Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 15
Do Mesenchymal Stem Cells Influence Keloid Recurrence?
Authors Nang'ole WF, Omu A, Ogeng'o JA, Agak GW
Received 9 May 2022
Accepted for publication 5 December 2022
Published 19 December 2022 Volume 2022:15 Pages 77—84
DOI https://doi.org/10.2147/SCCAA.S373551
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Bernard Binetruy
Wanjala Ferdinand Nang’ole,1 Anzala Omu,2 Julius A Ogeng’o,3 George W Agak4
1Department of Surgery, University of Nairobi, Nairobi, Kenya; 2Kenya Aids Vaccine Institute, University of Nairobi, Nairobi, Kenya; 3Department of Human Anatomy, University of Nairobi, Nairobi, Kenya; 4Division of Dermatology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
Correspondence: Wanjala Ferdinand Nang’ole, Department of Surgery, University of Nairobi, PO Box 30197-00100, Nairobi, Kenya, Tel +254-71-4342-214, Email [email protected]
Introduction: Mesenchymal stem cells (MSCs) have been postulated by a number of authors to be the precursor cells of fibroblasts and myofibroblasts in keloids. They have been seen as a regenerative pool that ensures a steady supply of cells. The objective of our study was to determine MSCs in keloids and normal skin as a determinant of keloid recurrence.
Methods: This was a longitudinal prospective study in which patients with keloid excisions of their specimens analyzed for MSC. A control group of patients matched for age, sex, and body-mass index (BMI) with no history of keloids admitted for elective surgical procedures had their skin samples taken and also analyzed for MSCs. Data collected were analyzed and compared using Student’s t, x2, and Fisher’s exact t tests.
Results: A total of 61 patients with keloids and a control group of 32 patients were recruited. The male:female ratio was 1:2 and mean age 29.5 and 29.7 years for keloids and controls, respectively. Patients with recurrent keloids had a mean density of 841.4 MSCs/g compared to 578 MSCs/g of tissue for those with no recurrence and 580 MSCs/g for patients with normal skin. Recurrent keloids had a significantly higher percentage of MSCs than those without.
Conclusion: Keloids compared to normal skin had a higher percentage of MSCs, with recurrent keloids demonstrating an even higher count, a possible indicator that MSCs might correlate with severity of keloid disease and recurrence.
Keywords: stem cells, skin, keloids, recurrence
A Letter to the Editor has been published for this article.
A Response to Letter by Mrs Noviantari has been published for this article.
Introduction
Keloids are a fibroproliferative disorder characterized by excessive deposition of collagen in the dermal layer of the skin.1–3 Keloids are characterized by high recurrence rates.1 The etiology of keloids is not clear. Theories on keloid pathogenesis include inflammatory, biomechanical tension theory, tissue hypoxia and abnormal fibroblasts.2,4 Keloid histology has demonstrated an abundance of inflammatory cells and fibroblasts.1–3 Keloid fibroblasts are considered the main cells in keloid formation. They have been shown to have high mitotic activity and a low apoptosis rate.1 Whether these properties are inherent in keloid fibroblasts or are due to the interaction with local tissue niche is still not clear. Myofibroblasts are other key cells identified in many keloid specimens and other fibrotic conditions, and are thought to differentiate from fibroblasts as a result of either mechanical stress or a hypoxic tissue environment.1 The source of both fibroblasts and myofibroblasts in keloids is not clear.
Mesenchymal stem cells (MSCs) have been postulated by a number of authors to be the precursor cells of fibroblasts and myofibroblasts in keloids.1,4–6 They have been seen as a regenerative pool that ensures a steady supply of proliferative cells. Their role in keloid recurrence is however not known: whether there are any differences in MSC in keloids that recur and those that do not has not been documented. We undertook this study to determine if there is any correlation between keloid MSC counts, proportions and recurrence.
Methods
This study was carried out at Kenyatta National Hospital and the Kenya Aids Vaccine Initiative, University of Nairobi, Kenya. Approval was obtained from the Kenyatta National Hospital/University of Nairobi ethic and review board (Ref no.P291/04/2018). The study complied with the Helsinki declaration. Consent and assent to participate in the study was taken from the patients and next of kin in cases of minnows respectively. Included in the study were patients with keloids amenable to surgery with primary wound closure. Excluded from the study were patients who had surgery within a year for the same keloid, patient with extensive keloids whose wounds could not be closed primarily, infected keloids and patients with chronic medical conditions such as diabetes mellitus, hypertension or HIV/AIDS. The surgery was by intra-lesional excision of the keloid tissue with about 3 mm of keloid tissue left behind (Figures 1 and 2). The wound was closed in two layers with the dermal sutures using polyglycolic acid 3/0 and nylon 3/0 for the skin. Post excision keloids patients were subjected to 12 grays of external beam radiotherapy within 24 hours of surgery. They were then followed up at a regular interval for at least two years.
 |
Figure 1 Marking for intralesional excision of keloids. |
 |
Figure 2 Postintralesional excision of keloids. Note remnants of keloid tissue. |
The control group comprised of patients admitted for elective surgical procedures with no keloids or any family history of keloids. These were matched for age, sex, BMI and anatomical location to those with keloid. Recurrence was determined as re-growth of the keloids or pruritus and pain within the follow up duration of requiring medical intervention. Post excision, the specimens were defatted, weighed, processed and analyzed for total cell counts and MSC using flow cyto-metric technique. MSC were characterized by the expression of stromal cell markers (CD73, CD105, CD44, CD29 and CD90) in the absence of hematopoietic markers (CD34, CD45 and CD14) and endothelial markers (CD34, CD31 and VWF).7,8
A human MSC analysis kit was sourced from R&D Biotechne systems, catalog number FM C020 (UK). Both keloid and skin specimens for the control group were weighed. The extracellular matrix was digested by collagenase solution of concentration 0.5 mg/mL by vibrating and heating at 37°C at 1200 revolutions per minute. This was followed for another 10−15 minutes with 1 mg/mL of collagenase. The cell suspension was transferred to the cell strainer. The cells were spun at 1600 revolutions per minute and the supernatant discarded. The cells were isolated for counting using a glass hemocytometer. The absolute number of cells and MSCs were counted. The absolute MSC count per specimen was divided by the weight to pbtain the density of MSCs per gram of tissue. In each specimen, MSC percentage was analyzed from the total cell count. Data captured were summarized and analyzed using SPSS 22. Pearson’s correlation tests were performed to compare the similarities between the two groups of patients. Students t, x2, and Fischer’s t tests were used to compare means and frequencies in the two groups of patients. Significance was taken as P<0.05.
Results
A total of 61 patients with keloids and a control group of 32 patients with no keloids were followed up in the study. The male:female ratio for both groups was 1:2. The age range for the keloid patients was 15–65 years, with a mean of 29.5 years and a median age of 20–25 years. The age range for the control group was 15.5–64 years, with a mean age of 29.7 years and a median age of 20–25 years (Table 1). The body-mass index for the patients with keloids was 24.3, while for the controls it was 24 0.6 (P=0.87). There was no statistical significance difference between the keloid group and the control group (R=0.997). The anatomical locations of the keloids and the control groups were similar (Table 2).
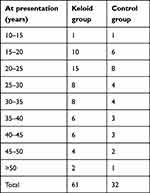 |
Table 1 Age of patients with keloids and the control group |
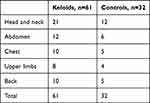 |
Table 2 Anatomical location of keloids in both groups |
About 40% of the keloids that recurred were from the head and neck region (Table 3). Mean MSCs in keloids was 664.5/g of tissue, with a range of 0 to 5180 and a median of 224. For the controls, the mean was 665.8/g of tissue with a range of 0 to 4440 and a median of 91.70 (P=0.847, Table 4). There was no statistically significant difference between the two groups (P=0.987). Mean MSC density for keloids with recurrence was 848.1 compared to 578.3 for those without recurrence (P<0.001, Table 5). MSCs in keloids and control were further analyzed by calculating the percentage of MSCs in the number of cells in the specimens. The mean proportion of MSCs in keloids was 0.701%, while in the controls it was 0.182% (P=0.885, Table 6).
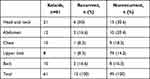 |
Table 3 Anatomical location of recurrent keloids that and those with no recurrence |
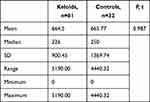 |
Table 4 MSC density per gram of tissue in keloids and normal skin |
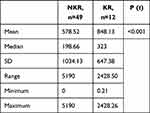 |
Table 5 MSC density per gram of tissue in keloids with no recurrence (NKR) and those with recurrence (KR) |
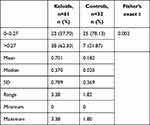 |
Table 6 MSC percentage in patients with keloids and controls |
There was a significant statistical difference between MSC percentage and the median percentiles of the two groups (P=0.004). The proportion of MSCs in the KR group was 1.123% compared to 0.427% in the NKR group (P=0.843, Table 7). Median MSC for both keloids and the control group was 0.27%. More than 62% of keloids had an MSC percentage >0.27 compared to 21.87% of the control group (P-<0.05), and 78% of keloids with recurrence had MSC of more than 0.27% compared to 21.8% of keloids that did not recur (P<0.05).
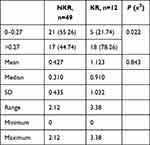 |
Table 7 MSC percentages in NKR and KR groups |
Discussion
Keloids are a fibroproliferative disorders characterized by pain, pruritus, and disfigurement with tendency to recur with no clear etiology. The disease has a strong familial and genetic influence, with some studies reporting up to 50% of patients with a positive family history.9 MSCs are pluripotent cells with the ability to regenerate into different cell lineages. They have been thought to play a critical role not only in wound healing and inflammation but in keloid formation as well.10–13 MSCs are thought to provide wounds with a steady supply of cells that differentiate into fibroblasts and myofibroblasts that synthesize the extracellular matrix responsible for healing. They are also thought to secrete cytokines that would mediate the inflammatory and proliferative process during wound healing.14 Though virtually present in any part of the body, a number of studies have demonstrated MSCs to be markedly elevated in keloids, suggesting that they could contribute to the pathology.10,12
The source of MSCs in keloids is not known. Whether the cells are resident in keloids or recruited in tissue as a result of injury or inflammation is not clear. They are, however, thought to originate from embryonic-like stem cells located in perivascular vessels within keloid-associated lymphoid tissue. These embryonic- like stem cells, under the influence of the local inflammatory environment, are thought to differentiate into resident MSCs.6,11,14 Resident MSCs have been implicated by a number of authors as a possible source of fibroblasts and myofibroblasts in fibrosis conditions, such as liver cirrhosis, pulmonary fibrosis, Dupuytren's disease, bone marrow fibrosis, and chronic kidney disease.15–17 Cell lineage–tracing studies have shown that the cellular source of myofibroblasts could partially originate from resident MSCs. Under the influence of inflammatory cytokines, such as IL6, IL17, and TGFB, these cells are thought to differentiate into fibroblasts and myofibroblasts that secrete collagen fibers responsible for fibrosis in keloids. An in vitro study by Qunzhou et al demonstrated an ability to reproduce keloid-like tissue in immunocompromised rats using keloid-derived stem cells and IL6, further emphasizing the role that MSCs and inflammatory cytokines could play in keloid pathogenesis. This activity was halted by antibodies against IL6.13
Our study demonstrated that there was no significant difference in MSC density in keloid and normal skin. However, a significantly higher density of MSCs was noted in keloids that recur than those that did not. The apparently similar counts of MSCs in keloids and normal skin should be taken in the context of high extracellular matrix in the keloids than the normal skin, since the study considered the density of cells per gram of tissue. This was illustrated by the fact that keloid specimens had a higher percentage of MSCs than normal skin. Also, keloids with recurrence had a higher percentage of MSCs than those with no recurrence, probably pointing to the fact that MSCs seem to influence keloid severity. It is, however, not clear whether the high MSC count is an inherent property of the keloid tissue or could be as a result of the homing concept by the MSCs in a zone of injury or inflammation.
Other than being the primary cells that can differentiate into fibroblasts and myofibroblasts, MSCs seem to play further roles in wound healing.3,4 They have been shown to produce a vast spectrum of paracrine factors, such as IL10, TGFβ2, PGE2, HGF, and IL6, which play critical roles in wound healing and inflammation.18 In addition, MSCs have been shown to exist in two forms: MSC1 and MSC2.19 MSC2 are predominantly anti-inflammatory, while MSC1 are proinflammatory. Exposure to sufficiently high levels of TNFα and IFNγ leads to formation of MSC2, while low levels of TNFα and IFNγ in tissue leads to MSC1. MSC1 secrete chemokines that recruit inflammatory cells, particularly Th1, Th17 cells, monocytes, and neutrophils, to the sites of inflammation, resulting in a strong inflammatory response, a possible precursor to keloid formation.19
Interestingly, experimental work on adipose-derived and amniotic-derived MSCs on keloid fibroblasts have demonstrated reduction in fibroblast activities with apoptosis in some cases, probably suggesting that the predominant MSCs in these tissue types could be MSC2 that has predominantly anti-inflammatory activity.20–23 Several authors have also pointed out that autologous or allogeneic MSCs seem to act in a completely different manner from resident MSCs.5
Conclusion
This study demonstrated that keloid tissues have a high proportion of MSCs, which seems to correlate with severity and tendency to recur. MSCs seem to play a critical role in keloid pathophysiology through the influence of inflammatory cytokines and other paracrine factors, such as TGFβ. The pro- or anti-inflammatory role of MSCs could be influenced by the presence of suchchemokines as TNFα and interferons in the local milieu. There is a need for further studies to determine the actual role that MSCs play in keloid formation and possible pharmacotherapeutic agents that could be utilized in treatment and manipulation. Further studies need to be done to categories the MSC types and their role in keloid pathogenesis.
Abbreviations
MSCs, mesenchymal stem cells; PG, prostaglandin.
Data Sharing
Data for this study are available and can be accessed through the corresponding author Dr Ferdinand W Nang’ole, email [email protected].
Ethics Approval and Informed Consent
This study was approved by the UON/KNH ethics and research committee. Confidentiality ruled supreme during the course of the study. Consent and assent to participate in the study was taken from the patients and next of kin for minors, respectively.
Author Contributions
All authors made a significant contribution to the work reported, whether in conception, study design, execution, acquisition of data, analysis, interpretation, or all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
GWA was supported by NIH K01AR071479.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Jumper N, Paus. R, Bayat. A. Functional histopathology of keloid disease. Histol histopathol. 2015;30:1033–1057. doi:10.14670/HH-11-624
2. Chike-Obi CJ, Patrick DC, Anthony EB. Keloids; Pathogenesis, clinical features and management. Plastic Surgery. 2009;23(3):34–40.
3. Ali MM, Karanja FW, Nang’ole FW, Opot EON, Mulehane KO, Mbithi DZ. Determination of the prevalence, clinical characteristics and histo-pathological features of keloids in patients managed at the Kenyatta National Hospital. East Afri Med J. 2019;96(1):2220–2229.
4. Nangole FW, George A. Keloids pathophysiology, Fibroblasts or inflammatory disorder. JPRAS Open. 2019;22:44–54. doi:10.1016/j.jpra.2019.09.004
5. Qin L, Liu N, Bao CL, et al. Mesenchymal stem cells in fibrotic diseases—the two sides of the same coin. Acta Pharmacol Sin. 2022. doi:10.1038/s41401-022-00952-0
6. Lim KH, Itinteang T, Davis PF, Tan ST. Stem cells in keloid lesions: a review. Plast Reconstr Surg Glob Open. 2019;7(5):e2228. PMID: 31333955; PMCID: PMC6571348. doi:10.1097/GOX.0000000000002228
7. Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi:10.1080/14653240500319234
8. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi:10.1080/14653240600855905
9. Kouotou EA, Nansseu JR, Omona Guissana E, et al. Epidemiology and clinical features of keloids in Black Africans: a nested case-control study from Yaoundé, Cameroon. Int J Dermatol. 2019;58(10):1135–1140. PMID: 31429935. doi:10.1111/ijd.14610
10. Moon J, Kwak SS, Park G, et al. Isolation and characterization of multipotent Human keloid derived mesenchymal like stem cells. Stem Cells Dev. 2007;17:713–724. doi:10.1089/scd.2007.0210
11. Lee WJ, Park JH, Shin JU, et al. Endothelial-to-mesenchymal transition induced by Wnt 3a in keloid pathogenesis. Wound Repair Regen. 2015;23:435–442. doi:10.1111/wrr.12300
12. Akino K, Akita S, Yakabe A, Mineda T, Hayashi T, Hirano A. Human mesenchymal stem cells may be involved in keloid pathogenesis. Int J Dermatol. 2008;47:1112–1117. PMID: 18986439. doi:10.1111/j.1365-4632.2008.03380.x
13. Zhang Q, Takayoshi YA, Kelly P. Tumor-like stem cells derived from human keloid are governed by the inflammatory niche driven by IL-17/IL-6 axis. PLoS One. 2009;4(11):e7798. doi:10.1371/journal.pone.0007798
14. Lovisa S. Epithelial-to-mesenchymal transition in fibrosis: concepts and targeting strategies. Front Pharmacol. 2021;12:1–9. doi:10.3389/fphar.2021.737570
15. Tan K, Withers AHJ, Tan ST, Itinteang T. The role of stem cells in Dupuytren’s disease: a review. Plast Reconstr Surg Glob Open. 2018;6(5):e1777. doi:10.1097/GOX.0000000000001777
16. El Agha E, Kramann R, Schneider RK, et al. Mesenchymal stem cells in fibrotic disease. Cell Stem Cell. 2017;21:166–177. doi:10.1016/j.stem.2017.07.011
17. Schneider RK, Mullally A, Dugourd A, et al. Gli1+ mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. Cell Stem Cell. 2017;20:785–800.e788. doi:10.1016/j.stem.2017.03.008
18. Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016. PMID: 25329189. doi:10.1038/ni.3002
19. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010;5(4):e10088. doi:10.1371/journal.pone.0010088
20. Sato C, Yamamoto Y, Funayama E, et al. Conditioned medium obtained from amnion-derived mesenchymal stem cell culture prevents activation of keloid fibroblasts. Plast Reconstr Surg. 2018;141(2):390–398. doi:10.1097/PRS.0000000000004068
21. Fong CY, Biswas A, Subramanian A, Srinivasan A, Choolani M, Bongso A. Human keloid cell characterization and inhibition of growth with human Wharton’s jelly stem cell extract. J Cell Biochem. 2014;115(5):826–838. doi:10.1002/jcb.24724
22. Ya J, Wang X, Zhang J, Yongjun Q, Gong H, Jiang D. Inhibiting function of human fetal dermal mesenchymal stem cells on bioactivities of keloid fibroblasts. Stem Cell Res Ther. 2017;8:170. doi:10.1186/s13287-017-0624-0
23. Wang X, Yan M, Gao Z, Yang J. Human adipose-derived stem cells inhibit bioactivity of keloid fibroblasts. Stem Cell Res Ther. 2018;9:40. doi:10.1186/s13287-018-0786-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
