Back to Journals » Vascular Health and Risk Management » Volume 12
Does the treatment of type 2 diabetes mellitus with the DPP-4 inhibitor vildagliptin reduce HbA1c to a greater extent in Japanese patients than in Caucasian patients?
Authors Foley JE , Bhosekar V, Kawamori R
Received 23 September 2015
Accepted for publication 23 December 2015
Published 21 January 2016 Volume 2016:12 Pages 9—12
DOI https://doi.org/10.2147/VHRM.S96971
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Daniel Duprez
James E Foley,1 Vaishali Bhosekar,2 Ryuzo Kawamori3
1Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 2Novartis Healthcare Pvt Ltd, Hyderabad, Telangana, India; 3Sportology Center, Juntendo University Graduate School of Medicine, Juntendo University, Tokyo, Japan
Background: Previous work suggests that Japanese patients with type 2 diabetes mellitus (T2DM) may respond more favorably to a DPP-4 (dipeptidyl peptidase-4) inhibitor than Caucasians. We aimed to compare the efficacy of the DPP-4 inhibitor vildagliptin (50 mg twice daily [bid]) between Japanese and Caucasian populations.
Methods: This analysis pooled data from 19 studies of drug-naïve patients with T2DM who were treated for 12 weeks with vildagliptin 50 mg bid as monotherapy. The pool comprised Japanese patients (n=338) who had been treated in Japan and Caucasian patients (n=1,275) who were treated elsewhere. Change from baseline (Δ) in glycated hemoglobin (HbA1c) at 12 weeks (in millimoles per mole) versus baseline HbA1c (both in percentage National Glycohemoglobin Standardization Program units [NGSP%] and millimoles per mole) for each population was reported. Universal HbA1c in millimoles per mole was calculated from either the Japanese Diabetes Society or the NGSP% HbA1c standards.
Results: At baseline, mean values for Japanese and Caucasian patients, respectively, were as follows: age, 59 years and 56 years; % male, 69% and 57%. The average HbA1c was reduced from 7.90% to 6.96% (Japanese Diabetes Society) and from 8.57% to 7.50% (United States National Glycohemoglobin Standardization Program), while HbA1c was reduced from 63 mmol/mol to 53 mmol/mol and from 70 mmol/mol to 58 mmol/mol in Japanese and Caucasians, respectively. ΔHbA1c increased with increasing baseline in both populations. The slopes were the same (0.41, r2=0.36; and 0.41, r2=0.15), and the intercepts were 15.4 mmol/mol and 17.2 mmol/mol, respectively. In Japanese patients, mean ΔHbA1c was greater by 1.7 mmol/mol (0.2% NGSP HbA1c) at any given baseline HbA1c than in Caucasians (P=0.01).
Conclusion: The present pooled analysis suggests that Japanese patients respond better to vildagliptin treatment compared with Caucasians. However, when glycemic control was corrected by using the same glycemic standard, the difference in HbA1c reduction between these populations was not clinically meaningful.
Keywords: baseline HbA1c, Caucasians, Japanese, vildagliptin, type 2 diabetes
Introduction
Evidence suggests that, on average, Japanese individuals have a lower capacity to secrete insulin than Caucasians. A greater β-cell deficiency predicts that Japanese patients with type 2 diabetes mellitus may respond more favorably to a dipeptidyl peptidase-4 (DPP-4) inhibitor than would Caucasian patients.1 In accordance with this hypothesis, studies conducted in Japan with the DPP-4 inhibitor vildagliptin reported greater reductions in glycated hemoglobin (HbA1c) from lower HbA1c baselines2 than those reported from non-Japanese studies, wherein the majority of the patients are Caucasians of European descent.3,4
However, apart from population differences, there exist methodological and analytic differences between the clinical trials’ results, which require reconciliation before reaching any conclusions. The most important among these are differences in the HbA1c standard used; the Japanese Diabetes Society (JDS) HbA1c standard yields HbA1c values that are ~0.4% lower than the international standard specified by the United States National Glycohemoglobin Standardization Program (NGSP), which was used in the non-Japanese studies.5,6 Furthermore, the baseline HbA1c is a very strong determinant of HbA1c reduction,7 and Japanese clinical trials often start from lower baseline HbA1c levels. In addition, Japanese clinical trials often last for 12 weeks rather than 24 weeks as seen in many other trials. This pooled analysis aimed to compare the efficacy levels of vildagliptin action in Japanese and Caucasian patients.
Materials and methods
Patients and study design
To directly compare the efficacy of the DPP-4 inhibitor vildagliptin (50 mg twice daily [bid]) in Japanese and Caucasian populations, we pooled data from all the randomized vildagliptin monotherapy studies (total of 19 studies) conducted in drug-naïve patients with T2DM, wherein HbA1c was assessed at 12 weeks. The pool comprised Japanese patients (n=338) treated in Japan and Caucasian patients (n=1,275) treated elsewhere. HbA1c (in millimoles per mole) was calculated from either the JDS or the NGSP% HbA1c standards.5,6 Because there was an active comparator instead of placebo in most studies, placebo-subtracted differences were not considered to be valid.
Study assessments and data analyses
Change in HbA1c (millimoles per mole) at 12 weeks from baseline was plotted versus baseline HbA1c (millimoles per mole) for each population. The data analysis was based on randomized population. A linear regression model fitted to the change from baseline HbA1c (millimoles per mole), with baseline HbA1c in millimoles per mole as covariate and race subgroup as factor, was used to analyze change in HbA1c from baseline to Week 12.
Ethics and good clinical practice
All studies included in the analysis were conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization/Good Clinical Practice guidelines. The study protocols were approved by an independent ethics committee/institutional review board at each site and all patients provided written informed consent.
Results
The two populations were similar with regard to sex and age. However, the HbA1c and fasting plasma glucose (FPG) were higher, and the body mass index as well as the homeostasis model of assessment for insulin resistance (HOMA-IR) score were much higher in the Caucasian population (Table 1).
The average HbA1c value ± standard error was reduced from 7.9%±0.05% to 6.96%±0.04% (JDS) and from 8.57%±0.03% to 7.50%±0.03% (NGSP), while HbA1c (in units of millimoles per mole) was reduced from 62.9±0.5 mmol/mol to 52.5±0.4 mmol/mol and from 70.1±0.3 mmol/mol to 58.5±0.4 mmol/mol in Japanese and Caucasian patients, respectively (Figure 1). The reduction in HbA1c increased with increasing baseline in both populations. The slopes for change in HbA1c (millimoles per mole) at Week 12 from baseline were the same for both the populations, but the intercepts were different (Table 2). When HbA1c was adjusted for mean change in change from baseline (in millimoles per mole) and for baseline HbA1c (in millimoles per mole) in the linear regression model, the mean change in HbA1c (in millimoles per mole) in Japanese versus Caucasian populations was –1.7±0.7 (95% confidence interval: –2.953 to –0.398; P=0.01). Thus, at any given baseline HbA1c, the reduction in HbA1c was greater by 1.7 mmol/mol (NGSP unit: 0.2% NGSP HbA1c) in Japanese patients compared with that in Caucasians.
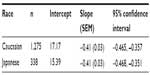  | Table 2 Linear regression analyses for change in HbA1c (millimoles per mole) at Week 12 from baseline |
Discussion
The data from this pooled analysis support the conclusion that, on average, Japanese physicians initiate treatment at lower levels of glucose, resulting in more Japanese than Caucasian patients reaching HbA1c goals with vildagliptin. Similar reductions from baseline in HbA1c from a lower baseline HbA1c also suggest better efficacy of vildagliptin in Japanese patients when compared with the results obtained in Caucasians. However, these differences were attenuated when glycemic control was corrected by using the same glycemic standard and the change in HbA1c was corrected for baseline HbA1c, resulting in only a small difference that was statistically significant but not clinically meaningful, based on the usual threshold for clinical relevance of 0.3% NGSP HbA1c. These results have limitations due to the pooled analysis design used and the lack of adequate numbers of placebo controls. However, in the studies wherein placebo values were available, the placebo effect was consistently greater in the Caucasian group, suggesting that differences between the groups would be greater and may actually rise to the usual threshold for clinical significance.
On the other hand, these data do not rule out that there could be important differences in the underlying pathologies of the two populations. There were important differences, for which we could not correct, in the baseline characteristics of the two populations indicative of a greater degree of insulin resistance in the Caucasian population; notably, the degree of variability in the HOMA-IR score was much higher in the Caucasian patients. Vildagliptin is known to work by improving β- and α-cell function, as well as through various extrapancreatic actions, which could together yield similar levels of glycemic control.8 The results are consistent with a recent mechanism study9 of glycemic control, which indicates a similar ability of Japanese and Caucasians to compensate for increased insulin resistance by increasing insulin secretion. In any case, further studies on the mechanism of glycemic control are needed to determine whether the underlying pathologies of insulin secretion and resistance are similar in Japanese and Caucasian patients.
Acknowledgments
The authors gratefully acknowledge the investigators and patients participating in all the studies included in this analysis. The authors thank Anil Dandu (Novartis Healthcare Private Limited, India) for technical help and editorial assistance with submission of the manuscript.
Author contributions
All authors had full access to all data and take responsibility for the integrity of the data and accuracy of analyses. All authors provided input to the analytical approach, interpretation of data, and preparation, revision, and final approval of the manuscript.
Disclosure
JEF is an employee of Novartis Pharmaceuticals Corporation and also owns company shares. VB is an employee of Novartis Healthcare Pvt Ltd. RK consulted for and received honorarium from Novartis Pharma AG. The authors report no other conflicts of interest in this work.
References
Yabe D, Sein Y, Fukushima M, Seino S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15:36. | |
Iwamoto Y, Kashiwagi A, Yamada N, et al. Efficacy and safety of vildagliptin and voglibose in Japanese patients with type 2 diabetes: a 12-week, randomized, double-blind, active-controlled study. Diabetes Obes Metab. 2010;12:700–708. | |
Foley JE, Sreenan S. Efficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naïve patients with type 2 diabetes. Horm Metab Res. 2009;41:905–909. | |
Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132–138. | |
Sacks DB. Measurement of hemoglobin A1c: a new twist on the path to harmony. Diabetes Care. 2012;35:2674–2680. | |
Kashiwagi A. Development of the HbA1c international standardization of HbA1c measurement in Japan Diabetes Society. Rinsho Byori. 2013;6:585–593. | |
Ahrén B, Mathieu C, Bader G, Schweizer A, Foley JE. Efficacy of vildagliptin versus sulfonylureas as add-on to metformin: comparison of results from randomized controlled trials and real-life conditions. Diabetologia. 2014;57:1304–1307. | |
Ahrén B, Foley JE. The islet enhancer vildagliptin: mechanisms of improved glucose metabolism. Int J Clin Pract Suppl. 2008(62); (suppl 159):8–14. | |
Møller JB, Dalla Man C, Overgaard RV, et al. Ethnic differences in insulin sensitivity, β-Cell function, and hepatic extraction between Japanese and Caucasians: a minimal model analysis. J Clin Endocrinol Metab. 2014;99:4273–4280. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


