Back to Journals » Journal of Inflammation Research » Volume 14
Dysiarenone from Marine Sponge Dysidea arenaria Attenuates ROS and Inflammation via Inhibition of 5-LOX/NF-κB/MAPKs and Upregulation of Nrf-2/OH-1 in RAW 264.7 Macrophages
Authors Hu TY , Zhang H, Chen YY, Jiao WH, Fan JT, Liu ZQ, Lin HW, Cheng BH
Received 21 November 2020
Accepted for publication 10 February 2021
Published 25 February 2021 Volume 2021:14 Pages 587—597
DOI https://doi.org/10.2147/JIR.S283745
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Tian-Yong Hu,1,* Hua Zhang,1,* Yan-Yan Chen,1 Wei-Hua Jiao,2 Jun-Ting Fan,3 Zhi-Qiang Liu,1 Hou-Wen Lin,2 Bao-Hui Cheng1
1Shenzhen Key Laboratory of ENT, Institute of ENT and Longgang ENT Hospital, Shenzhen, 518172, People’s Republic of China; 2Research Center for Marine Drugs, State Key Laboratory of Oncogene and Related Genes, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127, People’s Republic of China; 3Department of Pharmaceutical Analysis, School of Pharmacy, Nanjing Medical University, Nanjing, 211166, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hou-Wen Lin
Shanghai Jiao Tong University, 160 Pujian Road, Shanghai, 200127, People’s Republic of China
Email [email protected]
Bao-Hui Cheng
Shenzhen Key Laboratory of ENT, Institute of ENT and Longgang ENT Hospital, 3004, Longgang Avenue, Longgang District, Shenzhen, 518172, People’s Republic of China
Email [email protected]
Background: Marine natural products harbor a variety of pharmacological activities, and the sea species have been becoming a main source of new drug candidate. In pursuit of safer and more effective anti-inflammation drug, the anti-inflammatory activities, anti-oxygenation effects and underlying molecular mechanisms of compound dysiarenone from Dysidea arenaria were investigated via LPS-induced RAW 264.7 cell model.
Methods: Firstly, RAW 264.7 cells have been stimulated with LPS and treated with dysiarenone, and the cell viability of the LPS-treated RAW 264.7 cells was examined. One-step method, DCFH-DA fluorescence probe method was used to detect reactive oxygen species (ROS). The modulation of dysiarenone on anti-inflammation was detected by enzyme-linked immunosorbent assay by measuring the release of inflammatory cytokines (TNF-α and IL-6), and inflammatory mediators (LTB4). Further, the underlying anti-inflammatory mechanism of dysiarenone was explored by determining the expression of inducible 5-LOX, MAPKs, p-Akt, and p-NF-κB p65. Oxidative stress is tightly connected with inflammation, which was also evaluated through nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (OH-1) signaling pathway.
Results: Our study unraveled that dysiarenone between 2 and 8 μM reduces the inflammation responses via suppressing the production of inflammatory cytokines (TNF-α and IL-6) and inflammatory mediators (LTB4). Dysiarenone down-regulated the protein levels of inducible 5-LOX via the inhibition of phosphorylation of MAPKs (including p38, ERK), Akt and NF-κB p65. Additionally, dysiarenone decreases ROS accumulation by upregulating HO-1 expression via nuclear translocation of Nrf2.
Conclusion: In conclusion, we demonstrated that dysiarenone possesses anti-inflammation and anti-oxidation activity via inhibiting 5-LOX/NF-κB/MAPK and Nrf2/HO-1 signaling pathway. Dysiarenone might be a promising lead compound for inflammatory diseases.
Keywords: dysiarenone, Dysidea arenaria, Nrf2, HO-1, NF-κB
Introduction
Accumulating data indicates oxidative damage is a primary cause for the pathogenesis of neurodegenerative disorders, Alzheimer’s disease and autoimmune diseases.1 Overexpressed reactive oxygen species (ROS) induces nucleic acid damage consequently resulting in neurological senescence and aging.1,2 Inflammation is a self-protection act in response to injury being caused by harmful pathogens, irritants and damaged cells.3 However, prolonged inflammation leads to pathogenesis of a variety of diseases including arthritis, asthma, multiple sclerosis and many more.4–7 Exposure to lipopolysaccharide (LPS) initiates signaling cascade for inflammatory mediator expression including cytokines such as tumor necrosis factor α (TNF-α) and interleukin (IL)-6, Leukotriene (LT) B4 and nuclear factor-kappaB (NF-κB), and also produce ROS.8 Thus, intervention of LPS-induced responses is an effective strategy to suppress oxidative and inflammatory responses.
In pursuit of safer and more effective anti-inflammation drug, the sea species have been becoming a main source of new drug candidate.9 Natural products derived from marine harbor a variety of therapeutic effects for cancer, arthritis and neurodegenerative diseases.10 The genus Dysidea is well known for the production of active components with diverse structures.11–15 We isolated a C21 meroterpenoid, dysiarenone, from Dysidea arenaria, and demonstrated its inhibitory activities against expressions of prostaglandin E2 (PGE2) and cyclooxygenase-2 (COX-2).11 However, the detailed anti-inflammatory and anti-oxidative effects and related molecular mechanism pathways of dysiarenone have not been studied. Thus, the aim of this study was to evaluate the anti-inflammatory and anti-oxidative mechanisms of dysiarenone in LPS-induced RAW 264.7 cell model in this study.
Materials and Methods
Chemicals and Reagents
Lipopolysaccharide (LPS) (Catalogue Number: L2887) and dexamethasone (Catalogue Number: D4902) were obtained from Sigma-Aldrich (St Louis, MO, USA). 2.7-Dichlorodi-hydrofluorescein diacetate (DCFH-DA, Catalogue Number: S0033) was bought from Beyotime Biotechnology (Shanghai, China). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo (Tokyo, Japan). Antibodies for β-actin (Catalogue Number: 4967s), phosphorylated Erk (Catalogue Number: 9101s), Erk (Catalogue Number: 9102s), phosphorylated JNK (Catalogue Number: 9251s), JNK (Catalogue Number: 9252s), phosphorylated p38 (Catalogue Number: 9211s), p38 (Catalogue Number: 9212s), IKKβ (Catalogue Number: 4967s), phosphorylated-IKKβ (Catalogue Number: 2370s), phosphorylated-NF-κB p65 (Catalogue Number: 3033s) and NF-κB p65 (Catalogue Number: 8242s) were provided by Cell Signaling Technology, Inc. (Danvers, MA, USA). Bicinchoninic acid (BCA) assay (Catalogue Number: 23225), Thermo Scientific (Waltham, MA, USA) supplied relative peroxidase-conjugated secondary antibody (Catalogue Number: 31460), NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Catalogue Number: 78833) and Pierce™ ECL Western Blotting Substrate (Catalogue Number: 32106). IL-6 (Catalogue Number: 550950) and TNF-α (Catalogue Number: 560478) ELISA kits were from BD Biosciences (San Jose, CA, USA). Murine Leukotriene B4 (LTB4) ELISA kit (Catalogue Number: CSB-E08034m) was purchased from Cusabio (Wuhan, Hubei, China). Photographic film was purchased from Fujifilm Corporation (Tokyo, Japan). Anti-5-Lipoxygenase (LOX) antibody (Catalogue Number: ab39347), anti-Heme Oxygenase 1 antibody (Catalogue Number: ab189491), Anti-Nrf2 antibody (Catalogue Number: ab31163) and Phosphatase and Protease Inhibitor Cocktail (Catalogue Number: ab201120) were obtained from Abcam (Cambridge, MA, USA).
Extraction and Isolation
The extraction of D. arenaria (650 g) was performed with 95% EtOH to afford 3.4 g extract. A pair of interesting quasi-molecular ions (625.1 [M + H]+ in positive mode m/z and 623.3 [M - H]− in negative mode) were extracted from the LC-ESI-MS spectrum of the 6 organic extract. Guided by the molecular weight (MW 624), the extract was suspended in water (500 mL) and successively partitioned by n-Hexane and CH2Cl2 for three times. For the separation of CH2Cl2 fraction (2.5 g), a VLC on silica gel using n-hexane with increasing proportions of ethyl acetate (EtOAc) and then EtOAc with increasing proportions of MeOH were applied to give 11 fractions (DA-DK). The tenth fraction DJ (0.62 g, petroleum/EtOAc 5:1) was further separated by reversed-phase C18 column with gradient MeOH/H2O (60%-100%) to yield 15 subfractions (DJ1-DJ15). LC-DAD/MS analysis showed that the subfraction DJ13 (47.8 mg) contained the interesting molecular weight, and thus high-performance liquid chromatography equipped with YMC-Pack Pro C18 reversed-phase column (5 µm, 10 × 250 mm; ultraviolet absorbance measured at 220 nm) using 95% MeCN/H2O as eluting solvent at 2 mL/min was exploited for further purification to afford dysiarenone (tR = 49.2 min, 4.3 mg) (Jiao et al, 2018).
Cell Culture and Viability Assessment
RAW 264.7 macrophage cells, obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), were cultured with RPMI 1640 Medium (Life Technologies, Grand Island, NY, USA) supplemented with 10% FBS and 1% penicillin-streptomycin (Grand Island, NY, USA). The culture was incubated at 37°C in a humidified 5% CO2 atmosphere.
Cell Counting Kit-8 (CCK-8) assay was used for cell viability evaluation following previous literature.16 The cells were seeded into tissue culture 96-well plates (200 μL cell suspension per well) at a density of 1 × 105 cells/well for 2 h. The cells were then co-incubated with LPS (1 µM) and dysiarenone at concentrations ranging from 2 to 32 µM for 24 h. Ten μL solution of CKK-8 was added to the cell culture and further incubated for another 4 h. Absorbance at 450 nm was measured with SpectraMax Paradigm Multi-Mode Microplate Reader (Molecular Devices, San Jose, CA, USA). The cell viability was normalized to the non-LPS-treated group (control, CTL) group.
Reactive Oxygen Species (ROS) Assay
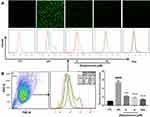
The ROS assay kit is mostly based on fluorescence intensity change of fluorescent dye DCFH-DA. DCFH-DA has no fluorescence and could cross the cell membrane freely. It can be hydrolyzed to DCFH which can be oxidized to fluorescent DCF by ROS in the cell. The real level of ROS can be analyzed by flow cytometry (BD FACSAriaTM II) and Laser Scanning Confocal Microscopy (LSCM, Leica TCS SP5 II), respectively. RAW264.7 cells were treated with dysiarenone at different concentrations of 4 μM and 8 μM in the presence of LPS (1 μg/mL) stimulation for 24 h. After washing twice with PBS, and the cells were stained with DCFH-DA (1:1000 dilution with FBS) at 37 °C with 5% CO2 in a humidified incubator for 30 min, finally, the fluorescence intensities were detected by flow cytometry and LSCM, respectively.
ELISA Assay for TNF-α and IL-6
RAW 264.7 cells were seeded into 96-well plates (3 × 105 cells/mL) and cultured for 24 h. The pro-inflammatory mediators (TNF-α and IL-6) produced by LPS-stimulated RAW 264.7 cells were quantified using ELISA Kits (TNF-α ELISA kit sensitivity: 10 pg/mL, intra-assay precision coefficient of variation (CV) % <5.8% and inter-assay precision CV% <8.4%; IL-6 ELISA kit sensitivity: 10 pg/mL, intra-assay precision CV% <5.4% and inter-assay precision CV% <4.0%). After 1 hour’s pretreatment with dysiarenone at concentrations ranging from 2 µM to 8 µM, the cells were further stimulated with LPS at 1 μg/mL for 12 h. As a positive control, the impacts of dexamethasone on TNF-α and IL-6 in LPS-induced RAW 264.7 cells were also determined parallelly. Preliminary experimental results reminded that the concentrations of TNF-α and IL-6 are beyond the detection range of these two ELISA kits. For the determination of TNF-α and IL-6, the cell supernatants were diluted 4-fold and 6-fold, respectively, for ELISA assay. ELISA was carried out according to the manufacturer’s instructions, and absorbance was measured at 450 nm.
ELISA Assay for LTB4 Production
RAW 264.7 cells were seeded at a density of 1 × 106 cells/mL into 24-well plates and cultured for 24 h. After treatment with dysiarenone at concentrations ranging from 2 µM to 8 µM for 1 h, the cells were further treated with the presence or the absence of LPS for 18 h. The concentration of LTB4 in cell-free supernatant was assayed following the manufacturer’s instructions (Sensitivity of the ELISA kit is 3.9 pg/mL; inter-assay precision is less than 10% and the intra-assay precision is less than 8%). Absorbance was measured using SpectraMax Paradigm Multi-Mode Microplate Reader at 450 nm. The modulations of dexamethasone on the production of LTB4 were also evaluated following the above procedure.
Western Blot of Inflammation-Related Enzymes
Western blot analysis was performed according to the previous method with some modifications.17 Briefly, the cells were plated and treated with dysiarenone in the presence of LPS for 24 h, and then extracted proteins were quantified using BCA assay. Protein samples were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electro-transferred onto PVDF membranes (GE Healthcare, Chicago, IL, USA). After blocking with 3% skim milk solution, the membranes were probed with primary antibodies including anti-COX-2, anti-Erk, anti-phosphorylated Erk, anti-JNK, anti-phosphorylated JNK, anti-p38, anti-phosphorylated p38, anti-phosphorylated-IKKβ, anti-IKKβ, anti-NF-κB p65, anti-phosphorylated-NF-κB p65, anti-HO-1 antibody, anti-Nrf2 and anti-β-actin, and then incubated with peroxidase-conjugated secondary antibodies. To determine the expressions of protein, the immunoblots were detected with enhanced chemiluminescent (ECL) reagent consistent with the manufacturer’s protocol. The band intensities were quantified using the Image J Software and normalized to the level of β-actin.
Statistical Analysis
All results are expressed as mean ± SD Multiple group comparisons of the data were performed using one-way analysis of variance (ANOVA) with post hoc test using GraphPad Prism 7.04, p < 0.05 was considered statistically significant.
Results
Effects of Dysiarenone on RAW 264.7 Cell Viability
After 24 h treatment with dysiarenone at concentrations ranging from 2 μM to 32 μM, the effects of dysiarenone on RAW 264.7 cell viability were examined (Figure 1). The results of CCK-8 assay showed that there were no significant changes in cell viabilities on RAW 264.7 cells by dysiarenone treatment below 8 μM. Subsequent experiments were performed at concentrations ranging from 2 μM to 8 μM of dysiarenone.
Dysiarenone Alleviated LPS-Induced Intracellular ROS in Macrophage
Oxidative damage plays an important role in the pathogenesis of various diseases, including diabetes, obesity, cancer, neurodegeneration, metabolic syndrome, cardiovascular disease, liver disease, and others. Therefore, whether dysiarenone pre-treatment could alleviate LPS-induced intracellular ROS was examined. As shown in Figure 2, the level of fluorescence emitted by DCF in LPS stimulated group raised significantly compared to normal group, but significantly decreased in dysiarenone pretreated groups. Therefore, the results suggested that dysiarenone pretreatment could effectively reduce the LPS-induced intracellular ROS (Figure 2) through anti-oxidative properties.
Impacts of Dysiarenone on IL-6, TNF-α and LTB4/5-LOX
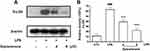
Pro-inflammatory cytokines (IL-6 and TNF-α) and LTB4 play vital roles in the pathogenesis of inflammatory diseases, and the effects of dysiarenone were evaluated on IL-6, TNF-α and LTB4 using ELISA. The release of IL-6, TNF-α and LTB4 in the supernatant of RAW 264.7 macrophages were significantly induced by LPS. As shown in Figure 3, dysiarenone at concentration ranging from 2 μM to 8 μM showed significant suppression on the release of TNF-α (*p < 0.05, Figure 3A), and inhibited on LTB4 at 4 μM and 8 μM significantly (**p < 0.01, ***p < 0.001, Figure 3C). Dysiarenone also significantly down-regulated IL-6 levels at 8 μM in cell cultural supernatant (***p < 0.001, Figure 3B).
For the remarkable inhibition of dysiarenone on LTB4 in LPS-treated RAW 264.7 cells, the inhibition of dysiarenone was further investigated on 5-LOX. The protein expression of 5-LOX in LPS-treated RAW 264.7 cells was detected by Western blot analysis (considering the weak inhibition of dysiarenone on IL-6, TNF-α and LTB4 at 2 µM, evaluation of dysiarenone on LPS-stimulated RAW 264.7 cells via Western blot was only performed at 4 µM and 8 µM). The protein expressions of 5-LOX were upregulated notably upon stimulated with LPS (1 μg/mL), and the up-regulated expressions of 5-LOX were significantly reduced by dysiarenone in a concentration-dependent manner between 4 μM and 8 μM (***p < 0.001, Figure 4). Compared to LPS-treated cells, treatment with dysiarenone (4 μM and 8 μM) decreased the protein expression of 5-LOX by 22.1% and 39.8%, respectively. These outcomes suggest that the inhibition of dysiarenone on LTB4 might be through inhibition of 5-LOX expression in LPS-stimulated RAW 264.7 macrophages.
Repression of Dysiarenone on LPS-Induced NF-κB Signaling Pathway
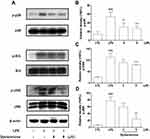
For its anti-inflammatory effect in LPS-induced RAW 264.7 macrophages, the inhibition of dysiarenone on LPS-induced nuclear translocation of NF-κB (p65) and related IκB kinase β (IKKβ) were analyzed by Western blot. Compared to the control group, the phosphorylation of NF-κB (p65) and IKKβ was enhanced by LPS (Figure 5). Pretreatment with dysiarenone for 1 h prevented the LPS-induced phosphorylation of IKKβ at 8 μM (**p < 0.01). Furthermore, in the case of LPS-stimulated phospho-NF-κB (p65), dysiarenone also showed significant inhibition (*p < 0.05, Figure 5) at concentration of 8 μM without interfering the total protein level of NF-κB (p65) in RAW 264.7 cells.
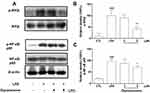
Suppression of Dysiarenone on LPS-Induced Phosphorylation MAPKs and Akt
Mitogen-activated protein kinase (MAPK) and Akt are widely known for their critical roles in various cellular processes including NF-κB activation. MAPKs (including JNK, ERK, and p38) and Akt play a vital role in activation of NF-κB during inflammatory process. Hence, we investigated the inhibition of dysiarenone on Akt phosphorylation induced by exposure of RAW 264.7 cells to LPS. As shown in Figure 6, the phospho-Akt was powerfully enhanced by LPS, and the phospho-Akt was markedly reduced by dysiarenone suggesting that dysiarenone could suppress LPS-induced Akt pathway signaling (Figure 6). Furthermore, we investigated the modulations of dysiarenone on ERK, JNK, and p38 signaling pathway in LPS-induced RAW 264.7 cells. Treatment of LPS rose phosphorylation levels of JNK, ERK, and p38 in macrophages significantly, and the increased phosphorylations of p38 and ERKs were lowered by 1 hour’s treatment of dysiarenone in a concentration-dependent manner at 4 μM and 8 μM (Figure 7B and C). Dysiarenone only showed significant inhibition on JNK protein kinase at 8 μM (Figure 7D).
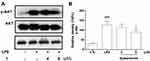
Up-Regulation of Dysiarenone on HO-1 in Macrophage Challenged with LPS
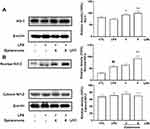
The roles of Nrf2 and HO-1 in protection against oxidation and inflammatory responses induced by LPS have been documented in previous study.18 To explore the mechanism underlying the anti-oxidant and anti-inflammatory efficacy of dysiarenone, protein expressions of Nrf2 and HO-1 in LPS stimulated macrophages were assessed. There were no significant variations of cytosol Nrf2 expressions among NC, LPS and dysiarenone (4–8 μM) treated group, and the expression of nuclear Nrf2 in RAW 264.7 cells was induced by treatment with LPS (1 μg/mL) 4–8 μM dysiarenone (Figure 8B). As shown in Figure 8B, treatment with dysiarenone (4–8 μM) resulted in the nuclear translocation of Nrf2. For the correlation between Nrf2 translocation with the expression of anti-oxidant proteins protecting against inflammation responses, whether dysiarenone regulates HO-1, a target gene of Nrf2, in LPS stimulated RAW 264.7 cells were examined. The result indicated that dysiarenone promoted the protein levels of HO-1 after dysiarenone (4–8 μM) treatment (Figure 8A). These results suggest that pretreatment with dysiarenone exhibits antioxidant and anti-inflammatory effects through the induction of HO-1 via Nrf2 nuclear translocation.
Discussion
Steroidal anti-inflammatory drugs are the current main therapies for inflammation, corticosteroids (dexamethasone and so on) are prevalently used in clinic.19 But the severe side effects such as fluid retention, high blood pressure, headache constrain the use of corticosteroids.20 As the main source for potential drug, natural products inspire the new drug discovery powerfully.21 Marine ecosystems contain enormous marine organisms with novel molecules possessing interesting pharmacological activities, and the discovery of bioactive natural products from the marine environment for the purpose of drug candidates has prevailed for decades.9 Marine sponges, as rich sources of structurally novel secondary metabolites, attract much attention for the yield of potential lead compounds for the development of new drugs.22 Sponges, a member of the genus Dysidea, are reported to be rich in bioactive secondary metabolites, and several sesquiterpene quinones and meroterpenes were isolated from this Dysidea species.11–15 Biological investigations study revealed that sesquiterpene quinones and meroterpenes isolated from Dysidea species could prevent degranulation of mast cell and lower PGE2 production via inhibiting COX-2 expression in RAW 264.7 cells.11,12
Inflammation and oxidation are tightly involved in pathogenesis of many diseases.8 Classical activation involves the exposure of the macrophage to LPS which will initiate TLR-4-mediated signaling pathway and cause inflammation. The anti-inflammation and anti-oxidation effect of dysiarenone was poorly demonstrated, so the anti-inflammation and anti-oxidation effect and underlying mechanism of dysiarenone on LPS-induced RAW 264.7 macrophages were explored in this study. As a steroidal anti-inflammatory drug, dexamethasone was chosen as a reference control in current experiment.23
Increasing evidence has demonstrated that the transcription factor Nrf2 plays a key role in antagonizing oxidative stress, and Nrf2 has been considered an emerging therapeutic target.24 Nrf2 was a transcription factor that binds to the promoter of the HO-1 gene leading to anti-inflammation and anti-oxidation responses.25 Dysiarenone significantly promoted the expression of Nrf2 and HO-1 protein in macrophages at a dose-dependent manner.
Inflammatory mediators, such as LTB4, TNF-α and IL-6, are tightly involved in the pathogenesis of inflammatory diseases.26 Interruption of these pro-inflammatory mediators could be an effective strategy for the prevention of inflammatory diseases.27 The arachidonate lipoxygenase enzyme system is in charge of the conversion of arachidonic acid into leukotriene. Arachidonate 5-lipoxygenase (5-LOX) converts of arachidonic acid to different leukotrienes such as LTB4.28 LTB4 is well documented in inflammation, and our experiment results showed that dysiarenone could lower the release of LTB4 in LPS-stimulated RAW 264.7 cells in a concentration-dependent manner at concentrations ranging from 4 μM to 8 μM.
NF-κB is well known for its participation in cell differentiation, and highly involved in the pathogenesis of inflammation.29 Multiple natural compounds have been reported to possess anti-inflammatory effects via downregulating the phosphorylation of NF-κB.30,31 Our studies demonstrated that dysiarenone attenuate LPS-induced IKKβ phosphorylation and NF-κB p-65 phosphorylation in RAW 264.7 macrophages. Accordingly, the suppression of dysiarenone on LPS-induced inflammation confirmed the inhibition of dysiarenone on NF-κB pathway in RAW 264.7 macrophages (Figure 2 and Figure 4). Compared to the reference control, dexamethasone (10 µM), compound dysiarenone also showed significant suppressions IL-6 and TNF-α at 8 µM (Figure 2).
Previous study indicated that Akt signaling pathway is activated by LPS-induced TLR-4-mediated pathway, and involved in NF-κB activation.32 This study showed that dysiarenone repressed LPS-induced phosphorylation of Akt at 8 μM, suggesting that dysiarenone restrains NF-κB activation via inhibition of Akt phosphorylation. MAPK, comprising of JNK, ERKs and p38, is an important upstream component of NF-κB.33,34 In this study, with treatment of dysiarenone, the phosphorylations of ERK and p38 MAP kinase were attenuated significantly at concentrations ranging from 4 μM to 8 μM, and JNK phosphorylation was also reduced significantly by dysiarenone at 8 μM (Figure 7).
Recent studies have indicated Nrf2, as an anti-oxidant gene regulator, encoding Phase II detoxifying enzymes, and this signaling pathway has critical importance in the mechanism of cellular protection and maintenance.18 HO-1 induction by activated Nrf2 protects the cells against oxidative stress.35 Dysiarenone has strong anti-oxidant activity via reduction of the intracellular ROS level in RAW 264.7 macrophages. Therefore, we determined whether the anti-oxidant activity of dysiarenone was mediated by Nrf2/HO-1 signaling. As expected, Nrf2 nuclear translocation from the cytoplasm was potentiated after the dysiarenone treatment. In addition, dysiarenone treatment increased the HO-1 protein expression levels in RAW 264.7 macrophages. The enhanced HO-1 production may result in the reduction of iNOS expression and decrease the amount of free radicals.36 In addition, these results suggest that dysiarenone has an anti-oxidative and anti-inflammatory effect through the Nrf2/HO-1 signaling pathway.
Conclusion
Dysiarenone reduces the LPS-induced high levels of ROS and suppresses inflammatory mediators (TNF-α and IL-6) and LTB4/5-LOX via interrupting LPS-stimulated NF-κB activation, and the inhibition of dysiarenone on NF-κB is via prevention of the phosphorylation of IKKβ, Akt and MAPK as well as Nrf2-mediated HO-1 induction in macrophages. This study demonstrates that dysiarenone from Dysidea arenaria possess potent anti-inflammation and anti-oxidation effects in LPS-stimulated RAW 264.7 cells. This is the first time to clarify anti-inflammation effects and underlying molecular mechanisms of dysiarenone, and our findings suggest dysiarenone, a marine-derived C21 meroterpenoid, can be further investigated as a potentially lead compound in anti-inflammation.
Data Sharing Statement
All the raw data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
This work was supported by grants Natural Science Foundation of China (No. 81973915, 81773978, and 82004046), Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515010391 and 2020A1515010592), Shenzhen Innovation of Science and Technology Commission (No. LGKCZSYS2019000046, JCYJ20170412103841386, and LGKCYLWS2019000864), and Shenzhen Key Medical Discipline Construction Fund (No. SZXK039). The funding bodies were not involved in the concept, design, analysis or writing the manuscript.
Disclosure
None of the authors has any conflict of interests regarding this study.
References
1. Lv J, Jiang S, Yang Z, et al. PGC-1α sparks the fire of neuroprotection against neurodegenerative disorders. Ageing Res Rev. 2018;44:8–21. doi:10.1016/j.arr.2018.03.004
2. Massaad CA, Pautler RG, Klann E. Mitochondrial superoxide: a key player in Alzheimer’s disease. Aging. 2009;1(9):758–761. doi:10.18632/aging.100088
3. Matsuzawa-Ishimoto Y, Hwang S, Cadwell K. Autophagy and Inflammation. Annu Rev Immunol. 2018;36(1):73–101. doi:10.1146/annurev-immunol-042617-053253
4. Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15(8):551–567. doi:10.1038/nrd.2016.39
5. Galkina E, Ley K. Immune and Inflammatory Mechanisms of Atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi:10.1146/annurev.immunol.021908.132620
6. Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Review Article. Nat Rev Neurosci. 2015;16:358. doi:10.1038/nrn3880
7. Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, Inflammation, and Cancer. Annu Rev Pathol. 2016;11:421–449. doi:10.1146/annurev-pathol-012615-044359
8. Ivashkiv LB. Inflammatory signaling in macrophages: transitions from acute to tolerant and alternative activation states. Eur J Immunol. 2011;41(9):2477–2481. doi:10.1002/eji.201141783
9. Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Drug development from marine natural products. Review Article. Nat Rev Drug Discov. 2008;8:69. doi:10.1038/nrd2487
10. Senthilkumar K, Kim S-K. Marine Invertebrate Natural Products for Anti-Inflammatory and Chronic Diseases. Evid Based Complementary Altern Med. 2013;2013:572859. doi:10.1155/2013/572859
11. Jiao W-H, Cheng B-H, Chen G-D, et al. Dysiarenone, a Dimeric C 21 Meroterpenoid with Inhibition of COX-2 Expression from the Marine Sponge Dysidea arenaria. Org Lett. 2018;20(10):3092–3095. doi:10.1021/acs.orglett.8b01148
12. Jiao W-H, Cheng B-H, Shi G-H, et al. Dysivillosins A–D, Unusual Anti-allergic Meroterpenoids from the Marine Sponge Dysidea villosa. Sci Rep. 2017;7(1):8947. doi:10.1038/s41598-017-04021-z
13. Jiao W-H, Huang X-J, Yang J-S, et al. Dysidavarones A–D, New Sesquiterpene Quinones from the Marine Sponge Dysidea avara. Org Lett. 2012;14(1):202–205. doi:10.1021/ol202994c
14. Jiao W-H, Shi G-H, Xu -T-T, et al. Dysiherbols A–C and Dysideanone E, Cytotoxic and NF-κB Inhibitory Tetracyclic Meroterpenes from a Dysidea sp. Marine Sponge. J Nat Prod. 2016;79(2):406–411. doi:10.1021/acs.jnatprod.5b01079
15. Jiao W-H, Xu -T-T, Yu H-B, et al. Dysideanones A–C, unusual sesquiterpene quinones from the south china sea sponge Dysidea avara. J Nat Prod. 2014;77(2):346–350. doi:10.1021/np4009392
16. Li KK, Shen SS, Deng X, et al. Dihydrofisetin exerts its anti-inflammatory effects associated with suppressing ERK/p38 MAPK and Heme Oxygenase-1 activation in lipopolysaccharide-stimulated RAW 264.7 macrophages and carrageenan-induced mice paw edema. Int Immunopharmacol. 2018;54:366–374. doi:10.1016/j.intimp.2017.11.034
17. Liu J-Q, Lian C-L, Hu T-Y, et al. Two new farnesyl phenolic compounds with anti-inflammatory activities from Ganoderma duripora. Food Chem. 2018;263:155–162. doi:10.1016/j.foodchem.2018.04.097
18. Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):585–597. doi:10.1016/j.bbadis.2016.11.005
19. Kapugi M, Cunningham K. Corticosteroids. Orthop Nurs. 2019;38(5):336–339. doi:10.1097/nor.0000000000000595
20. Yasir M, Goyal A, Bansal P, Sonthalia S. Corticosteroid Adverse Effects. StatPearls. StatPearls Publishing. Copyright © 2020. StatPearls Publishing LLC; 2020.
21. Kingston DGI. Modern Natural Products Drug Discovery and Its Relevance to Biodiversity Conservation. J Nat Prod. 2011;74(3):496–511. doi:10.1021/np100550t
22. Shady N, El-Hossary E, Fouad M, Gulder T, Kamel M, Abdelmohsen U. Bioactive Natural Products of Marine Sponges from the. Genus Hyrtios Molecules. 2017;22(5):781.
23. Jeon YJ, Han SH, Lee YW, Lee M, Yang KH, Kim HM. Dexamethasone inhibits IL-1β gene expression in LPS-stimulated RAW 264.7 cells by blocking NF-κB/Rel and AP-1 activation. Immunopharmacology. 2000;48(2):173–183. doi:10.1016/S0162-3109(00)00199-5
24. Copple IM, Dinkova-Kostova AT, Kensler TW, Liby KT, Wigley WC. NRF2 as an Emerging Therapeutic Target. Oxid Med Cell Longev. 2017;2017:8165458. doi:10.1155/2017/8165458
25. Zhang X, Ding M, Zhu P, et al. New Insights into the Nrf-2/HO-1 signaling axis and its application in pediatric respiratory diseases. Oxid Med Cell Longev. 2019;2019:3214196. doi:10.1155/2019/3214196
26. Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediators Inflamm. 2014;2014:
27. Rider P, Carmi Y, Cohen I. Biologics for Targeting Inflammatory Cytokines, Clinical Uses, and Limitations. Int J Cell Biol. 2016;11:9259646. doi:10.1155/2016/9259646
28. Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res. 2010;86(2):243–253. doi:10.1093/cvr/cvq016
29. Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13(5):738–747. doi:10.1038/sj.cdd.4401877
30. Menghini L, Ferrante C, Leporini L, et al. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother Res. 2016;30(9):1513–1518. doi:10.1002/ptr.5655
31. Locatelli M, Macchione N, Ferrante C, et al. Graminex pollen: phenolic pattern, colorimetric analysis and protective effects in immortalized prostate cells (PC3) and rat prostate challenged with LPS. Molecules. 2018;23(5):1145.
32. Zhou L-T, Wang K-J, Li L, Li H, Geng M. Pinocembrin inhibits lipopolysaccharide-induced inflammatory mediators production in BV2 microglial cells through suppression of PI3K/Akt/NF-κB pathway. Eur J Pharmacol. 2015;761:211–216. doi:10.1016/j.ejphar.2015.06.003
33. Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology. 2008;47(4):409–414. doi:10.1093/rheumatology/kem297
34. Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi:10.1186/1476-4598-12-86
35. Deshmukh P, Unni S, Krishnappa G, Padmanabhan B. The Keap1-Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys Rev. 2017;9(1):41–56. doi:10.1007/s12551-016-0244-4
36. Datta PK, Koukouritaki SB, Hopp KA, Lianos EA. Heme oxygenase-1 induction attenuates inducible nitric oxide synthase expression and proteinuria in glomerulonephritis. J Am Soc Nephrol. 1999;10(12):2540–2550.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.