Back to Journals » Risk Management and Healthcare Policy » Volume 16
Drug Pricing of Domestic Anti-PD-L1 Antibody Adebrelimab: Cost-Effectiveness Analysis of the First-Line ES-SCLC Treatment in China
Authors Zhou D, Dong X, Zhou Z, Liu Q
Received 7 September 2023
Accepted for publication 15 November 2023
Published 22 November 2023 Volume 2023:16 Pages 2521—2529
DOI https://doi.org/10.2147/RMHP.S439119
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gulsum Kubra Kaya
Dongchu Zhou,1 Xinrui Dong,1 Zhen Zhou,2 Qiao Liu1
1Department of Pharmacy, The Second Xiangya Hospital of Central South University, Changsha, People’s Republic of China; 2School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia
Correspondence: Qiao Liu, Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, Hunan, 410000, People’s Republic of China, Email [email protected]
Purpose: The market price of adebrelimab, the first Chinese-developed anti-PD-L1 antibody used as a first-line treatment for extensive stage-small-cell lung cancer (ES-SCLC), has garnered significant public attention. This study sought to investigate the affordable price of adebrelimab for Chinese patients with untreated ES-SCLC through a cost-effectiveness analysis.
Patients and Methods: We conducted a cost-effectiveness analysis using a Markov model, incorporating a what-if scenario of adding adebrelimab to first-line etoposide/platinum (EP) chemotherapy is cost-effective for ES-SCLC patients from the perspective of the Chinese healthcare system. The model included three mutually exclusive health states, with transition probabilities derived from the CAPSTONE-1 trial. Health state utilities and costs were acquired from a myriad of authoritative sources. We compared the incremental cost-effectiveness ratios (ICERs) for adebrelimab plus EP chemotherapy (AEP) versus EP with a willingness-to-pay threshold of $37,654 per quality-adjusted life-years (QALYs) to estimate the affordable price ceiling of the upcoming adebrelimab.
Results: For the entire ES-SCLC population, the estimated price ceiling of adebrelimab/mg was $0.542 (95% CI, $0.542-$0.552). Subgroup analyses found that the highest price ceiling of adebrelimab/mg was observed in ES-SCLC patients with lactate dehydrogenase concentration ≤ upper normal limit [$0.824 (95% CI, $0.815-$0.830)]; and the lowest was found in ES-SCLC patients with liver metastasis [$0.252 (95% CI, $0.250- $0.256)]. Sensitivity analysis revealed a heightened probability of cost-effectiveness for the first-line AEP as the price of adebrelimab decreased, encompassing both the entire ES-SCLC population and its subgroups.
Conclusion: The affordable price range for adebrelimab/mg Chinese patients with untreated ES-SCLC was estimated to be between $0.252 and $0.824, with variations observed across different subgroups. In the context of universal healthcare coverage, our study provides valuable evidence to inform the implementation of a value-based pricing strategy for cancer treatment.
Keywords: cost-effectiveness, extensive stage-small-cell lung cancer (ES-SCLC), adebrelimab, drug pricing, China
Introduction
Lung cancer remains a serious public health issue in China, with 870,982 new cases and 766,898 deaths recorded in 2020.1,2 Small cell lung cancer (SCLC), which is a fraction of all lung cancers (~15%), represented an aggressive malignancy with a five-year survival rate of around 7%.3 SCLC is characterized by an exceptionally rapid tumor growth and strong predilection for early metastasis. SCLC is classified into limited stage disease and extensive-stage (ES) disease, with the latter accounting for more than two thirds of the SCLC cases.4 In recent years, major milestones have been achieved in ES-SCLC immuno-chemotherapy with significant survival benefits and favorable safety, leading to the approval of the combination of programmed cell death protein-1/ligand-1 (PD-1/L1) inhibitors and first-line etoposide/platinum (EP) chemotherapy for ES-SCLC.5–8 As of now, two imported PD-1/L1 inhibitors (atezolizumab and durvalumab) in combination of EP chemotherapy have been listed as the standard-of-care for the management of ES-SCLC in the latest Chinese society of clinical oncology (CSCO) Guidelines.9 However, the widespread use of these two immuno-chemotherapies in China is impractical, because of their unfavorable cost-effectiveness driven by the prohibitive price of imported anti-PD-1/L1 agents.10,11
To improve the accessibility to PD-1/L1 inhibitors in patients with ES-SCLC, Chinese government has been pouring large sums of funding into supporting the independent research for the development of domestic anticancer drugs.12 Adebrelimab (SHR-1316) is the first China-developed anti-PD-L1 antibody that has shown anti-tumor activity as the first-line treatment of ES-SCLC.13 A recent clinical trial named CAPSTONE-1 investigated the efficacy and safety of adding adebrelimab to standard first-line EP chemotherapy in participants with untreated ES-SCLC (ClinicalTrials.gov Identifier: NCT03711305).13 The study reported that the combination therapy significantly improved overall survival (OS) compared with chemotherapy alone (median OS: 15.3 vs 12.8 months; hazard ration [HR]: 0.72 [95% CI:0.58–0.90]; p=0.0017), with acceptable safety profile. These favorable clinical evidence supports the combination of adebrelimab and EP chemotherapy (AEP) used as a new first-line treatment option for ES-SCLC.
The upcoming market price of adebrelimab has generated significant public attention due to its compelling clinical efficacy and the crucial role that the price of anticancer drugs plays in determining their widespread use.14 Cost-effectiveness analysis serves as a valuable tool for generating evidence to inform payers about therapy value and guiding drug pricing decisions.15,16 In this study, we conducted a cost-effectiveness analysis to investigate the affordable price of adebrelimab for Chinese patients with untreated ES-SCLC. Our primary objective was to provide insightful information for informed decision-making regarding the pricing of adebrelimab, with the ultimate aim of maximizing its potential benefits for the target patient population in China. The findings from this rigorous cost-effectiveness analysis will offer valuable evidence to guide the implementation of a value-based pricing strategy for cancer treatment.
Materials and Methods
Analytical Overview
To find the appropriate price for the upcoming adebrelimab, we designed a Markov model with a what-if scenario. That is, adding adebrelimab to the first-line EP chemotherapy is cost-effective for ES-SCLC patients from the perspective of the Chinese healthcare system. In this model, the clinical trajectory of ES-SCLC patients was simulated using 3 mutually exclusive health states: progression-free disease (PFD), post-progression disease (PD) and death (Figure 1). The Markov cycle length was purposefully established as 21 days to align with the clinical dosing interval for ES-SCLC treatment.9,13 Additionally, a 10-year time horizon was chosen to ensure that nearly 99% of participants reach the terminal health state representing death. This extended timeframe enabled a comprehensive understanding of ES-SCLC’s trajectory and its long-term implications on patients’ health.
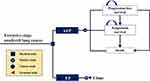 |
Figure 1 Markov model diagram. ES-SCLC extensive stage-small-cell lung cancer, AEP adebrelimab combined with etoposide and platinum, EP etoposide plus platinum. |
All patients entered in the model with the PFD health state and were randomly assigned to either the AEP group or the EP group. Patients in the AEP group received 4–6 cycles of EP chemotherapy (etoposide, 100mg/m2 administered on days 1–3 of each cycle; carboplatin, area under the curve of 5mg/mL/min administered on day 1 of each cycle) and up to 35 cycles of adebrelimab (20 mg/kg on day 1 of each cycle).13 Patients in the EP group received EP chemotherapy with the identical dosage and schedule, while being assigned to placebo instead of adebrelimab. Patients experiencing disease progression during the period of first-line treatment will move to the PD health state, in which certain patients were proceeded to subsequent anticancer therapy. As per the CAPSTONE-1 trial, subsequent anticancer therapies used in our analyses were chemotherapy, targeted therapy, antiangiogenic therapy and immunotherapy. Finally, patients were provided end-of-life care before death as recommended by the CSCO Guidelines.9
We calculated the incremental cost-effectiveness ratio (ICER) as the incremental medical cost consumed for adebrelimab added to the first-line EP chemotherapy for each additional quality-adjusted life-year (QALY) gained. Using a willingness-to-pay (WTP) threshold of $37,654 per QALY (defined as three times of China’s per capita GDP in 2021),17 we then determined the cost-effectiveness of AEP under different hypothetical market prices of adebrelimab, so as to to investigate the affordable price of adebrelimab for Chinese ES-SCLC patients. In this study, both costs and QALYs were discounted at an annual rate of 5%.17
The statistical tools used in this study were treeAge Pro Healthcare software (version 2021, https://www.treeage.com/) and R software (version 4.0.4, http://www.r-project.org). Our study collected and analyzed published data and was therefore deemed exempt from the approval of Clinical Ethics Committee of the Second Xiangya Hospital of Central South University.
Clinical Effectiveness
In the Markov model, the clinical effectiveness was quantified by QALY, which was determined by transition probability between health states and utility assigned to each health state.
We estimated the transition probabilities between health states using the Kaplan-Meier (KM) curves from the CAPSTONE-1 trial publication.13 First, survival data for each treatment strategy were digitized via the GetData Graph Digitizer software (version 2.26; http://www.getdata-graphdigitizer.com/index.php), and then fitted with five commonly used parametric survival models: exponential, Weibull, log-normal, log-logistic and Gompertz distributions. To determine the best-fit survival models, we conducted goodness-of-fit tests using Akaike information criterion (AIC) and Bayesian information criterion (BIC). The survival model with the lowest AIC and BIC values was selected. We subsequently used the parameters of these best-fit survival models to estimate the transition probabilities between health states. The results of these goodness-of-fit tests are presented in Tables S1, S2 and Figures S1–S4.
Due to the dearth of health state utilities specific to Chinese ES-SCLC patients, the model employed utilities from a previous literature conducted on Chinese patients with non-small cell lung cancer.18 The utility scores obtained from this literature were 0.856 for the PFD health state and 0.768 for the PD health state, respectively.18 Experiencing grade III/IV adverse events (AEs) during the period of first-line treatments was considered to be associated with a decrement in utility (otherwise known as disutility).19 To calculate the disutility of each first-line treatment, we assumed that all AEs occurred in the first cycle and then multiplied the disutilities reported for specific AEs by the corresponding AEs frequencies observed in the CAPSTONE-1 clinical trial. The disutility of each AE was summed up to obtain the disutility for the first-line treatment of interest (Table S3). All clinical effectiveness inputs are summarized in Table S4.
Medical Costs
We analyzed data from the perspective of the Chinese healthcare system. In this study, all costs were inflated to 2021 prices using domestic inflation rates derived from China’s healthcare consumer price index in 2021 and then exchanged into US dollars using the exchange rate in 2021 (1 USD was equivalent to 6.4514 CNY).20
We considered the costs for drug acquisition and treating AEs. Etoposide and carboplatin acquisition prices were obtained from the National Health Industry Data Platform.21 To calculate the dosages of etoposide and carboplatin, we assumed that the model patients have a mean body surface area of 1.72 m2 and a mean creatinine clearance rate of 70mL/min.22 The treatment costs of all grade III/IV AEs were included in the model, and these AEs costs were estimated locally (Table S3). Other direct costs such as routine follow-up costs, subsequent therapy costs, best supportive care costs and end-of-life care costs that were derived from previous literature were also considered in the model.22
Price Simulation
The model set the price of adebrelimab/mg to vary between $0 and $4.21. The lower price limit of $0 was set to investigate the lowest price of the upcoming adebrelimab that Chinese ES-SCLC patients could afford. The upper price limit of $4.21 was set according to the bid-winning price of durvalumab that is available on the National Health Industry Data Platform as of December 30, 2021, because durvalumab was the only PD-L1 inhibitor recommended by the CSCO Guidelines for the first-line treatment of ES-SCLC. We used a pre-specified average body weight of 65.0 kg of Chinese ES-SCLC patients when calculating the dosage of treatment.22 Based on the latest bid-winning price of durvalumab of $ 2804 per 500mg,21 and the dosage of 15mg/kg per 21-day cycle,23 we estimated that the cost of durvalumab per cycle would be $ 5468. Because the cost of domestic anticancer drugs is generally much lower than that of similar imported products,24 we assumed that the cost of adebrelimab per cycle would not exceed $ 5468. Accordingly, we calculated the upper price limit of adebrelimab/mg by dividing the upper cost limit of adebrelimab/cycle ($ 5468) by the dosage of adebrelimab/cycle (20mg/kg*65kg), which was $ 4.21/mg.
Subgroup and Sensitivity Analysis
In conducting subgroup analyses, initially, we gathered HRs for OS and PFS comparing first-line AEP with first-line EP in different subgroups from the CAPSTONE-1 clinical trial. As there was a lack of survival data specifically pertaining to first-line EP in these subgroups, we made the assumption that the survival benefit observed in the entire group was consistent across all subgroups within the EP group. Subsequently, the HR of OS or PFS for each subgroup was individually incorporated into the model to adjust the survival probabilities for first-line AEP, utilizing the following formula: 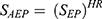 . In the sex-specific subgroup, we assumed male and female patients having an average body weight of 69.6kg and 59.0kg, respectively.25
. In the sex-specific subgroup, we assumed male and female patients having an average body weight of 69.6kg and 59.0kg, respectively.25
Deterministic sensitivity analyses (DSA) were implemented to test the impact of individual parameters on our results. During DSA, the discount rate was tested between 0%~8%,17 utilities were tested within the reported ±95% confidence intervals (CIs),18 and other parameters were tested within ±20% of the baseline value. Please refer to Table S4 for specific details of the parameter variations during the DSA.
Probabilistic sensitivity analyses (PSA) were carried out to determine the probabilities of AEP being cost-effective under different hypothetical market prices of adebrelimab. In PSA, 10,000 Monte Carlo simulations were performed using model parameters randomly sampled from the distribution listed in Table S4.
Results
Base-Case Analysis
For the entire ES-SCLC population, within the assumed market price range of adebrelimab/mg, the ICERs between first-line AEP and first-line EP varied from $11,236/QALY to $216,409/QALY (Table 1), with the value decreasing with a lower price of adebrelimab (Figure 2). A price of adebrelimab/mg less than $0.542 would allow the ICER for AEP versus EP below the WTP threshold of $ 37,654 per QALY (Figure 2)
 |
Table 1 Summary of Simulation Results |
Subgroup Analyses
Subgroup analysis results are detailed in Table 1. Figure 2 shows the relationship between the price of adebrelimab/mg and the ICER of first-line AEP versus first-line EP. To make the first-line AEP more cost-effective than the first-line EP, the price ceiling of adebrelimab per mg was $0.824 ($0.815-$0.830) in the subgroups of ES-SCLC patients with lactate dehydrogenase (LDH) concentration ≤upper normal limit (ULN), which was highest among all subgroups; the subgroups of ES-SCLC patients with liver metastasis (LDH) gained the lowest price ceiling of adebrelimab per mg, which is $0.252 ($0.250-$0.256).
Sensitivity Analysis
Figure S5 showed the results of DSA for entire patient population. We found that the price ceiling of adebrelimab/mg, which render AEP cost-effective, fluctuated between $0.41 to $0.68 as a result of parameter uncertainty. The mean patient weight had the greatest impact on model results, followed by the proportion of of subsequent anticancer therapies. Changes in other parameters have little impact on the model results.
Figure S6 illustrated the probability of first-line AEP to be cost-effective compared with first-line EP under different marketing prices of adebrelimab/mg. In general, the probability increased with the decreasing adebrelimab price in both the entire ES-SCLC population and subgroups.
Discussion
In 2008, the Chinese government launched the “major new drug development” project to improve the accessibility and affordability of cancer treatments for patients.26 The program has had a significant and far-reaching impact on reducing the national burden of cancer by expediting the discovery and development of domestic anticancer agents.24 Notably, remarkable achievements have been made in the field of lung cancer therapeutic drugs, primarily due to China having the largest population of lung cancer cases globally, accounting for more than one-third of all cases.1,2 Out of the 26 domestically approved anticancer drugs in China since 2016, nine are specifically designed for the treatment of lung cancer.27 Adebrelimab stands out as the first domestically developed anti-PD-L1 antibody used as a first-line treatment for ES-SCLC.
Domestic anticancer drugs have significant market potential due to their cheaper price compared to imported alternatives and their successful performance in trials against active controls. However, the market price remains a critical factor in determining their widespread use among a large population.14 In the past few decades, the treatment of ES-SCLC has primarily relied on traditional platinum-based chemotherapy, which has shown undesirable efficacy.9 Although imported PD-1/L1 inhibitors like atezolizumab and durvalumab have been introduced into China for ES-SCLC treatment in recent years, their cost of over $5000 per 21-day cycle make them unaffordable for most of Chinese patients.21 The emergence of adebrelimab is expected to revolutionize the therapeutic paradigm for ES-SCLC, generating significant interest from the government, academia and the public regarding its upcoming price. In our current study, we explored the affordable price for adebrelimab by constructing a Markov model from the perspective of the Chinese healthcare system. Our findings suggest that, for the entire ES-SCLC population, at a WTP threshold of $37,654 per QALY, first-line AEP was cost-effective when the price of adebrelimab/mg was below $0.542. Subgroup analyses revealed that first-line AEP outperformed first-line EP in all subgroups when the price of adebrelimab/mg was lower than $0.252; conversely, first-line AEP was inferior to first-line EP when the price of adebrelimab/mg exceeded $0.824. Within the price range of $0.252 and $0.824 per mg of adebrelimab, first-line AEP demonstrated improved performance in specific subgroups. For instance, at a price of $0.4 per mg of adebrelimab, first-line AEP was not cost-effective in the subgroups of ES-SCLC patients with ECOG performance status score of 0, LDH concentration >ULN and liver metastasis. Our sensitivity analysis indicated an increased probability of first-line AEP being cost-effective compared to first-line EP as the price of adebrelimab decrease, both for the entire ES-SCLC population and its subgroups.
This systematic cost-effectiveness study provides valuable insights into the pricing of the upcoming anticancer drug, adebrelimab. In 2009, China implemented the renowned “Universal Medical Insurance System”, achieving coverage of over 95% of the population by 2020.28,29 As a result, the expenditure of the medical insurance fund has quintupled over the past 11 years, reaching $331.7 billion from $52.6 billion.29 The sustainable development of the “Universal Medical Insurance System” not only requires the coverage of medical expenditure but also necessitates addressing the significant treatment demand from the vast population base of approximately 1.4 billion.28 In this context, reasonable pricing of cancer drugs becomes an crucial. The value-based pricing informed by this cost-effectiveness study will provide useful guidance for determining the price of the upcoming adebrelimab.
Reasonably priced adebrelimab offers several positive impacts at both the individual level and societal level. Firstly, compared to similar imported drugs, a lower cost of adebrelimab increases the likelihood that Chinese ES-SCLC patients can afford this crucial life-saving treatment. Secondly, treating ES-SCLC with reasonably priced adebrelimab significantly reduces the medical expenditure associated with this disease and alleviate the budgetary strains on the Chinese healthcare system. Thirdly, the saved medical expenditure can be used to subsidize and reimburse treatments for other types of cancer, thereby promoting the sustainable development of the Universal Medical Insurance System.
This study has several limitations.First, due to the lack of survival data for first-line EP in different subgroups, we assumed that the survival benefit was consistent across all subgroups by applying the survival data from the entire EP group. Secondly, potential biases may be introduced by incorporating non-native data, such as some literature-based costs, into the model. Thirdly, Thirdly, the lack of health state utilities specifically tailored to Chinese ES-SCLC patients introduces a level of uncertainty. In order to address this, utility scores from previous literature evaluating Chinese patients with non-small cell lung cancer were employed in the model. However, it is important to note that acquiring more accurate and relevant data would further enhance the precision and accuracy of the model. Fourthly, the inherent bias of model-based analysis lied in the uncertainty surrounding post-trial outcomes for patients. Lastly, while our conclusion may not be generalized to other counties due to the unique perspectives and data used in this study, it is worth noting that China has the highest distribution of new lung cancer cases. Therefore, the valuable evidence yielded from this study holds the potential to alleviate disease and financial burdens on both a national and global scale.
Conclusion
In conclusion, our study has determined that the affordable price range of adebrelimab/mg for Chinese patients with untreated ES-SCLC is estimated to be between $0.252 and $0.824, with variations observed among different subgroups. These findings provide valuable evidence to guide the development of a value-based pricing strategy for cancer treatment.
Data Sharing Statement
The original contributions presented in the study are included in the article and Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
All authors contributed to this publication were listed.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the Hunan Province Natural Science Foundation (NO. 2023JJ60140).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–590. doi:10.1097/CM9.0000000000002108
3. Rudin CM, Ismaila N, Hann CL, et al. Treatment of small-cell lung cancer: American society of clinical oncology endorsement of the American college of chest physicians guideline. J Clin Oncol. 2015;33(34):4106–4111. doi:10.1200/JCO.2015.63.7918
4. van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741–1755. doi:10.1016/S0140-6736(11)60165-7
5. Horn L, Mansfield AS, Szczęsna A, et al. First-Line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi:10.1056/NEJMoa1809064
6. Liu SV, Reck M, Mansfield AS, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. 2021;39(6):619–630. doi:10.1200/JCO.20.01055
7. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, Phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi:10.1016/S0140-6736(19)32222-6
8. Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. doi:10.1016/S1470-2045(20)30539-8
9. Guidelines Working Committee of Chinese society of Clinical Oncology. Guidelines of Chinese Society of Clinical Oncology (CSCO) for Small Cell Lung Cancer. Beijing: People’s Medical Publishing House; 2021:141.
10. Li LY, Wang H, Chen X, et al. First-line atezolizumab plus chemotherapy in treatment of extensive small cell lung cancer: a cost-effectiveness analysis from China. Chin Med J. 2019;132(23):2790–2794. doi:10.1097/CM9.0000000000000536
11. Liu G, Kang S. Cost-effectiveness of adding durvalumab to first-line chemotherapy for extensive-stage small-cell lung cancer in China. Expert Rev Pharmacoecon Outcomes Res. 2022;22(1):85–91. doi:10.1080/14737167.2021.1888717
12. Central People’s Government of the People’s Republic of China. China has strengthened the research and development of anti-cancer drugs and promoted the supply guarantee of patented drugs. Available from: http://www.gov.cn/xinwen/2018-08/06/content_5311957.htm.
13. Wang J, Zhou C, Yao W, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739–747. doi:10.1016/S1470-2045(22)00224-8
14. Liu Q, Zhou Z, Luo X, et al. First-Line ICI monotherapies for advanced non-small-cell lung cancer patients With PD-L1 of at least 50%: a cost-effectiveness analysis. Front Pharmacol. 2021;12:788569. doi:10.3389/fphar.2021.788569
15. Neumann PJ, Cohen JT, Ollendorf DA. Drug-pricing debate redux - should cost-effectiveness analysis be used now to price pharmaceuticals? N Engl J Med. 2021;385(21):1923–1924. doi:10.1056/NEJMp2113323
16. Hammad EA. The use of economic evidence to inform drug pricing decisions in Jordan. Value Health. 2016;19(2):233–238. doi:10.1016/j.jval.2015.11.007
17. Chinese Pharmaceutical Association. China guidelines for pharmacoeconomic evaluations; 2020. Available from: https://www.cpa.org.cn/cpadmn/attached/file/20201203/1606977380634185.pdf.
18. Shen Y, Wu B, Wang X, et al. Health state utilities in patients with advanced non-small-cell lung cancer in China. J Comp Eff Res. 2018;7(5):443–452. doi:10.2217/cer-2017-0069
19. Nafees B, Lloyd AJ, Dewilde S, et al. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13(5):e195–e203. doi:10.1111/ajco.12477
20. National Bureau Of Statistics. National annual data. Available from: https://data.stats.gov.cn/easyquery.htm?cn=C01.
21. National Health Industry Data Platform. Bid winning information of drugs. Available from: https://www.yaozh.com/.
22. Luo X, Liu Q, Zhou Z, et al. Cost-effectiveness of bevacizumab biosimilar ly01008 combined with chemotherapy as first-line treatment for Chinese patients with advanced or recurrent nonsquamous non-small cell lung cancer. Front Pharmacol. 2022;13:832215. doi:10.3389/fphar.2022.832215
23. U.S. FDA National Drug Code DataBase. IMFINZI® (durvalumab) injection, for intravenous use. Available from: https://www.drugfuture.com/fda-ndc/label.aspx?ProductNDC=0310-4500.
24. Li G, Qin Y, Xie C, et al. Trends in oncology drug innovation in China. Nat Rev Drug Discov. 2021;20(1):15–16. doi:10.1038/d41573-020-00195-w
25. National Health Commission of the People’s Republic of China. Press conference of the information office of the state council on December 23, 2020. Available from: http://www.nhc.gov.cn/xcs/s3574/202012/bc4379ddf4324e7f86f05d31cc1c4982.shtml.
26. National Health Commission of the People’s Republic of China. Notice on printing and distributing major science and technology projects of “major new drug creation”. http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=37578.
27. National Health Industry Data Platform. Domestic drugs. https://db.yaozh.com/pijian.
28. Yip W, Fu H, Chen AT, et al. 10 years of health-care reform in China: progress and gaps in universal health coverage. Lancet. 2019;394(10204):1192–1204. doi:10.1016/S0140-6736(19)32136-1
29. National Healthcare security Administration. Statistical bulletin on the development of national medical security in 2020. Available from: http://www.nhsa.gov.cn/art/2021/6/8/art_7_5232.html.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

