Back to Journals » Journal of Inflammation Research » Volume 14
Donor-Specific Antibodies Against Donor Human Leukocyte Antigen are Associated with Graft Inflammation but Not with Fibrosis Long-Term After Liver Transplantation: An Analysis of Protocol Biopsies
Authors Gül-Klein S , Hegermann H, Röhle R, Schmelzle M, Tacke F , Schöning W, Öllinger R , Dziodzio T, Maier P, Plewe JM, Horst D, Sauer IM, Pratschke J, Lachmann N, Eurich D
Received 24 February 2021
Accepted for publication 4 May 2021
Published 23 June 2021 Volume 2021:14 Pages 2697—2712
DOI https://doi.org/10.2147/JIR.S307778
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Safak Gül-Klein,1 Henriette Hegermann,1 Robert Röhle,2– 4 Moritz Schmelzle,1 Frank Tacke,5 Wenzel Schöning,1 Robert Öllinger,1 Tomasz Dziodzio,1 Patrick Maier,1 Julius M Plewe,1 David Horst,6 Igor Maximilian Sauer,2 Johann Pratschke,1 Nils Lachmann,7 Dennis Eurich1
1Charité – Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Department of Surgery, Campus Charité Mitte, Campus Virchow-Klinikum, Berlin, Germany; 2Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Institute of Biometry and Clinical Epidemiology, Berlin, Germany; 3Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Coordinating Center for Clinical Studies, Berlin, Germany; 4Berlin Institute of Health (BIH), Berlin, Germany; 5Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Department of Hepatology & Gastroenterology, Campus Charité Mitte, Campus Virchow-Klinikum, Berlin, Germany; 6Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Institute of Pathology, Charité – Universitätsmedizin Berlin, Berlin, Germany; 7Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, HLA Laboratory, Institute of Transfusion Medicine, Histocompatibility and Immunogenetics, Charité - Universitätsmedizin, Berlin, Germany
Correspondence: Safak Gül-Klein
Department of Surgery, Campus Charité Mitte and Campus Virchow-Klinikum, Charité – Universitätsmedizin Berlin, Augustenburger Platz 1, Berlin, 13353, Germany
Tel +49 30 450 652 195
Fax +49 30 450 552 900
Email [email protected]
Background: Donor-specific antibodies (DSA) against donor human leukocyte antigen after liver transplantation, which are associated with histological changes, have been widely studied with respect to their sustained impact on transplant function. However, their long-term impact after liver transplantation remains unclear.
Methods: We performed a cross-sectional analysis from June 2016 to July 2017 that included all patients who presented themselves for scheduled follow-up after receiving a liver transplantation between September 1989 and December 2016. In addition to a liver protocol biopsy, patients were screened for human leukocyte antigen antibodies (HLAab) and donor-specific antibodies. Subsequently, the association between human leukocyte antigen antibodies, donor-specific antibodies, histologic and clinical features, and immunosuppression was analyzed.
Results: Analysis for human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen was performed for 291 and 271 patients. A significant association between higher inflammation grades and the presence of human leukocyte antigen antibodies and donor-specific antibodies was detected, while fibrosis stages remained unaffected. These results were confirmed by multivariate logistic regression for inflammation showing a significant increase for presence of human leukocyte antigen antibodies and donor-specific antibodies (OR: 4.43; 95% CI: 1.67– 12.6; p=0.0035). Furthermore, the use of everolimus in combination with tacrolimus was significantly associated with the status of negative human leukocyte antigen antibodies and donor-specific antibodies. Viral etiology for liver disease, hepatocellular carcinoma (HCC) and higher steatosis grades of the graft were significantly associated with a lower rate of human leukocyte antigen antibodies. The impact of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen was associated with higher levels of laboratory parameters, such as transaminases and bilirubin.
Conclusion: Donor-specific antibodies against donor human leukocyte antigen are associated with histological and biochemical graft inflammation after liver transplantation, while fibrosis seems to be unaffected. Future studies should validate these findings for longer observation periods and specific subgroups.
Keywords: human leukocyte antigen antibodies, donor-specific antibodies, liver biopsy, liver transplantation
Introduction
Routine protocol liver biopsies after liver transplantation allow the observation and evaluation of parenchymatous changes and their dynamics via specific histological determinants (inflammation, steatosis, fibrosis). Together with donor-specific antibodies against donor human leukocyte antigen they can serve as a diagnostic tool in patients with antibody-mediated changes of the liver graft. Such changes in liver biopsies can be silent alarms and indicate ongoing immunological processes, even if they remain clinically unremarkable at first.1–6 The occurrence of donor-specific antibodies against donor human leukocyte antigen after organ transplantation has gained enormous attention in the field as a potential pathological mechanism involved in mediating graft dysfunction.7–10 Donor-specific antibodies against donor human leukocyte antigen after liver transplantation may have a negative impact on graft and patient survival according to previously published studies.11–15 Chronic rejection after liver transplantation has been shown to be associated with the presence of donor-specific antibodies against donor human leukocyte antigen.16,17 Understanding the interaction between circulating donor-specific antibodies against donor human leukocyte antigen and histologic changes could have a profound impact on prevention of graft dysfunction and graft loss, acute therapeutical intervention, as well as long-term graft survival and could also influence further medical decisions.18 Especially regarding fibrosis after liver transplantation, there is still a need to better understand and assess cellular processes and potential risk factors.19–21
In the long term, the clinical relevance of donor-specific antibodies against donor human leukocyte antigen after liver transplantation is not conclusively clear.22 There are indications that acute unclear organ loss is associated with the presence of human leukocyte antigen antibodies, on the other hand, the presence of donor-specific antibodies against donor human leukocyte antigen may not be associated with any graft pathology.23,24
The relevance of positive detection of donor-specific antibodies against donor human leukocyte antigen and practical consequences for clinical management are currently unclear.25 Therefore, we have specifically collected and classified histological features of protocol liver biopsies and correlated them with donor-specific antibodies against donor human leukocyte antigen in order to determine the relevance of donor-specific antibodies against donor human leukocyte antigen and human leukocyte antigen antibodies on the biochemical, histological and clinical level including biliary complications.
Patients and Methods
Study Population
We analyzed 291 patients between June 2016 to July 2017, who were on routine follow-up after liver transplantation at the Surgical Department, Campus Virchow-Klinikum, Charité - Universitätsmedizin Berlin. The allocation of donor organs in Germany was the responsibility of Eurotransplant. The German Foundation for Organ Transplantation (DSO) coordinated and organized the post-mortem organ donations from the registration of a potential donor by a hospital until the transfer of the organs to the transplant centers. In this context, organ donation was always voluntarily with written informed consent in accordance with the Istanbul Declaration. All patients were tested for human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen. Relevant data (clinical course, laboratory parameters, human leukocyte antigen antibodies as well as donor-specific antibodies against donor human leukocyte antigen results and pathology reports from protocol liver biopsies) for this patient cohort – who underwent liver transplantation between January 1989 and December 2016 – were collected in a prospective manner during this period. Five patients, who were originally transplanted externally, were also in our follow-up care at this time. The cross-sectional analysis focused on the evaluation of HLAab and DSA. The crossmatch detects cytotoxic DSA. In our study, we report non-cytotoxic DSA detected by Luminex. Differentiation between de novo and preformed DSA was not possible based on the current data.
The study was conducted according to the principles of good scientific practice and the Declaration of Helsinki. Patient data were anonymized, retrospectively analyzed. The institutional review board Ethikkommission of the Charité – Universitätsmedizin Berlin (EA2/150/13) approved the study.
Data Collection
Demographic characteristics and clinical variables, such as donor and recipient age at the time of routine follow-up, gender of the recipient, underlying diseases, and laboratory and histological data were collected and evaluated. Furthermore, we evaluated the impact of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen on the incidence of biliary complications in terms of non-anastomotic biliary strictures (Table 1).
 |
Table 1 Patient Characteristics |
Immunosuppression
Immunosuppressive therapy was mainly based on calcineurin inhibitors (CNI) - with tacrolimus (tac) being used more frequently than cyclosporin A (CyA) – and was differentiated into mono- and dual-therapy. Adjustments in immunosuppressive therapy were made on an individual basis. Dosage of steroids were routinely reduced based on a standardized scheme and discontinued 2 months after liver transplantation or were continued with a maintenance dose of 5mg if the patients had autoimmune hepatitis. Combination therapy with antimetabolites, such as mycophenolate mofetil (MMF), was administered on an individual basis, including cases of reduced renal function. Mammaliantarget of rapamycin-inhibitors (mTORi), such as sirolimus and everolimus, were used predominantly in patients with hepatocellular carcinoma (HCC) in the explanted liver and in case of impairment of kidney function. Average doses of IS and trough levels were recorded according to clinical standard during hospital stay as well as during aftercare. For this purpose, the trough levels of immunosuppression were determined and controlled against the target value. Correlation analysis of the donor-specific antibodies against donor human leukocyte antigen was performed (Table 2).
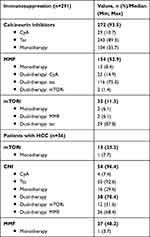 |
Table 2 Immunosuppression |
Post-Liver Transplantation Aftercare
Standardized follow-up included per-protocol liver biopsies, alpha-1-fetoprotein (AFP) determination if hepatocellular carcinoma was present, and flow measurement as well as evaluation of liver parenchyma for steatosis, fibrosis and abnormal lesions using ultrasound monitoring, Follow-up was at 6 months, 1, 3, 5, 7, 10 and 13 or more years after liver transplantation for lifetime.
Histopathology
As part of routine follow-up, patients retrospectively examined in the cross-sectional study received scheduled protocol biopsies, which were evaluated in a standardized fashion by senior pathologists specialized and experienced in transplantation. We then evaluated these final histopathologic findings as part of our retrospective analysis. For the histopathological evaluation of liver tissue, the following were considered: signs of acute rejection, fibrosis, mesh wire fibrosis, inflammation, steatosis and hemosiderosis as well as bile duct changes. Fibrosis stages were determined according to Desmet et al (0: absent, 1: mild without septa, 2: moderate with few septa, 3: numerous septa without cirrhosis, 4: cirrhosis). For comparability reasons, stages 0 and 1 were summarized and compared with significant and advanced fibrosis stages containing septa (F2–F4). Degrees of inflammation were classified according to Desmet and Scheuer (0: none, 1: minimal, 2: mild, 3: moderate, 4: severe) and for comparability reasons evaluated by groups with no or minimal inflammation versus mild, moderate and severe. The Desmet and Scheuer classification was chosen because of better reproducibility.26 Further histological features such as steatosis (0: no steatosis, 1: low grade (<1/3 of cells), 2: moderate (>1/3 of cells), 3: high grade (>2/3 of cells)) and hemosiderosis (0: none, 1: low grade, 2: moderate, 3: severe/ high grade) were categorized and later evaluated (Table 3).
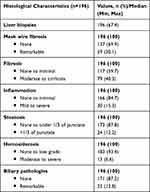 |
Table 3 Histological Characteristics of Protocol Liver Biopsies |
Human Leukocyte Antigen Antibodies and Donor-Specific Antibodies Against Donor Human Leukocyte Antigen Analysis
Commercial Luminex-based tests were used to detect and specify human leukocyte antigen antibodies in the recipient serum. These assays are solid-phase immunoassays in which the human leukocyte antigen molecules are bound to a solid matrix. Contrary to the less sensitive enzyme-mediated ELISA technique, fluorescence-impregnated latex beads are used. These beads are impregnated with a mixture of two fluorescent dyes with an adsorption maximum at 635 nm and emission maxima of 580 and 660 nm graduated so that they can be individually distinguished from each other with a flow cytometer (Luminex® 100/200 or LABScan3D) designed for this purpose.
Each of these up to 100 beads carries different human leukocyte antigen molecules against which antibodies can be detected. For this purpose, 5µL beads are incubated with 20 µL patient serum for 30 min at room temperature (20–25°C) under gentle shaking, then washed 3 times with 150–200µL wash buffer and centrifuged for 5 min at 1300g. Antibodies can bind to corresponding HLA molecules on the beads and are detected by addition of 100µL of a detection antibody (goat anti-human IgG) coupled to R-Phycoerythrin (PE) after a further 30 min light protected incubation at room temperature followed by 2 washing steps (as described above) and measurement in Luminex® 100/200 or LABScan3D. Qualitative screening for human leukocyte antigen antibodies was performed with LABSCreen® Mixed Beads (One Lambda, West Hills, CA, USA) carrying antigen pools from 3 to 5 different individuals, separated according to human leukocyte antigen class I and II. Specification was performed downstream in a stepwise diagnostic procedure using LABScreen® Single Antigen Beads (One Lambda), each carrying a single human leukocyte antigen molecule. The amount of PE-coupled detection antibody is directly proportional to the intensity of the emitted fluorescence signal at 578 nm, which is expressed as mean fluorescence intensity (MFI). However, due to steric hindrances, the MFI signal does not correlate directly with the amount of bound human leukocyte antigen antibody. Nevertheless, this is the only marker that allows at least indirect conclusions about the amount of human leukocyte antigen antibody using Luminex® technology. Human leukocyte antigen antibodies with an MFI >1000 were evaluated as positive. Human leukocyte antigen antibodies detectable against human leukocyte antigen mismatches between donor and recipient were declared as so-called donor-specific antibodies against donor human leukocyte antigen.
Statistical Analysis
We compared categorical parameters by the Chi-square test, while we used the Mann–Whitney U-test to compare the antibody effect on the laboratory parameters of the recipient (continuous variables). In order to adjust the analyses of the occurrence of histopathological parameters for potential confounders, we performed multivariate logistic regression models. For this, we grouped human leukocyte antigen antibodies (HLAab) and donor-specific antibodies against donor human leukocyte antigen (DSA) into “no HLAab & no DSA”, “only HLAab” and “HLAab and DSA”. Besides human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen status, we considered hepatocellular carcinoma status, viral status and gender as covariates into the models depending on the number of occurrences. We chose the number of covariates so that about ten occurrences per parameter were available. The selection of variables was based on clinical expertise and results from the univariate testing. Further covariates (donor age, alcohol status, tacrolimus intake) were also considered but not included due to the small sample sizes and less relevance. We considered p values within the analyses as purely exploratory. All data were analyzed using IBM SPSS Statistics Version 26 statistical software and R (R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/; last use 01.11.2020).
Results
Patient Characteristics
In total, 291 patients after liver transplantation were characterized during their regular follow-up visits in our outpatient department for clinical events, biochemical and histological changes and for the presence of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen. There was an equal gender distribution (female: 122, 41.9%; male: 169, 58.1%). Main indications for liver transplantation included alcohol-related (83, 28.5%) and viral cirrhosis (73, 25.1%). Within this group, 56 (19.2%) patients also suffered hepatocellular carcinoma. The median time between liver transplantation and study inclusion was 8 years on average (range 0–28 years) and 4 years on average (range 0–22 years) for 23 (7.9%) patients after re-liver transplantation.
History of acute rejections occurring prior to the study period were documented in 141 (48.5%) patients and separated according to frequency (1: 89, 63.1%; >1: 39, 27.7%; >3: 13, 9.2%) (Table 1).
From the cohort of 291 patients a total of 147 (50.5%) patients were tested positive for human leukocyte antigen antibodies positive for class I (42, 27.9%) and class II (64, 43.5%). Of those 147 (50.5%) patients, 41 (27.9%) patients presented HLA class I and II antibodies. In 20 of 291 (6.9%) patients, donor-specific antibodies against donor human leukocyte antigen could not be determined due to incomplete donor typing. Presence of donor-specific antibodies against donor human leukocyte antigen was detected in 80 (29.5%) patients, while 191 (70.5%) patients had a negative status for donor-specific antibodies against donor human leukocyte antigen (Table 1).
Donor Characteristics
The majority of donors were 50 years or older (153/285, 53.7%) and male (157/285, 55.1%), no data were available for 4 (0.4%) donors. Specific donor characteristics are listed in detail in Supplemental Table 1. No associations between positive or negative results for human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen compared to donor gender, age or BMI were demonstrated (data not shown).
Immunosuppression
Immunosuppression was recorded for 289 (99.3%) patients, while two (0.7%) patients were free of IS. Calcineurin inhibitors (CNI) were baseline medication in 272 (93.5%) patients (tac: 243, 89.3%; CyA: 29, 10.7%), while Calcineurin inhibitors monotherapy was applied to 104 (35.7%) patients (Table 2). Trough levels (ng/mL) for calcineurin inhibitors (tacrolimus, cyclosporine A) (265, 97.4%) were divided into three groups for low dose, standard dose and high dose (Supplemental Table 2).
Immunosuppressive therapy with Mycophenolate mofetil (154, 52.9%) was considered separately for monotherapy (13, 8.4%) and in any combination as dual-therapy with calcineurin inhibitors and mammalian target of rapamycin-inhibitors (Table 2). MMF levels were determined by averaging the dose per day over the last six months and divided into two groups (low dose (<1g): 101, 66.0%; high dose (>1g): 52, 34.0%).
Antiproliferative medication (mammalian target of rapamycin-inhibitors: everolimus and sirolimus) was documented in 33 (11.3%) patients, mostly in combination therapy (tac: 29, 87.8%; MMF: 2, 6.1%) and only in 2 (6.1%) as monotherapy. Three mammalian target of rapamycin-Inhibitors groups based on the trough levels (ng/mL) of the last 6 months were divided into low dose (<3 ng/mL: 5, 15.2%), standard dose (3–5 ng/mL: 7, 21.2%) and high dose (>5 ng/mL: 17, 51.5%).
A detailed review of immunosuppression for 56 (19.2%) patients with hepatocellular carcinoma was additionally performed as a subgroup (Table 2).
Protocol Liver Biopsy and Histological Characteristics
Protocol liver biopsies during the study period were performed in 196 (67.4%) patients out of the patient cohort (n=291). Histologic characteristics such as fibrosis, mesh wire fibrosis, inflammation, steatosis, hemosiderosis, and biliary duct pathologies (Table 3) were confirmed. According to the stage and grade of those six histologic features, a grouping was performed.
Analyses for Human Leukocyte Antigen Antibodies and Donor-Specific Antibodies Against Donor Human Leukocyte Antigen Analysis
Patient Characteristics
An analysis for association of patient characteristics with positive results for human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen was performed. An equal distribution of gender, age and etiologies for positive and negative results for human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen is shown in Table 4.
 |
Table 4 Association of Patient Characteristics with HLA-AB and DSA |
Numerically more patients younger than 50 years of age showed positive versus negative results for donor-specific antibodies against donor human leukocyte antigen (21 (26.2%) versus 32 (16.8%)) compared to patients over 50 years of age (59 (73.8%) versus 159 (83.2%)), but this difference did not reach statistical significance (p=0.072). The distribution of human leukocyte antigen antibodies was equal.
Interestingly, the subgroup analysis presented significant differences regarding a viral etiology with a significantly lower prevalence of human leukocyte antigen antibodies (27 (18.4%) versus 46 (31.9%); p=0.008) as well as of donor-specific antibodies against donor human leukocyte antigen (12 (15.0%) versus 58 (30.4%); p=0.008) in patients with hepatitis B virus (HBV) and hepatitis C virus (HCV) induced end-stage liver disease compared to non-viral etiologies for end-stage liver disease.
In the subgroup analysis for hepatocellular carcinoma, significant differences were observed in the distribution of human leukocyte antigen antibodies. Presence of hepatocellular carcinoma in the explant liver was significantly associated with the absence of human leukocyte antigen antibodies (20 (13.6%) versus 36 (25.0%); p=0.014). However, there was no significant difference in the distribution of donor-specific antibodies against donor human leukocyte antigen status among patients with and without hepatocellular carcinoma as displayed in Table 4.
Remaining patient characteristics, such as body-mass index (BMI), age groups, re-transplant status and number of episodes of acute rejection did not show any association to the human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen status (Table 4).
Biliary abnormalities were also considered. They tended to be more frequent, though without statistical significance, in the presence of donor-specific antibodies against donor human leukocyte antigen (31 (38.8%) versus 52 (27.2%); p=0.060). Within this group, specific consideration of ischemic type biliary lesions (ITBL), as a non-anastomotic stricture, showed nearly significant values for positive donor-specific antibodies against donor human leukocyte antigen results (8 (42.1%) versus 8 (19.5%); p=0.066) (Table 4).
Immunosuppression
A consideration of both groups showed no significant differences in immunosuppressive therapy with respect to either calcineurin inhibitors (tacrolimus, cyclosporine A) and mycophenolate mofetil or for dual- and monotherapy or dosage of immunosuppressive medication. However it was found that patients with mammalian target of rapamycin-inhibitors medication (HLAab: n=33, DSA: n=32) had tested more frequently negative for human leukocyte antigen antibodies (10 (6.8%) versus 23 (16.0%); p=0.014) and negative for donor-specific antibodies against donor human leukocyte antigen (4 (5.0%) versus 28 (14.7%); p=0.025) (Table 5).
 |
Table 5 Association of Immunosuppression with HLA-AB and DSA |
Histological Features
Protocol biopsies and a grading of all examined histopathological features, such as fibrosis, inflammation, steatosis, hemosiderosis, bile duct pathologies and presence of mesh wire fibrosis, were assessed for a total of 196 patients and consecutively analyzed for its association with positivity and negativity of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen (Table 6).
 |
Table 6 Association of Histopathologic Parameters with HLA-AB and DSA |
Fibrosis stages 0–I were diagnosed in 117 patients and stages II–IV were found in 79 patients (Table 3). There was no significant difference between the presence and absence of human leukocyte antigen antibodies in the group with fibrosis stages 0-I (56 (57.1%) versus 61 (62.2%); p=0.467), as well as in the group with fibrosis stages II–IV (42 (42.9%) versus 37 (37.8%); p=0.467).
Furthermore, fibrosis was not associated with the presence of donor-specific antibodies against donor human leukocyte antigen, since higher fibrosis stages were distributed equally regarding positive versus negative results for donor-specific antibodies against donor human leukocyte antigen (21 (38.9%) versus 48 (38.1%); p=0.920).
The proportion of patients with mesh wire fibrosis was higher, but without statistical significance, in the presence of human leukocyte antigen antibodies (35 (35.7%) versus 24 (24.5%); p=0.087) as well as for positive donor-specific antibodies against donor human leukocyte antigen results (18 (33.3%) versus 32 (25.4%); p=0.276).
Higher inflammation grades (II–IV) were found in 30 (15.3%) patients. In total, adjusted logistic regression for inflammation is given in Table 7 and showed a non-significant increase for human leukocyte antigen antibodies only (OR versus no HLAab and no DSA: 3.14; 95% CI: 0.94–10.13; p=0.0553) and a significant increase for human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen-positive patients (OR versus no HLAab & no DSA: 4.43; 95% CI: 1.67–12.6; p=0.0035). The results for the remaining parameters given in Supplemental Tables 3–5 showed no further significant associations.
 |
Table 7 Logistic Regression for Inflammation |
Inverse and as well statistically significant associations were seen in higher grades (II–III) of steatosis for negative human leukocyte antigen antibodies results (7 (7.1%) versus 17 (17.3%); p=0.029) and negative donor-specific antibodies against donor human leukocyte antigen results (3 (5.6%) versus 21 (16.7%); p=0.044).
Analysis for hemosiderosis of any grade revealed an equal distribution between negative and positive human leukocyte antigen antibodies (grades 0–I: 92 (93.9%) versus 91 (92.9%); p=0.774) and as well donor-specific antibodies against donor human leukocyte antigen results (grades 0–I: 51 (94.4%) versus 118 (93.7%); p=0.839).
Interestingly, analysis of biliary pathologies presented no association to positive or negative human leukocyte antigen antibodies results (14 (14.3%) versus 11 (11.2%); p=0.521) or donor-specific antibodies against donor human leukocyte antigen results (6 (11.1%) versus 16 (12.7%); p=0.766) (Table 6).
Laboratory Values
Laboratory values, such as alanine-aminotransferase (ALT), aspartate-aminotransferase (AST), gamma-glutamyltransferase (GGT) and Bilirubin are visualized in boxplots and compared by the Mann–Whitney U-test (Figures 1 and 2). Significant higher mean values of alanine-aminotransferase (150.41 versus 129.22; p=0.042) (Figure 2), aspartate-aminotransferase (154.59 versus 127.46; p=0.009) (Figure 2), and gamma-glutamyltransferase (155.42 versus 127.11; p=0.007) (Figure 2) were seen for positive results of donor-specific antibodies against donor human leukocyte antigen. In addition, significant higher levels of mean values for bilirubin were found in association with positive results for human leukocyte antigen antibodies (0.59 mg/dl versus 0.49 mg/dl; p=0.006) and donor-specific antibodies against donor human leukocyte antigen (0.67 mg/dl versus 0.49 mg/dl; p=0.000) (Figure 2).
 |
Figure 1 ALT, AST, GGT, bilirubin values in association to HLAab. |
 |
Figure 2 ALT, AST, GGT, bilirubin values in association to DSA. |
Discussion
Clinical variables were collected in the sense of a cross-sectional study over 1 year and evaluated retrospectively. The association between human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen to specific histological features was investigated by protocol biopsies. Some of the examined patients had been transplanted several decades ago as well as only 1 year ago. This background implies that there have been changes in therapies and associated courses, eg, due to the conversion of immunosuppressive therapies. The present study was designed to evaluate the pragmatic relevance of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen in the daily aftercare of the outpatient clinic and to identify differences between patients after liver transplantation depending on their human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen status as well as potential risk of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen on histological changes in the allograft. In our cross-sectional analysis, we could show that positive results for human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen were significantly associated with histopathological inflammation. An adjusted logistic regression analysis confirmed significance and independence for the occurrence of inflammation in the presence of DSA. According to our results, suitable data have already been shown in pediatric liver transplantation.27,28 Remarkably, we found opposite dynamics regarding the association of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen to inflammation and vice versa to steatosis. This included on the one hand a significant association for absence of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen in higher steatosis grades (II–III), while on the other hand contrary to this, the presence of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen was significantly associated with higher inflammation grades (II–IV). It has been shown that inflammation affects insulin resistance and dyslipidemia.29 As Furman et al reported, acute inflammation should be distinguished from systemic chronic inflammation. It is possible that the nature of the immune response triggered by the presence of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen has effects, whose magnitude is not assessable given to our current knowledge.30 The kind of permanent state of low-grade, non-infectious (“sterile”) systemic chronic inflammation described by Furman et al, proven to be significantly associated with human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen in our analysis, may reduce insulin resistance, may lead to an increase in insulin sensitivity and thus exert an effect on steatosis.
There was no correlation between immunosuppressive therapy to human leukocyte antigen antibodies as well as to donor-specific antibodies against donor human leukocyte antigen. However, there seemed to be a significant association between mammalian target of rapamycin-inhibitors medication and the absence of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen, also accompanied by a significant association between absence of human leukocyte antigen antibodies and hepatocellular carcinoma. Schnitzbauer et al have demonstrated an improvement of results in liver transplantation due to hepatocellular carcinoma, remarkably with alpha-1-fetoprotein-evidence of higher tumor activity, following mammalian target of rapamycin-inhibitors treatment with Sirolimus for ≥3 months. An anti-cancer effect and an advantage of Sirolimus in younger patients and in active hepatocellular carcinoma (increased alpha-1-fetoprotein), lying within the Milan criteria, was proven.31 Here, a possible protective effect of mammalian target of rapamycin-inhibitors on biologically active hepatocellular carcinoma could be explained by suppression of inflammation.
A prevention of developing donor-specific antibodies against donor human leukocyte antigen was shown by Willuweit et al and would also be conceivable in the interaction between mammalian target of rapamycin-inhibitorsuse and its significant association with absence of human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen.32 Furthermore Narumi et al as well as Perbos et al have shown results supporting the protective role mammalian target of rapamycin-inhibitors play on developing donor-specific antibodies against donor human leukocyte antigen after kidney transplantation and solid organ transplantation.33,34 Overall, the small sample size in the groups hampered a valid analysis.
Associations between acute rejection and subclinical T-cell-mediated rejection after liver transplantation and kidney transplantation have already been shown, and subclinical T-cell-mediated rejection seemed to have a good prognosis even without specific therapy.35,36 Correspondingly, Loupy et al have shown for kidney transplantation that late donor-specific antibodies against donor human leukocyte antigen worsens the prognosis of subclinical T-cell-mediated rejection if in addition a histopathologically confirmed graft glomerulopathy is present.37 The role of donor-specific antibodies against donor human leukocyte antigen positivity after kidney transplantation with respect to graft damage is also described after liver transplantation.17,38
Many authors have shown an association between donor-specific antibodies against donor human leukocyte antigen and progressive fibrosis leading to graft dysfunction and graft loss.39–43 Nevertheless, donor-specific antibodies against donor human leukocyte antigen positivity in stable graft function and also in immunological tolerance remains controversial and needs further investigation.44 It should also be considered, as Höfer et al were able to show, that a donor-specific antibodies against donor human leukocyte antigen-positive status is not automatically associated with a C4d detection just as a C4d detection can also be present without donor-specific antibodies against donor human leukocyte antigen-positive status.45 The significant association between donor-specific antibodies against donor human leukocyte antigen positivity and the occurrence of inflammation in our cross-sectional study was striking, as it was also associated with significantly less steatosis. Pathophysiologically, persistent donor-specific antibodies against donor human leukocyte antigen in the liver parenchyma seem to cause changes due to the inflammation. The question of whether creeping damage caused by remodelling of the parenchyma is also primarily triggered by donor-specific antibodies against donor human leukocyte antigen or other causalities, such as the “second hit” hypothesis, plays an important role as yet to be clarified in this setting.13,38 Since this is a cross-sectional analysis, we did not exclude etiologies, as authors do, eg, for viral genesis, in order to better investigate chronic inflammation.45 Moreover, all previously hepatitis C virus positive patients had been successfully treated with modern antiviral substances, so that this inflammation compound could definitively be ruled out.
Patients with a history of known bile duct changes and/or biliary complications were particularly observed. Analysis was performed after exclusion of obstructive causes. Associations between humoral alloreactivity and biliary complications have been described in the literature.46–49 Den Dulk et al showed that both non-anastomotic strictures (NAS) and de novo Class II donor-specific antibodies against donor human leukocyte antigen after liver transplantation are independent factors associated with graft loss.50 The extent of the inflammation could be influenced by silent humoral and cellular mechanisms and needs to be investigated in further studies.51 The significant association of circulating donor-specific antibodies against donor human leukocyte antigen with histological inflammation described in our study seems to occur without apparent fibrosis. We need more information from larger patient cohorts that evaluate protocol biopsies in a standardized way to improve our understanding in the subject. To this extent, protocol biopsies should be performed regularly and analyzed systematically over a long period of time. The use of additional methods, eg, immunohistochemistry for specific queries and the complementary analysis and determination of donor-specific antibodies against donor human leukocyte antigen should be considered in special, vulnerable patients prone to antibody-mediated rejection and/or graft damage and to a lesser extent in the vast majority of immunologically stable patients of the daily practice.
The immunological mechanisms leading to graft failure are still not sufficiently understood. The slow dynamics, which can be the basis of chronic rejection processes that lead up to transplant failure, are not primarily evident, but rather can take up to years in manifesting themselves. Certain aspects of liver transplantation, such as the recurrence of underlying diseases (recurrence of hepatocellular carcinoma, earlier hepatitis C infection), donor shortage, the use of marginal organs and the use of immunosuppression are ever more present and tangible, and hence have gained recent notoriety.
The decisive factor is the inflammation, which acts in the background and is a permanent stimulus for fibrogenesis in the liver graft tissue.
Histological changes often appear late and run in the background, especially when liver function and laboratory chemistry seem to be unaffected. The histopathological inflammatory changes may induce “hidden” or subclinical graft damage. They may be associated with rejection and later with a slow fibrosis progression.
The characterization of histopathological changes such as inflammation, fibrosis associated with donor-specific antibodies against donor human leukocyte antigen positivity could act as a biomarker and in some cases have an impact on graft survival. Clinical monitoring could be expanded and refined by the targeted determination of donor-specific antibodies against donor human leukocyte antigen and adaptation of immunosuppressive therapy. Patients with signs of histological inflammation and donor-specific antibodies against donor human leukocyte antigen positivity should be more thoroughly investigated. Immunosuppression should be adjusted and not reduced too early.
Based on the present results, no conclusions regarding a clear causal relationship can be drawn from the presence of donor-specific antibodies against donor human leukocyte antigen and higher levels of inflammation. The study in its current form did not distinguish between preformed donor-specific antibodies against donor human leukocyte antigen and de novo donor-specific antibodies against donor human leukocyte antigen, as only one screening for donor-specific antibodies against donor human leukocyte antigen was performed regardless of the timepoint after liver transplantation. In this respect, causality regarding the pathophysiology (donor-specific antibodies against donor human leukocyte antigen inducing inflammation versus inflammation inducing donor-specific antibodies against donor human leukocyte antigen) cannot be drawn from the data. In summary, there is an association between donor-specific antibodies against donor human leukocyte antigen detection and graft inflammation.
Future prospective randomized studies should establish a baseline status quo with regard to preformed recipient donor-specific antibodies against donor human leukocyte antigen immediately before liver transplantation. Then distinguish them during follow-up for the occurrence of de novo donor-specific antibodies against donor human leukocyte antigen and possible associated complications. In addition, the aspect of cellular senescence as well as marginal organ quality should also be considered. Under certain circumstances, “healthy” grafts may have the resources to eliminate occurring donor-specific antibodies against donor human leukocyte antigen, whereas “damaged” grafts may no longer have this ability.
Conclusion
Human leukocyte antigen antibodies and donor-specific antibodies against donor human leukocyte antigen have gained recent notoriety. A hypothesis was drawn associating it with the triggering of severe graft damage. This effect has been demonstrated in some literature reports. However, in the present study, which handels with the clinical relevance of donor-specific antibodies against donor human leukocyte antigen determination in the long-term follow-up after liver transplantation, a lack of association with the main determinant of liver disease – fibrosis – was shown. Patients who did not develop donor-specific antibodies against the donor’s human leukocyte antigen showed significant differences in laboratory chemistry as well as histopathological inflammation, so a certain relevance might be concluded here. It can be assumed that the risk may affect marginal groups such as younger transplant patients. For the main cohort, donor-specific antibodies against donor human leukocyte antigen and human leukocyte antigen antibodies seem to be rather insignificant and should be reserved for special cases.
Abbreviations
AFP, alpha-1-fetoprotein; ALT, alanine-aminotransferase; AST, aspartate-aminotransferase; CI, confidence interval; CNI, calcineurin inhibitors; CyA, cyclosporin A; DSA, donor-specific antibodies; ELISA, enzyme-linked immunosorbent assay; ESLD, end-stage liver disease; GGT, gamma-glutamyltransferase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HLA, human leukocyte antigen; HLAab, human leukocyte antigen antibodies; ITBL, ischemic type biliary lesions; MFI, mean fluorescence intensity; MMF, mycophenolate mofetil; mTORi, mammalian target of rapamycin-inhibitors; OR, odds ratio; Sub-TCMR, subclinical T-cell-mediated rejection; tac, tacrolimus.
Data Sharing Statement
Demographic data is given in this manuscript. Detailed data can be accessed on request.
Funding
The authors have no funding to declare for this work.
Disclosure
Professor Moritz Schmelzle reports personal fees from Merck Serono, Bayer, non-financial support from ERBE, Amgen, non-financial support from Johnson & Johnson, Takeda, Olympus, Medtronic, Intuitive, outside the submitted work. Dr Nils Lachmann reports personal fees from BmT GmbH, outside the submitted work. The authors declare no other conflicts of interest. All authors have approved the final article.
References
1. Londoño M-C, Souza LN, Lozano -J-J, et al. Molecular profiling of subclinical inflammatory lesions in long-term surviving adult liver transplant recipients. J Hepatol. 2018;69(3):626–634. doi:10.1016/j.jhep.2018.04.012
2. Yoshitomi M, Koshiba T, Haga H, et al. Requirement of protocol biopsy before and after complete cessation of immunosuppression after liver transplantation. Transplantation. 2009;87(4):606–614. doi:10.1097/TP.0b013e318195a7cb
3. Vergani D, Mieli-Vergani G. Autoimmunity after liver transplantation: autoimmunity after liver transplantation. Hepatology. 2002;36(2):271–276. doi:10.1053/jhep.2002.35339
4. Sebagh M. All liver recipients benefit from the protocol 10-year liver biopsies. Hepatology. 2003;37(6):1293–1301. doi:10.1053/jhep.2003.50231
5. Kerkar N, Hadzic N, Davies ET, et al. De-novo autoimmune hepatitis after liver transplantation. Lancet. 1998;351(9100):409–413. doi:10.1016/S0140-6736(97)06478-7
6. Heneghan M. Graft dysfunction mimicking autoimmune hepatitis following liver transplantation in adults. Hepatology. 2001;34(3):464–470. doi:10.1053/jhep.2001.26756
7. O’Leary JG, Kaneku H, Jennings L, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific alloantibodies are associated with fibrosis progression after liver transplantation in hepatitis C virus-infected patients: donor-specific antibodies and fibrosis progression. Liver Transpl. 2014;20(6):655–663. doi:10.1002/lt.23854
8. Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplant J. 2013;95(1):19–47. doi:10.1097/TP.0b013e31827a19cc
9. Colvin RB. Dimensions of antibody-mediated rejection: editorial. Am J Transplant. 2010;10(7):1509–1510. doi:10.1111/j.1600-6143.2010.03172.x
10. Witt CA, Gaut JP, Yusen RD, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2013;32(10):1034–1040. doi:10.1016/j.healun.2013.07.004
11. Demetris AJ, Bellamy COC, Gandhi CR, Prost S, Nakanuma Y, Stolz DB. Functional immune anatomy of the liver-as an allograft. Am J Transplant. 2016;16(6):1653–1680. doi:10.1111/ajt.13749
12. Halloran PF, Venner JM, Madill-Thomsen KS, et al. Review: the transcripts associated with organ allograft rejection. Am J Transplant. 2018;18(4):785–795. doi:10.1111/ajt.14600
13. Demetris AJ, Bellamy C, Hübscher SG, et al. 2016 comprehensive update of the Banff working group on liver allograft pathology: introduction of antibody-mediated rejection. Am J Transplant. 2016;16(10):2816–2835. doi:10.1111/ajt.13909
14. Miyagawa-Hayashino A, Yoshizawa A, Uchida Y, et al. Progressive graft fibrosis and donor-specific human leukocyte antigen antibodies in pediatric late liver allografts: posttransplant Hla DSA
15. O’Leary JG, Kaneku H, Demetris AJ, et al. Antibody-mediated rejection as a contributor to previously unexplained early liver allograft loss: antibody-mediated liver transplant rejection. Liver Transpl. 2014;20(2):218–227. doi:10.1002/lt.23788
16. Kaneku H, O’Leary JG, Taniguchi M, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with chronic rejection and graft loss after liver transplantation: i
17. O’Leary JG, Cai J, Freeman R, et al. Proposed diagnostic criteria for chronic antibody-mediated rejection in liver allografts: chronic AMR in liver transplant. Am J Transplant. 2016;16(2):603–614. doi:10.1111/ajt.13476
18. Cousin VL, Rougemont A-L, Rubbia-Brandt L, et al. Peripheral donor specific antibodies are associated with histology and cellular subtypes in protocol liver biopsies of pediatric recipients. Transplantation. 2019. doi:10.1097/TP.0000000000003099
19. Yamada H, Kondou H, Kimura T, et al. Humoral immunity is involved in the development of pericentral fibrosis after pediatric live donor liver transplantation: pericentral fibrosis and humoral immunity. Pediatr Transplant. 2012;16(8):858–865. doi:10.1111/j.1399-3046.2012.01781.x
20. Teegen EM, Dürr M, Maurer MM, et al. Evaluation of histological dynamics, kidney function and diabetes in liver transplant patients after antiviral treatment with direct-acting antivirals: therapy of HCV-recurrence. Transpl Infect Dis. 2019;21(1):e13020. doi:10.1111/tid.13020
21. Del Bello A, Danjoux M, Congy-Jolivet N, et al. Histological long-term outcomes from acute antibody-mediated rejection following ABO-compatible liver transplantation: outcome of antibody mediated rejection. J Gastroenterol Hepatol. 2017;32(4):887–893. doi:10.1111/jgh.13613
22. Legaz I, Boix F, López M, et al. Influence of preformed antibodies in liver transplantation. JCM. 2020;9(3):708. doi:10.3390/jcm9030708
23. Höfer A, Jonigk D, Hartleben B, et al. Non-invasive screening for subclinical liver graft injury in adults via donor-specific anti-HLA antibodies. Sci Rep. 2020;10(1):14242. doi:10.1038/s41598-020-70938-7
24. Koch M, Marget M, Sterneck M, Fischer L, Thude H, Nashan B. Limited impact of pre-existing donor specific HLA-antibodies (DSA) on long term allograft survival after first adult liver transplantation. Hum Immunol. 2018;79(7):545–549. doi:10.1016/j.humimm.2018.04.009
25. Muro M. The endless history or search for the true role of alloantibodies in liver transplantation. Clin Res Hepatol Gastroenterol. 2020;101544. doi:10.1016/j.clinre.2020.09.005
26. Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19(6):1513–1520. doi:10.1002/hep.1840190629
27. Ekong UD, Melin-Aldana H, Seshadri R, et al. Graft histology characteristics in long-term survivors of pediatric liver transplantation. Liver Transpl. 2008;14(11):1582–1587. doi:10.1002/lt.21549
28. Varma S, Ambroise J, Komuta M, et al. Progressive fibrosis is driven by genetic predisposition, allo-immunity, and inflammation in pediatric liver transplant recipients. EBioMedicine. 2016;9:346–355. doi:10.1016/j.ebiom.2016.05.040
29. Straub RH, Schradin C. Chronic inflammatory systemic diseases: an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol Med Public Health. 2016;2016(1):37–51. doi:10.1093/emph/eow001
30. Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi:10.1038/s41591-019-0675-0
31. Schnitzbauer AA, Filmann N, Adam R, et al. mTOR inhibition is most beneficial after liver transplantation for hepatocellular carcinoma in patients with active tumors. Ann Surg. 2020;272(5):855–862. doi:10.1097/SLA.0000000000004280
32. Willuweit K, Heinold A, Rashidi-Alavijeh J, et al. Immunosuppression with mTOR inhibitors prevents the development of donor-specific antibodies after liver transplant. Clin Transplant. 2017;31(6):e12974. doi:10.1111/ctr.12974
33. Narumi S, Watarai Y, Goto N, et al. Everolimus-based immunosuppression possibly suppresses mean fluorescence intensity values of de novo donor-specific antibodies after primary kidney transplantation. Transplant Proc. 2019;51(5):1378–1381. doi:10.1016/j.transproceed.2019.03.019
34. Perbos E, Juinier E, Guidicelli G, et al. Evolution of donor-specific antibodies (DSA) and incidence of de novo DSA in solid organ transplant recipients after switch to everolimus alone or associated with low dose of calcineurin inhibitors. Clin Transplant. 2014;28(9):1054–1060. doi:10.1111/ctr.12418
35. Bartlett AS, Ramadas R, Furness S, Gane E, McCall JL. The natural history of acute histologic rejection without biochemical graft dysfunction in orthotopic liver transplantation: a systematic review. Liver Transpl. 2002;8(12):1147–1153. doi:10.1053/jlts.2002.36240
36. Taubert R, Pischke S, Schlue J, et al. Enrichment of regulatory T cells in acutely rejected human liver allografts: treg enrichment in rejected liver allografts. Am J Transplant. 2012;12(12):3425–3436. doi:10.1111/j.1600-6143.2012.04264.x
37. Loupy A, Vernerey D, Tinel C, et al. Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts. JASN. 2015;26(7):1721–1731. doi:10.1681/ASN.2014040399
38. Kim PTW, Demetris AJ, O’Leary JG. Prevention and treatment of liver allograft antibody-mediated rejection and the role of the ‘two-hit hypothesis’. Curr Opin Organ Transplant. 2016;21(2):209–218. doi:10.1097/MOT.0000000000000275
39. Levitsky J, Kaneku H, Jie C, Walsh RC, Abecassis M, Tambur AR. Donor-specific HLA antibodies in living versus deceased donor liver transplant recipients. Am J Transplant. 2016;16(8):2437–2444. doi:10.1111/ajt.13757
40. O’Leary JG, Kaneku H, Banuelos N, Jennings LW, Klintmalm GB, Terasaki PI. Impact of IgG3 subclass and C1q-fixing donor-specific HLA alloantibodies on rejection and survival in liver transplantation: igG3 and C1q-fixing DSA in liver transplantation. Am J Transplant. 2015;15(4):1003–1013. doi:10.1111/ajt.13153
41. Ohe H, Uchida Y, Yoshizawa A, et al. Association of anti-human leukocyte antigen and anti-angiotensin II type 1 receptor antibodies with liver allograft fibrosis after immunosuppression withdrawal. Transplantation. 2014;98(10):1105–1111. doi:10.1097/TP.0000000000000185
42. Kaneku H, O’Leary JG, Banuelos N, et al. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients: de novo DSA in liver transplantation. Am J Transplant. 2013. doi:10.1002/ajt.12212
43. O’Leary JG, Klintmalm GB. Impact of donor-specific antibodies on results of liver transplantation. Curr Opin Organ Transplant. 2013;18(3):279–284. doi:10.1097/MOT.0b013e3283614a10
44. Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60(6):2109–2117. doi:10.1002/hep.27254
45. Höfer A, Jonigk D, Hartleben B, et al. DSA are associated with more graft injury, more fibrosis, and upregulation of rejection-associated transcripts in subclinical rejection. Transplantation. 2020;104(3):551–561. doi:10.1097/TP.0000000000003034
46. Musat AI, Agni RM, Wai PY, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation: HLA alloantibodies in liver allograft rejection. Am J Transplant. 2011;11(3):500–510. doi:10.1111/j.1600-6143.2010.03414.x
47. O’Leary JG, Kaneku H, Susskind BM, et al. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection postliver transplant: DSA found in post-OLT chronic rejection. Am J Transplant. 2011;11(9):1868–1876. doi:10.1111/j.1600-6143.2011.03593.x
48. Takaya S, Jain A, Yagihashi A, et al. Increased bile duct complications and/or chronic rejection in crossmatch positive human liver allografts. Transplant Proc. 1999;31(5):2028–2031. doi:10.1016/s0041-1345(99)00256-0
49. Iacob S, Cicinnati VR, Dechêne A, et al. Genetic, immunological and clinical risk factors for biliary strictures following liver transplantation. Liver Int. 2012;32(8):1253–1261. doi:10.1111/j.1478-3231.2012.02810.x
50. den Dulk AC, Shi X, CorneliaJ V, et al. Donor-specific anti-HLA antibodies are not associated with nonanastomotic biliary strictures but both are independent risk factors for graft loss after liver transplantation. Clin Transplant. 2018;32(2):e13163. doi:10.1111/ctr.13163
51. Muro M, R Moya-Quiles M, Mrowiec A. Humoral response in liver allograft transplantation: a review of the role of anti-Human Leukocyte Antigen (HLA) antibodies. CPPS. 2016;17(8):776–784. doi:10.2174/1389203717666160226145101
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
