olanzapine in rats induced significant weight gain over control beginning at day 5.30 In addition, chronic olanzapine treatment did not significantly increase liver mass or visceral adiposity in our study. A number of rodent studies have reported that chronic olanzapine treatment does not increase triglyceride and cholesterol plasma levels or induce weight gain independent increases in triglyceride levels.2,3,8,33 Intriguingly, our results showed that the serum level of triglycerides was decreased in olanzapine but had no effect on total cholesterol. Considering that the liver and adipose tissue are the two main organs related to the physiopathology of metabolic dysfunction, the effects of olanzapine on target genes in the lipogenic and lipolytic pathways were investigated in adipose tissues and the liver.
The adipose tissue is known to play critical roles in several physiological processes through secretion of adipokines, signaling lipids and inflammatory mediators.34 It is known that adipocyte and immune cell dysfunction are involved in the etiology of schizophrenia.35,36 Several studies have shown an elevation of the inflammatory cytokines, chemokines and adipokines in the serum of patients with chronic schizophrenia, which are highly linked to the incidence of disease and metabolic syndrome.35,36 A previous study demonstrated that induced inflammation in WAT was associated with a significant increase in TNFα expression in olanzapine-treated rats.37 However, when we examined the levels of inflammatory mediators, olanzapine decreased TNF-α and MCP-1 expression in WAT. Of interest, a significantly enhanced brown fat-specific marker UCP-1 was found in BAT following olanzapine treatment. These findings can also partially explain why olanzapine treatment did not change body weight and decreased the triglycerides of rats to an appreciable extent.
Regarding direct mechanisms, we and others have reported that atypical antipsychotics alter SREBP transcription factors and related target genes involved in lipid and cholesterol biosynthesis.15,38,39 In this study, we did not find any differences in hepatic lipid accumulation or damage between the groups. Olanzapine was observed to influence the direct transcriptional downregulation of SREBP1 and its downstream target gene such as ACC, but SCD1 expression was increased. SCD1 mediates oleic acid (18:1 n-9) synthesis from stearic acid (18:0), and 18:1 is a substrate required for lipid biosynthesis. The desaturation index (18:1/18:0 ratio), a validated index of SCD1 enzyme activity, is correlated with triglyceride levels and insulin resistance.40 A number of studies have demonstrated that SCD1 plays a key role in regulating lipogenesis, but its specific role in hepatic lipid accumulation is still inconsistent.41,42 In fact, several studies have shown that SCD1 expression is enhanced during diet-induced obesity;41 in contrast, other studies have found that SCD1 expression is significantly decreased.42 Interestingly, Stefan et al showed that SCD1 mRNA expression was not upregulated in fatty liver and that hepatic fat deposition is negatively correlated with hepatic SCD1 activity.43 McNamara et al reported that atypical antipsychotics increased plasma triglyceride levels and the plasma 18:1/18:0 ratio but did not affect liver SCD1 mRNA expression.44 The effect of olanzapine on SCD1 activity needs to be considered in the future in our chronic olanzapine treatment rat model. In addition to de novo lipid synthesis, the uptake of circulating fatty acids by the liver is an important contribution to hepatic lipid accumulation. Our results showed that the triglyceride synthetic elements, including CD36, FATP5, ACSL3, ACSL5 and DGAT1, were upregulated in the olanzapine group. In the present study, in response to olanzapine treatment, transcriptional activation patterns were different between de novo lipogenic genes and the triglyceride synthetic apparatus. Hepatic lipid levels are controlled by the balance between lipogenesis and lipolysis in terms of lipid acquisition and disposal.45 Recent studies have shown that ATGL influences triglyceride catabolism and is downstream of fatty acid products in the liver.18,20 Numerous studies have reported that activation of PPARα and oxidative gene expression in multiple tissues, including liver and adipose tissue, depends on lipolytic catabolism mediated through manipulations of ATGL or other fatty acid binding proteins.18–20,46–48 Moreover, a reduction in ATGL and CGI-58 protein levels in the liver was observed in insulin-resistant patients with fatty liver disease,49 and genetic variations within ATGL are associated with glucose and triglyceride levels.50 These data suggest a regulatory role of ATGL activity in hepatic steatosis and insulin resistance. Stefanidis et al demonstrated that olanzapine induced fat accumulation and caused insulin sensitivity associated with a reduction in lipolysis and inhibited the protein expression of ATGL and its critical coactivator. CGI-5851 However, our results showed that the mRNA expression of CPT1α, ATGL and PPARα, regulators of lipolysis and β-oxidation of fatty acids, was increased in the olanzapine groups. In addition, carbohydrate metabolism was also enhanced by olanzapine. The discrepancy with our finding might be explained by the fact that the duration of olanzapine exposure was shorter (14 days) and under a higher dose (6 mg/kg) in previous studies and was assured to cause metabolic disturbances.51 In contrast, olanzapine did not induce significant weight gain or metabolic disturbances at the end of our study.
There are several limitations in this study. Firstly, we used a lower dose of olanzapine in the animal model and found that this dosage was not sufficient to induce metabolic abnormalities although it corresponds to 50% of the commonly prescribed maximal physiological human dosage. In other reports, different delivery routes (oral gavage and intraperitoneal injection) and higher doses (up to 10 mg/kg) were used.7,30 In future studies we will include various doses and harvest the tissue samples at several time points for comparison. Secondly, the use of animal models of psychiatric disorders may also provide more information. Moreover, more factors could be involved in the olanzapine-induced metabolic abnormalities, including inflammation. In the current study, we did not evaluate the levels of plasma pro-inflammatory cytokines, chemokines and adipokines, which could give more precise evaluation of the metabolic status. It is well known that the adipose tissue acts as a multifunctional organ through secretion of various soluble factors that communicate with other tissues and the immune system to regulate systemic metabolic homeostasis.52 Lastly, the expression of proteins involved in thermogenesis and lipid biosynthesis was not assessed.
This study helps us conclude that olanzapine has multifaceted effects on hepatic lipid metabolism by balancing lipogenesis and lipolysis. However, some results contrary to our expectations are different from the previous indicants that olanzapine induce weight gain and dyslipidemia under conditions of metabolic stress. The effect of olanzapine on metabolic disturbances might be more intricate than previously thought, but our study also elucidates the clinical effects of atypical antipsychotic agents for the development of metabolic disturbances.
Acknowledgments
The research was supported by the Chang Gung Medical Foundation (CMRPG8H0652 and CMRPG8L1091 to CC-C.).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rojo LE, Gaspar PA, Silva H, et al. Metabolic syndrome and obesity among users of second generation antipsychotics: a global challenge for modern psychopharmacology. Pharmacol Res. 2015;101:74–85.
2. Svensson CK, Larsen JR, Vedtofte L, et al. One-year follow-up on liraglutide treatment for prediabetes and overweight/obesity in clozapine- or olanzapine-treated patients. Acta Psychiatr Scand. 2019;139(1):26–36.
3. Albaugh VL, Henry CR, Bello NT, et al. Hormonal and metabolic effects of olanzapine and clozapine related to body weight in rodents. Obesity. 2006;14(1):36–51.
4. Milano W, De Rosa M, Milano L, Capasso A. Antipsychotic drugs opposite to metabolic risk: neurotransmitters, neurohormonal and pharmacogenetic mechanisms underlying with weight gain and metabolic syndrome. Open Neurol J. 2013;7:23–31.
5. Endomba FT, Tankeu AT, Nkeck JR, Tochie JN. Leptin and psychiatric illnesses: does leptin play a role in antipsychotic-induced weight gain? Lipids Health Dis. 2020;19(1):22.
6. Mukherjee S, Skrede S, Milbank E, Andriantsitohaina R, López M, Fernø J. Understanding the Effects of Antipsychotics on Appetite Control. Front Nutr. 2022;8:815456.
7. Li H, Peng S, Li S, et al. Chronic olanzapine administration causes metabolic syndrome through inflammatory cytokines in rodent models of insulin resistance. Sci Rep. 2019;9(1):1582.
8. Horska K, Ruda-Kucerova J, Babinska Z, et al. Olanzapine-depot administration induces time-dependent changes in adipose tissue endocrine function in rats. Psychoneuroendocrinology. 2016;73:177–185.
9. Ren L, Zhou X, Huang X, Wang C, Li Y. The IRS/PI3K/Akt signaling pathway mediates olanzapine-induced hepatic insulin resistance in male rats. Life Sci. 2019;217:229–236.
10. Jiang T, Zhang Y, Bai M, et al. Up-regulation of hepatic fatty acid transporters and inhibition/down-regulation of hepatic OCTN2 contribute to olanzapine-induced liver steatosis. Toxicol Lett. 2019;316:183–193.
11. Ferno J, Vik-Mo AO, Jassim G, et al. Acute clozapine exposure in vivo induces lipid accumulation and marked sequential changes in the expression of SREBP, PPAR, and LXR target genes in rat liver. Psychopharmacology. 2009;203(1):73–84.
12. Shamshoum H, Medak KD, Townsend LK, et al. AMPK beta1 activation suppresses antipsychotic-induced hyperglycemia in mice. FASEB J. 2019;33(12):14010–14021.
13. Minet-Ringuet J, Even PC, Valet P, et al. Alterations of lipid metabolism and gene expression in rat adipocytes during chronic olanzapine treatment. Mol Psychiatry. 2007;12(6):562–571.
14. Deh M, Schreurs V, Vancampfort D, Vanw R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15–22.
15. Chen CC, Hsu LW, Huang KT, Goto S, Chen CL, Nakano T. Overexpression of Insig-2 inhibits atypical antipsychotic-induced adipogenic differentiation and lipid biosynthesis in adipose-derived stem cells. Sci Rep. 2017;7(1):10901.
16. Sarvari AK, Vereb Z, Uray IP, Fesus L, Balajthy Z. Atypical antipsychotics induce both proinflammatory and adipogenic gene expression in human adipocytes in vitro. Biochem Biophys Res Commun. 2014;450(4):1383–1389.
17. Zimmermann R, Strauss JG, Haemmerle G, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–1386.
18. Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53(1):116–126.
19. Haemmerle G, Moustafa T, Woelkart G, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17(9):1076–1085.
20. Wu JW, Wang SP, Alvarez F, et al. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54(1):122–132.
21. Fuchs CD, Radun R, Dixon ED, et al. Hepatocyte-specific deletion of adipose triglyceride lipase (adipose triglyceride lipase/patatin-like phospholipase domain containing 2) ameliorates dietary induced steatohepatitis in mice. Hepatology. 2022;75(1):125–139.
22. Reid BN, Ables GP, Otlivanchik OA, et al. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem. 2008;283(19):13087–13099.
23. Smith J, Griffiths LA, Band M, Horne D. Cardiometabolic Risk in First Episode Psychosis Patients. Front Endocrinol (Lausanne). 2020;11:564240.
24. Sugawara N, Yasui-Furukori N, Yamazaki M, et al. Attitudes toward metabolic adverse events among patients with schizophrenia in Japan. Neuropsychiatr Dis Treat. 2016;12:427–436.
25. Sahpolat M, Ari M. Higher prevalence of metabolic syndrome and related factors in patients with first-episode psychosis and schizophrenia: a cross-sectional study in Turkey. Nord J Psychiatry. 2021;75(1):73–78.
26. Cohen JA, Perel JM. Adolescent weight loss during treatment with olanzapine. J Child Adolesc Psychopharmacol. 2004;14(4):617–620.
27. Ferno J, Ersland KM, Duus IH, et al. Olanzapine depot exposure in male rats: dose-dependent lipogenic effects without concomitant weight gain. Eur Neuropsychopharmacol. 2015;25(6):923–932.
28. Zhang X, Zhao Y, Liu Y, Yuan Y, Shao H, Zheng X. Regulation of obesity-associated metabolic disturbance by the antipsychotic drug olanzapine: role of the autophagy-lysosome pathway. Biochem Pharmacol. 2018;158:114–125.
29. Cunningham JI, Eyerman DJ, Todtenkopf MS, et al. Samidorphan mitigates olanzapine-induced weight gain and metabolic dysfunction in rats and non-human primates. J Psychopharmacol. 2019;33(10):1303–1316.
30. Yang CP, Wang YY, Lin SY, et al. Olanzapine Induced Dysmetabolic Changes Involving Tissue Chromium Mobilization in Female Rats. Int J Mol Sci. 2019;20(3):34.
31. Liu JH, Chen N, Guo YH, et al. Metabolomics-based understanding of the olanzapine-induced weight gain in female first-episode drug-naive patients with schizophrenia. J Psychiatr Res. 2021;140:409–415.
32. Stogios N, Smith E, Bowden S, et al. Metabolic adverse effects of off-label use of second-generation antipsychotics in the adult population: a systematic review and meta-analysis. Neuropsychopharmacology. 2022;47(3):664–672.
33. Arivazhahan A, Bairy LK, Nayak V, Kunder SK. A Study to Assess the Therapeutic Effect of Enalapril on Olanzapine Induced Metabolic Syndrome in Wistar Rats. J Clin Diagn Res. 2017;11(2):FF01–FF06.
34. Li Y, Yun K, Mu R. A review on the biology and properties of adipose tissue macrophages involved in adipose tissue physiological and pathophysiological processes. Lipids Health Dis. 2020;19(1):164.
35. Beumer W, Drexhage RC, HJd W, Versnel MA, Drexhage HA, Cohen D. Increased level of serum cytokines, chemokines and adipokines in patients with schizophrenia is associated with disease and metabolic syndrome. Psychoneuroendocrinology. 2012;37:1901–1911.
36. Sahpolat M, Ari M, Kokacya MH. Plasma Apelin, Visfatin and Resistin Levels in Patients with First Episode Psychosis and Chronic Schizophrenia. Clin Psychopharmacol Neurosci. 2020;18(1):109–115.
37. Guo C, Liu J, Li H. Metformin ameliorates olanzapine-induced insulin resistance via suppressing macrophage infiltration and inflammatory responses in rats. Biomed Pharmacother. 2021;133:110912.
38. Lauressergues E, Staels B, Valeille K, et al. Antipsychotic drug action on SREBPs-related lipogenesis and cholesterogenesis in primary rat hepatocytes. Naunyn Schmiedebergs Arch Pharmacol. 2010;381(5):427–439.
39. Cai HL, Tan QY, Jiang P, et al. A potential mechanism underlying atypical antipsychotics-induced lipid disturbances. Transl Psychiatry. 2015;5:e661.
40. Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009;297(1):E28–37.
41. Hu CC, Qing K, Chen Y. Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and -resistant mice. Obes Res. 2004;12(8):1264–1270.
42. Fernandez Gianotti T, Burgueno A, Gonzales Mansilla N, Pirola CJ, Sookoian S. Fatty liver is associated with transcriptional downregulation of stearoyl-CoA desaturase and impaired protein dimerization. PLoS One. 2013;8(9):e76912.
43. Stefan N, Peter A, Cegan A, et al. Low hepatic stearoyl-CoA desaturase 1 activity is associated with fatty liver and insulin resistance in obese humans. Diabetologia. 2008;51(4):648–656.
44. McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Atypical antipsychotic medications increase postprandial triglyceride and glucose levels in male rats: relationship with stearoyl-CoA desaturase activity. Schizophr Res. 2011;129(1):66–73.
45. Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75(18):3313–3327.
46. Ong KT, Mashek MT, Davidson NO, Mashek DG. Hepatic ATGL mediates PPAR-alpha signaling and fatty acid channeling through an L-FABP independent mechanism. J Lipid Res. 2014;55(5):808–815.
47. Khan SA, Sathyanarayan A, Mashek MT, Ong KT, Wollaston-Hayden EE, Mashek DG. ATGL-catalyzed lipolysis regulates SIRT1 to control PGC-1alpha/PPAR-alpha signaling. Diabetes. 2015;64(2):418–426.
48. Tang S, Wu F, Lin X, Gui W, Zheng F, Li H. The Effects of New Selective PPARalpha Agonist CP775146 on Systematic Lipid Metabolism in Obese Mice and Its Potential Mechanism. J Diabetes Res. 2020;2020:4179852.
49. Kato M, Higuchi N, Enjoji M. Reduced hepatic expression of adipose tissue triglyceride lipase and CGI-58 may contribute to the development of non-alcoholic fatty liver disease in patients with insulin resistance. Scand J Gastroenterol. 2008;43(8):1018–1019.
50. Schoenborn V, Heid IM, Vollmert C, et al. The ATGL gene is associated with free fatty acids, triglycerides, and type 2 diabetes. Diabetes. 2006;55(5):1270–1275.
51. Stefanidis A, Watt MJ, Cowley MA, Oldfield BJ. Prevention of the adverse effects of olanzapine on lipid metabolism with the antiepileptic zonisamide. Neuropharmacology. 2017;123:55–66.
52. Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest. 2019;129(10):3990–4000.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.