Back to Journals » Patient Related Outcome Measures » Volume 12
Do Generic Preference-Based Measures Accurately Capture Areas of Health-Related Quality of Life Important to Individuals with Amyotrophic Lateral Sclerosis: A Content Validation Study
Authors Peters N, Dal Bello-Haas V , Packham T , Chum M, O'Connell C, Johnston WS, MacDermid JC, Turnbull J, Van Damme J , Kuspinar A
Received 30 March 2021
Accepted for publication 4 June 2021
Published 25 June 2021 Volume 2021:12 Pages 191—203
DOI https://doi.org/10.2147/PROM.S313512
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lynne Nemeth
Nicole Peters,1 Vanina Dal Bello-Haas,1 Tara Packham,1 Marvin Chum,2 Colleen O’Connell,3 Wendy S Johnston,4 Joy C MacDermid,5 John Turnbull,2 Jill Van Damme,1 Ayse Kuspinar1
1School of Rehabilitation Science, McMaster University, Hamilton, ON, Canada; 2Department of Medicine, McMaster University, Hamilton, ON, Canada; 3Stan Cassidy Centre for Rehabilitation, Fredericton, NB, Canada; 4Department of Medicine, University of Alberta, Edmonton, AB, Canada; 5School of Physical Therapy, Western University, London, ON, Canada
Correspondence: Ayse Kuspinar
School of Rehabilitation Science, McMaster University, Hamilton, ON, Canada
Tel +1 905-525-9140 Ext. 27823
Email [email protected]
Objective: The objectives of this study were to 1) assess the content validity of generic preference-based measures (GPBMs), and (2) examine the convergent validity of the EuroQol 5 Dimension 5 Level (EQ-5D-5L), against the Patient Generated Index (PGI) in Amyotrophic Lateral Sclerosis (ALS).
Methods: Participants were recruited from 3 clinical sites across Canada. The PGI, EQ-5D-5L and Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) were administered through an online or hardcopy survey and scores compared for convergent validation. Domains nominated by participants as important to their health-related quality of life were generated using the PGI, classified using the International Classification of Functioning, Disability and Health (ICF) and mapped onto GPBMs to determine content coverage.
Results: Fifty-two participants (N=28 female; 61.3 ± 11.6 mean age ± standard deviation (SD); 3.5 ± 2.9 mean ± SD years since diagnosis) completed this study. The top three ICF domains identified by participants were recreation and leisure, lower limb mobility, and interpersonal relationships. The Quality of Well-Being Self-Administered (QWB-SA) scale had the highest content coverage (87%) and the Health Utilities Index 3 (HUI3) had the lowest (33%). Two domains were covered by all GPBMs and no GPBM included all domains identified as important by participants. A moderate correlation coefficient of 0.52 between the PGI and EQ-5D-5L was found.
Conclusion: The majority of GPBMs covered only approximately half of the domains important to individuals with ALS suggesting the need for an ALS specific preference-based measure to better reflect the health-related quality of life of this population.
Keywords: amyotrophic lateral sclerosis, health-related quality of life, patient reported outcome measures, psychometric properties, economic evaluation
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by selective and progressive loss of voluntary motor neurons.1 Individuals with ALS experience a range of symptoms related to the loss of muscle control in limb, bulbar and respiratory functions.2–4 Consequently, activities of daily living, independence, and health-related quality of life (HRQL) are all impacted by the disease and as no curative treatment is currently available, optimal treatment includes addressing symptoms and improving HRQL.2,5–8
Improvement in HRQL, “an individual’s perception of how an illness and its treatment affect the physical, mental and social aspects of his or her life,”9,10 is often considered the ultimate goal in healthcare,11 and measures of HRQL can be used for treatment decision-making and outcome evaluation purposes.11,12 Generic preference-based measures (GPBMs) are a type of HRQL measure designed to assess the cost-effectiveness of interventions due to their ability to produce a single index score, typically anchored from 0.0 (death) to 1.0 (perfect health).13–15 This value can be used to calculate quality-adjusted life years (QALYs) by capturing the effect of an intervention on one’s quality of life (morbidity) and length of life (mortality).13,16 GPBMs have been used to assess the HRQL of individuals with ALS and to aid researchers, policymakers and health care professionals in evaluating the cost-effectiveness of different treatment options.17
While generic measures include a set of common domains relevant across a variety of health conditions, they may not capture all the domains that are impacted by specific health conditions. When this occurs, scores from GPBMs may be higher than the true impact, resulting in incorrect comparisons across interventions and populations.18
Before a measure can be applied in practice, it must be tested to ensure that it is both reliable and valid.19,20 Content validity is the degree to which the content of an instrument accurately reflects the construct to be measured: a fundamental aspect in considering whether a measure can be used in a population.20 However, the content validity of GPBMs in individuals with ALS has not yet been evaluated. The Patient Generated Index (PGI) is an individualized measure that was developed to focus on the impact of a specific health condition on HRQL.21 It has been previously utilized to identify areas of quality of life important to patients in studies of content validity.18,22 Therefore, the primary objective of this study was to assess the content validity of GPBMs in ALS. The secondary objective of this study was to examine the convergent validity of the EuroQol 5 Dimension 5 Level (EQ-5D-5L), a widely used GPBM for HRQL, against the Patient Generated Index (PGI), in ALS.
Methods
Participants
Participants were recruited from the Canadian ALS Research Network (CALS) outpatient clinics across Western (Edmonton, AB), Central (Hamilton, ON), and Eastern (Fredericton, NB) Canada. Participants were eligible for inclusion if they : 1) were 18 years of age or older, 2) had a clinical diagnosis of ALS, and 3) able to communicate, verbally or electronically, in English. Individuals with severe frontotemporal dementia were excluded.
Outcome Measures
This study involved the administration of an online or hard copy (paper and pen) questionnaire consisting of sociodemographic and clinical information, the PGI, the EQ-5D-5L and the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R).
Sociodemographic and Clinical Information
Sociodemographic information consisting of age, sex, gender, residing region, highest education level, marital status, living situation and employment status was obtained. Clinical information consisting of year of diagnosis, ALS symptom onset location, clinic location and clinic visitation frequency was recorded.
Patient Generated Index (PGI)
The PGI21 is an individualized measure previously utilized to identify areas of quality of life important to individuals in studies of content validity. It has been used with chronic conditions such as cancer,23–25 Parkinson’s disease,22 Multiple Sclerosis (MS)18 and Ankylosing Spondylitis.26 It is completed in three stages. First, participants are asked to identify up to five of the most important areas of their lives affected by their health condition (ie, ALS). Second, they are asked to rate the extent of impact of each area on their lives from 0 (the worst you could imagine) to 10 (exactly as you would like it to be). A supplementary sixth item is provided to rate all other areas of life not mentioned. This can include additional areas of life affected by the health condition, as well as non-health related areas. In the third phase, participants are asked to imagine that they could improve some or all their chosen areas. Participants are given twelve weighting points to distribute across the five potential areas they would like to have improved, as well as the sixth item indicative of all other areas not mentioned. They can distribute these weighting points in any manner they choose but cannot use more than 12 points in total. More points allocated to an area indicate greater importance and hope of improvement. An average of 2 or more weighting points per area is considered meaningful.22
The rating and weighting points allocated to each area are then multiplied and summed to produce a single index score of overall HRQL from 0 to 10, with higher scores indicating better HRQL.21 This score is typically reported as a percentage27 and is intended to represent the extent to which reality matches expectations of perceived quality of life for those areas of life patients most value an improvement.21,24 If there are missing data, an overall PGI score cannot be calculated.
EQ-5D-5L
The EQ-5D-5L,28 developed by the EuroQol Group, is a well-established and widely used GPBM of HRQL that consists of two parts.15,29 The first part (the descriptive system) assesses health in five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each domain has five response levels, scored from 1 (no problems) to 5 (unable/extreme).28 A health utility value is derived from the five domains and is transformed into a single index score using a time-trade off (TTO) derived scoring system; a method of assigning values to health states from the population by asking respondents to choose between a shorter life in a state of perfect health or a longer life in a state of impaired health.30 Canadian health utilities for the EQ-5D-5L range from −0.148 for the worst possible health state (55,555; a score of 5 for each of the health domains) to 0.949 (11,111; a score of 1 for each of the health domains) for the best possible health state.30 The EQ-5D-5L describes a total of 3125 health states (55), has been translated into more than 170 languages world-wide, and takes only a few minutes for participants to complete.31 The second part of the EQ-5D-5L consists of a visual analogue scale (VAS) of self-rated health, scored from 0 to 100.31,32 The scores from the VAS cannot be used directly as weights in QALY calculations, as they not produce a single index value from a preference-based scoring system; however, the scores can be used as a subjective measure of one’s self-perceived health.32
Self-Administered ALSFRS-R
The ALSFRS-R33 quantifies degree of functional impairment in ALS, and consists of 12 questions across 3 domains: bulbar, motor and respiratory. The questions are rated on a 5-point scale from 0 (complete dependence) to 4 (normal function) and a total ALSFRS-R score, ranging from 0 to 48, is produced through summation of the individual item scores; with higher scores indicating better health and increased predicted survival.33 The self-administered version of the ALSFRS-R has demonstrated excellent reliability (intra-class correlation = 0.93, 95% CI0.88 to 0.96) and sensitivity to change over time.34
More recently, studies have evaluated the measurement properties of the ALSFRS-R using Rasch analyses35,36 and longitudinal and survival analyses.37 From their findings, researchers caution the reporting of a global score and recommend reporting domain-specific subscale scores organized into bulbar, motor and respiratory domains.33,35–37 A total subscale score for each domain is produced through summation of the corresponding items: items 1 to 3 (bulbar), items 4 to 9 (motor) and items 10 to 12 (respiratory).35 Bulbar and respiratory domains range in score from 0 to 12 whereby the motor domain ranges in score from 0 to 24; with higher scores indicating better function.
Procedure
Ethics approval for this cross-sectional study was obtained from McMaster University (HiREB #5664) and all sites in accordance with their respective research ethics boards. This study was conducted in accordance with the Declaration of Helsinki and all participants provided written informed consent. A designated clinician or research nurse located in clinic, identified, and recruited eligible participants. All participants were diagnosed with ALS using valid diagnostic criteria by board-certified neurologists specialized in motor neuron disorders. If interested, participants were given a hard copy of information including the invitation letter, consent form, and survey. Alternatively, interested participants could provide their email and a link to complete the LimeSurvey38 was then sent by the research team. Participants could complete the questionnaire package by themselves or with the aid of a caregiver.
The domains generated from the PGI were classified independently by two reviewers (NP and JVD) using the World Health Organization’s International Classification of Functioning, Disability and Health (ICF).39 The ICF was used as it provided a framework for coding and a standardized description of health-related problems at various levels (impairments, activity limitations and participation restrictions). A third and fourth reviewer (AK and VDBH) were consulted if consensus was not reached or ambiguity in responses were present. Established guidelines from Cieza and Stucki40 were used for the coding process to ensure accuracy of coding between reviewers and to capture all relevant domains:
- The domain nominated by the participant was coded to the most specific ICF code; if the reported area covered more than one code, then all codes were assigned.
- To eliminate subjectivity, reviewers coded all possible interpretations of the domains.
- Broader categories were used for coding if there were inconsistencies between reviewers in order to be as conservative as possible.
All individual and overarching ICF domains were then mapped onto the seven leading GPBMs: the EQ-5D-5L,28 the SF-6D,41 the Health Utilities Index Mark 2 and 3 (HUI 2 and 3),42 the Assessment of Quality of Life 8-Dimension (AQoL-8D),43 the 15-Dimension (15D)44 and the Quality of Well-Being Self-Administered (QWB-SA) scale.45 Mapping was performed by two independent reviewers (NP and JVD), with a third reviewer (AK) for consultation, if needed. The content coverage of GPBMs was determined by the percentage of domains included in the GPBM that were also nominated by individuals with ALS using the PGI. High and low percentages correspond with high and low content coverage, respectively. This methodology, as outlined in Figure 1, followed that of similar studies assessing content validity of GPBMs using the PGI.18,22,46
Sample Size
There are no sample size recommendations for content validation.47 Therefore, our sample size calculation was based on: the number of participant responses needed to achieve data saturation (when no new relevant knowledge is being obtained from participants)48 and; the recommended sample size for construct validation studies, which is a minimum of 50 patients total.20 Studies have demonstrated that sample sizes around 15 to 20 are sufficient for saturation,49–51 therefore, to satisfy both recommendations we aimed to recruit between 45 and 60 participants across the 3 clinical sites.
Data Analysis
Descriptive statistics consisting of parametric measures such as mean, standard deviation, frequency and percentage were calculated to analyze participants’ sociodemographic and clinical information. Scores for the PGI, EQ-5D-5L, and ALSFRS-R were computed according to their respective guidelines. Pearson’s correlation coefficient was used to measure the strength of the association between PGI and EQ-5D-5L scores, as the data were normally distributed. Only complete data were used to assess association. Correlations with instruments measuring similar constructs should be greater than or equal to 0.50,48 therefore to assess convergent validity, a correlation of at least 0.5 was hypothesized between the PGI and EQ-5D-5L.52
Results
Table 1 presents the sociodemographic and clinical characteristics for the sample (N=52). No participants were excluded due to severe frontotemporal disorder. A total of 35 participants completed the PGI in full. The mean age of the sample was 61 years old and 54% were females. Approximately half of the sample (52%) completed the questionnaire in hard copy format, with the remaining completing the online format. Of the study participants, 67% completed the questionnaire without the assistance of a caregiver. Participants were distributed across Western (46%), Central (37%) and Eastern (17%) Canada, with 33% of individuals visiting their designated clinic every 3 months. The mean time since diagnosis was 3.5 (2.95) years. The time since diagnosis ranged from less than 1 year ago (27%) to five or more years (12%). For our sample, ALS symptoms first began to appear in the upper and/or lower limbs for 75% of the sample. The mean total ALSFRS-R score was 30.4 (9.4). The following subdomain scores were calculated for the ALSFRS-R: bulbar was 8.5 (3.5), scored out of 12; motor was 12.2 (6.2), scored out of 24; and respiratory was 9.8 (2.8), scored out of 12. The mean PGI score was 25.4 (14.1) and the mean EQ-5D-5L score was 0.55 (0.24).
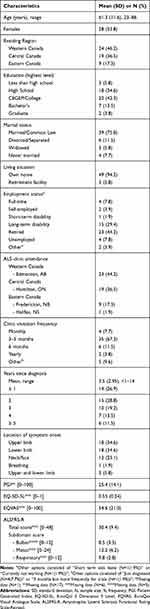 |
Table 1 Sociodemographic and Clinical Characteristics of Sample (N=52) |
Table 2 outlines the ICF domains identified by participants to be most affected by ALS and their frequency of appearance (n). There were 78 individual domains identified which resulted in 25 overarching ICF domains. The top 10 overarching domains identified were: recreation and leisure (17%), lower limb mobility (11%), interpersonal relationships (9%), self-care (7%), housework and preparing meals (6.5%), speaking (6%), eating and swallowing (5%), work and employment (4%), upper limb mobility (4%), and daily routine and independence (4%).
 |
Table 2 Overarching ICF Domains Identified More Than Once by Participants from the Patient Generated Index, Organized from Most Frequent to Least Frequent (Total n=291) |
Figure 2 outlines the mean impact scores for each overarching ICF domain identified by participants. The three least impacted HRQL domains were upper limb mobility (mean score of 4.4), self-care (mean score of 3.6) and lower limb mobility (mean score of 3.2). The domains work and employment (mean score of 1.3) and recreation and leisure (mean score of 1.7) were the most impacted.
Figure 3 outlines the mean number of points (out of 12 points) that participants allocated to each of the overarching ICF domains. The most desired areas for improvement were interpersonal relationships (30% of points, mean of 3.0 points), muscle and movement functions (29% of points, mean of 2.9 points) and speaking (29% of points, mean of 2.9 points). The area with the least desire for improvement was housework and preparing meals (15% of points, mean of 1.5 points).
Table 3 presents the mapping of overarching ICF domains identified by individuals with ALS against GPBMs. The GPBM that covered the highest number of ICF domains identified by participants was the QWB-SA scale at 87% coverage. The HUI3 addressed the least number of domains with 33% coverage. The remaining GPBMs identified between 53% and 67% of domains. The domains covered by all GPBMs were lower limb mobility and emotions. The domains most commonly missing from GPBMs were: structures involved in voice and speech, which was only included in the QWB-SA scale; and caring for household objects, only included in the AQoL-8D. Supplementary Figure 1 presents a scatter plot of EQ-5D-5L scores plotted against PGI scores. A positive moderate Pearson’s correlation coefficient of 0.52 was observed between the two measures.
 |
Table 3 Mapping of Overarching ICF Domains, Identified by ALS Patients, Onto Generic Preference-Based Measures |
Discussion
This was the first study evaluating the content validity of GPBMs in individuals with ALS. Participants completed an individualized measure, the PGI, to evaluate the impact of ALS on their HRQL. Commonly reported domains, identified as areas impacted by ALS and rated in terms of desire for improvement, were classified using the ICF and consequently mapped onto GPBMs to estimate the extent to which these generic measures captured domains that were important to individuals with ALS.
Individualized measures provide a standardized method to identify aspects of a health condition that impact patients’ HRQL.53,54 The PGI21 allowed individuals with ALS to identify the areas of their lives affected by their condition and assign a weight to each identified domain. The majority of GPBMs included approximately half of the areas reported on the PGI. The domains lower limb mobility and emotions were the only two areas identified by all GPBMs, however there was no one GPBM that included all the domains nominated by participants.
Domains self-identified as being affected by ALS encompassed three out of four ICF components - body structures (7%), body functions (13%), and activities and participation (80%). Domains nominated were relatively severely impacted, rated as very poor to poor21 with an average score of 2.7 out of 10 (See Figure 2), and important to their quality of life with an average of 2.4 weighting points out of 12 (See Figure 3) allocated across domains for desire for improvement. For example, recreation and leisure was the most commonly reported domain and not only was it severely impacted by ALS but the desire for improvement in this domain was heavily weighted. This was true to some fashion for all domains nominated by participants as the average impact of ALS on domains was rated as severe. For example, it is well known that ALS progression results in a decline in physical health.2 Studies have shown that HRQL is not necessarily dependent on patient’s physical well-being but on their mental and social well-being.55–57 The results of this study further demonstrate the impact of ALS on patients’ social well-being and independence. Therefore, we would thus expect GPBMs used in ALS to capture these nominated domains, yet this was not the case.
In assessing the content coverage of GPBMs, the GPBM with the least coverage was the HUI3; evolved from the HUI/HUI1 and HUI2.42 The HUI3 has been widely used in clinical populations, including neurological conditions.58–60 It includes eight HRQL domains that focus on bodily functions: vision, speech, hearing, dexterity, ambulation, cognition, emotion and pain.61 Only a third of the domains identified in our sample were covered by this measure ie, lower limb mobility, speaking, upper limb mobility, emotions and undertaking a task. The HUI3 does not include domains relevant to social well-being, which was identified as important in our sample, and is missing many areas identified as important to their HRQL.
The QWB-SA scale had the highest percentage of included domains, with 87% of the domains deemed relevant to individuals with ALS. The QWB-SA scale is a comprehensive measure of HRQL that combines scales of functioning with a measure of symptoms and problems.45 However, the measure is very symptom and limitation focused, and did not seem to translate to our sample. ALS affects all areas of life including participation areas, and our study showed that the effects of the symptoms, rather than the symptoms themselves, were the most impacted in our sample. The QWB-SA scale did not capture this well. Furthermore, the administration of the QWB-SA scale is lengthy, ie, takes around 14-minutes to complete,62 compared to the EQ-5D which only takes a few minutes to complete;31 this may be one reason why it is not as widely used. Additionally, a study by Smith et al (2000)63 utilized the QWB-SA in ALS and found evidence corroborating decreased usage of the measure in this population, which may contribute further insight into the low utilization in ALS. Specifically, the measure demonstrated poor convergent validity with other measures of HRQL (ie, the SF-36 and Sickness Impact Profile/ALS-19). The authors explained their results in relation to the general makeup of the test; with items and valuations that may not accurately capture the physical symptoms of individuals with ALS or provide equal weighting to items that are associated with ALS (such as self-care).
The EQ-5D-5L is a widely used GPBM and has previously been used in the ALS population.17,64–68 It covered 53% of domains identified by individuals with ALS (8/15 domains). However, it did not cover relevant and important domains such as speaking, eating, and swallowing or upper limb mobility, which are affected by ALS. For instance, domains comprised by the EQ-5D-5L (Mobility, Self-care, Usual Activities, Pain/Discomfort and Anxiety/Depression)28 are comparable to the impairments and activity limitations identified by our sample. Nonetheless, the social aspects of ALS identified by our sample as being impacted, were not explicitly addressed by the EQ-5D-5L as a distinctive domain, which resulted in a lower coverage than expected.
The mean EQ-5D-5L score in our sample indicates a moderate health state, whereas the mean PGI score indicates poor HRQL. A lower HRQL score on the PGI in comparison to the EQ-5D-5L, suggests that GPBMs may underestimate the effects of ALS on the HRQL of patients. Furthermore, a moderate Pearson’s correlation between the PGI and the EQ-5D-5L was found in our study. A higher correlation was anticipated between the two HRQL measures,13 however it is not surprising considering there were identified domains not included in the EQ-5D-5L. Therefore, both the magnitude of correlation and percentage of content coverage in the EQ-5D-5L provide evidence of an overall lack of items relevant to individuals with ALS.
Strengths and Limitations
Our study is not without its strengths and limitations. To strengthen the generalizability of our study, participants were recruited from 3 different regions across Canada. Furthermore, we had a fairly equal distribution of men and women in our sample. An additional strength is in the number of years since diagnosis in that it is reflective of the ALS population in large epidemiological studies;69 with the majority of individuals diagnosed within two to five years. Moreover, in our sample 75% of participants indicated upper and/or lower symptoms as the primary symptom recognized at onset; and limb-onset ALS affects 65% to 75% of individuals diagnosed.70,71 Lastly, there was a wide range of ALSFRS-R scores in our sample, indicating mild to severe functional impairment, which is again reflective of the ALS population.69
One limitation to this study was in utilizing the ICF as a coding framework. While the ICF has been used in similar studies, the framework is not all encompassing; therefore, some domains nominated by the sample, such as balance, were difficult to code. The second limitation was the amount of missing data present (N=17), particularly related to the completion of the hardcopy version of the PGI. As a result, we could only determine the magnitude of the association between the EQ-5D-5L and PGI for the 67% of the sample that had complete data.
Future Research
As this was a cross-sectional study, we did not evaluate the impact of symptom progression on HRQL and ALSFRS-R scores. It has been shown that rate of disease progression is a simple and sensitive clinical prognostic biomarker in ALS, when reflected over time by ALSFRS-R change score.72 This is an area for future research to investigate whether slow or fast progression differentially impact the type and order of domains generated by the ICF procedure and PGI. Another area for future research would be to compare ALSFRS-R scores to GPBMs and the PGI. As the ALSFRS-R is not a measure of HRQL, this calculation was not within the scope of this study. Such analysis by future studies could strengthen findings from this study by comparing quality of life and degree of patient ability.
In addition, due to a limited sample size, analysis on clinical information of the sample was restricted. For instance, epidemiological characteristics of ALS such as ALS phenotype (classic, bulbar, respiratory, flail arm, flail leg, pyramidal, pure lower motor neuron, and pure upper motor neuron)73 was not established by researchers a priori. Scores from the ALSFRS-R may therefore be impacted due to perceived burden of disease which may vary with ALS phenotype. This is an area for future research as the impact of ALS and perceived burden of the disease may be reflected by GPBMs.
Conclusion
Content of preference-based measures needs to be reflective of the population’s values for accurate economic evaluation of treatments. Our results demonstrated that the majority of well-recognized GPBMs included only approximately half of the domains important to those living with ALS. The most commonly used GPBM in ALS, the EQ-5D-5L, correlated moderately with the PGI, however it underestimated the impact of ALS on the HRQL of patients. Likewise, in assessing the content validity of GPBMs, there were domains that were not identified, or that were inaccurately represented and not relevant to our sample. Findings from this study suggest the need for the development of an ALS specific preference-based measure with items that will capture the areas of life important to people with ALS and provide population-specific values that can be utilized for the assessment of treatment implications.
Ethics
Ethics approval for this cross-sectional study was obtained from McMaster University (HiREB #5664) and all sites in accordance with their respective research ethics boards. This study was conducted in accordance with the Declaration of Helsinki and all participants provided written informed consent.
Acknowledgments
The authors would like to thank the research staff at each clinical site for their assistance with recruitment of participants.
Funding
This work was supported by the 2018 ALS Project Grant awarded by the ALS Society of Canada.
Disclosure
Vanina Dal Bello-Haas reports grants from ALS Society of Canada Project Grant, outside the submitted work. Colleen O’Connell reports personal fees from MT Pharma, outside the submitted work. Wendy S Johnston reports grants and personal fees from Biogen Inc., Cytokinetics Inc, and Mitsubishi Tanabe Canada Inc.; personal fees from Amylyx Inc.; and grants from Alexion Inc., Annexion Inc., and Orion Inc., outside the submitted work . The authors report no other potential conflicts of interest in this work.
References
1. Hardiman O, Al-Chalabi A, Chio A, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primer. 2017;3(1):17071. doi:10.1038/nrdp.2017.71
2. Zarei S, Carr K, Reiley L, et al. A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int. 2015;6(1):171. doi:10.4103/2152-7806.169561
3. Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7(9):710–723. doi:10.1038/nrn1971
4. Andersen PM, Borasio GD, Dengler R, et al. EFNS task force on management of amyotrophic lateral sclerosis: guidelines for diagnosing and clinical care of patients and relatives. An evidence-based review with good practice points. Eur J Neurol. 2005;12(12):921–938. doi:10.1111/j.1468-1331.2005.01351.x
5. Simmons Z. Patient-perceived outcomes and quality of life in ALS. Neurotherapeutics. 2015;12(2):394–402. doi:10.1007/s13311-014-0322-x
6. Handy CR, Krudy C, Boulis N, Federici T. Pain in amyotrophic lateral sclerosis: a neglected aspect of disease. Neurol Res Int. 2011;2011:1–8. doi:10.1155/2011/403808
7. Swash M. Health outcome and quality-of-life measurements in amyotrophic lateral sclerosis. J Neurol. 1997;244(S2):S26–S29. doi:10.1007/BF03160578
8. Bensimon G, Lacomblez L, Meininger V, Controlled A. Trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994;330(9):585–591. doi:10.1056/NEJM199403033300901
9. Mayo NE. ISOQOL dictionary of quality of life and health outcomes measurement. ISOQOL; 2015. Available from: https://books.google.ca/books?id=cKjksgEACAAJ.
10. Karimi M, Brazier J. Health, health-related quality of life, and quality of life: what is the difference? PharmacoEconomics. 2016;34(7):645–649. doi:10.1007/s40273-016-0389-9
11. Ruta DA, Garratt AM. Health status to quality of life measurement. In: Jenkinson C, editor. Measuring Health and Medical Outcomes. Routledge; 2013:138–159. Available from: https://www.taylorfrancis.com/books/e/9781315072159.
12. Guyatt GH. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622. doi:10.7326/0003-4819-118-8-199304150-00009
13. Brazier J, Ratcliffe J, Salomon JA, Tsuchiya A. Measuring and Valuing Health Benefits for Economic Evaluation.
14. Torrance GW. Utility approach to measuring health-related quality of life. J Chronic Dis. 1987;40(6):593–600. doi:10.1016/0021-9681(87)90019-1
15. Brauer CA, Rosen AB, Greenberg D, Neumann PJ. Trends in the measurement of health utilities in published cost-utility analyses. Value Health. 2006;9(4):213–218. doi:10.1111/j.1524-4733.2006.00116.x
16. Neumann PJ, Goldie SJ, Weinstein MC. Preference-based measures in economic evaluation in health care. Annu Rev Public Health. 2000;21(1):587–611. doi:10.1146/annurev.publhealth.21.1.587
17. Lopez-Bastida J, Perestelo-Perez L, Monton-Alvarez F, Serrano-Aguilar P, Alfonso-Sanchez JL. Social economic costs and health-related quality of life in patients with amyotrophic lateral sclerosis in Spain. Amyotroph Lateral Scler. 2009;10(4):237–243. doi:10.1080/17482960802430781
18. Kuspinar A, Mayo NE. Do generic utility measures capture what is important to the quality of life of people with multiple sclerosis? Health Qual Life Outcomes. 2013;11(1):71. doi:10.1186/1477-7525-11-71
19. Souza AC, Alexandre NM, Guirardello ED. Psychometric properties in instruments evaluation of reliability and validity. Epidemiol Serv Saude. 2017;26(3):649–659. doi:10.5123/S1679-49742017000300022
20. de Vet HCW, editor. Measurement in Medicine: A Practical Guide. Cambridge University Press; 2011.
21. Ruta DA, Garratt AM, Leng M, Russell IT, MacDonald LM, New A. Approach to the measurement of quality of life: the patient-generated index. Med Care. 1994;32(11):1109–1126. doi:10.1097/00005650-199411000-00004
22. Kuspinar A, Mate K, Lafontaine A-L, Mayo N. Evaluating the content validity of generic preference-based measures for use in Parkinson’s disease. Parkinsonism Relat Disord. 2019;62:112–116. doi:10.1016/j.parkreldis.2019.01.014
23. Tavernier SS, Totten AM, Beck SL. Assessing content validity of the patient generated index using cognitive interviews. Qual Health Res. 2011;21(12):1729–1738. doi:10.1177/1049732311420169
24. Aburub AS, Gagnon B, Rodríguez AM, Mayo NE. Using a personalized measure (Patient Generated Index (PGI)) to identify what matters to people with cancer. Support Care Cancer. 2016;24(1):437–445. doi:10.1007/s00520-015-2821-7
25. Tavernier SS, Beck SL, Clayton MF, Pett MA, Berry DL. Validity of the patient generated index as a quality-of-life measure in radiation oncology. Oncol Nurs Forum. 2011;38(3):319–329. doi:10.1188/11.ONF.319-329
26. Haywood KL, Garratt AM, Dziedzic K, Dawes PT. Patient centered assessment of ankylosing spondylitis-specific health related quality of life: evaluation of the patient generated index. J Rheumatol. 2003;30(4):764–773.
27. Martin F, Camfield L, Rodham K, Kliempt P, Ruta D. Twelve years–experience with the Patient Generated Index (PGI) of quality of life: a graded structured review. Qual Life Res. 2007;16(4):705–715. doi:10.1007/s11136-006-9152-6
28. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. doi:10.1007/s11136-011-9903-x
29. Brazier J, Ara R, Rowen D, Chevrou-Severac HA. Review of generic preference-based measures for use in cost-effectiveness models. PharmacoEconomics. 2017;35(S1):21–31. doi:10.1007/s40273-017-0545-x
30. Xie F, Pullenayegum E, Gaebel K, et al. A time trade-off-derived value set of the EQ-5D-5L for Canada. Med Care. 2016;54(1):98–105. doi:10.1097/MLR.0000000000000447
31. van Reenen M, Janssen B. EQ-5D-5L user guide: basic information on how to use the EQ-5D-5L instrument; 2019. Avaialble from: https://euroqol.org/publications/user-guides.
32. Brooks R. EuroQol: the current state of play. Health Policy (New York). 1996;37(1):53–72. doi:10.1016/0168-8510(96)00822-6
33. Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1–2):13–21. doi:10.1016/S0022-510X(99)00210-5
34. Montes J, Levy G, Albert S, et al. Development and evaluation of self-administered version of the ALSFRS-R. Neurology. 2006;67:1294–1296. doi:10.1212/01.wnl.0000238505.22066.fc
35. Franchignoni F, Mora G, Giordano A, Volanti P, Chiò A. Evidence of multidimensionality in the ALSFRS-R Scale: a critical appraisal on its measurement properties using Rasch analysis. J Neurol Neurosurg Psychiatry. 2013;84(12):1340–1345. doi:10.1136/jnnp-2012-304701
36. Franchignoni F, Mandrioli J, Giordano A, Ferro S, ERRALS Group. A further Rasch study confirms that ALSFRS-R does not conform to fundamental measurement requirements. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(5–6):331–337. doi:10.3109/21678421.2015.1026829
37. Rooney J, Burke T, Vajda A, Heverin M, Hardiman O. What does the ALSFRS-R really measure? A longitudinal and survival analysis of functional dimension subscores in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(5):381–385. doi:10.1136/jnnp-2016-314661
38. Limesurvey GmbH, Schmitz C. LimeSurvey: an open source survey tool. Limesurvey GmbH; 2020. Available from: http://www.limesurvey.org.
39. World Health Organization. International Classification of Functioning, Disability and Health: ICF. World Health Organization; 2001.
40. Cieza A, Stucki G. The international classification of functioning disability and health: its development process and content validity. Eur J Phys Rehabil Med. 2008;44(3):303–313.
41. Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292. doi:10.1016/S0167-6296(01)00130-8
42. Horsman J, Furlong W, Feeny D, Torrance GW. The Health Utilities Index (HUI®): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1(1):54. doi:10.1186/1477-7525-1-54
43. Richardson J. Monash University, centre for health economics, Monash University, faculty of business and economics. Modelling the Utility of Health States with the Assessment of Quality of Life (AQoL) 8D Instrument: Overview and Utility Scoring Algorithm. Monash University, Business and Economics, Centre for Health Economics; 2011. Available from: http://www.buseco.monash.edu.au/centres/che/pubs/researchpaper63.pdf.
44. Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33(5):328–336. doi:10.3109/07853890109002086
45. Seiber WJ, Groessl EJ, David KM, Ganiats TG, Kaplan RM. Quality of well-being self-administered scale (QWB-SA); 2008. Available from: https://www.researchgate.net/profile/Kristin_Kistler/publication/252316672_Quality_of_well_being_self-administered_QWB-SA_scale/links/5437d6990cf2590375c55a65/Quality-of-well-being-self-administered-QWB-SA-scale.pdf.
46. Mayo NE, Moriello C, Asano M, van der Spuy S, Finch L. The extent to which common health-related quality of life indices capture constructs beyond symptoms and function. Qual Life Res. 2011;20(5):621–627. doi:10.1007/s11136-010-9801-7
47. Mason M. Sample size and saturation in PhD studies using qualitative interviews. Forum Qual Soc Res. 2010;11(3). Methods for Qualitative Management Research in the Context of Social Systems Thinking. doi:10.17169/FQS-11.3.1428
48. Prinsen CAC, Mokkink LB, Bouter LM, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147–1157. doi:10.1007/s11136-018-1798-3
49. Bertaux D, editor. Biography and Society: The Life History Approach in the Social Sciences. Sage Publications; 1981.
50. Guest G, Bunce A, Johnson L. How many interviews are enough?: an experiment with data saturation and variability. Field Methods. 2006;18(1):59–82. doi:10.1177/1525822X05279903
51. Green J, Thorogood N. Qualitative Methods for Health Research.
52. Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71.
53. Dijkers MP. Individualization in quality of life measurement: instruments and approaches. Arch Phys Med Rehabil. 2003;84:S3–S14. doi:10.1053/apmr.2003.50241
54. Joyce CRB, McGee HM, OBoyle CA. Individual quality of life: approaches to conceptualisation and assessment. Harwood Academic Publishers; 1999. Available from: http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=526533.
55. Chiò A. A cross sectional study on determinants of quality of life in ALS. J Neurol Neurosurg Psychiatry. 2004;75(11):1597–1601. doi:10.1136/jnnp.2003.033100
56. O’Doherty LJ, Hickey A, Hardiman O. Measuring life quality, physical function and psychological well-being in neurological illness. Amyotroph Lateral Scler. 2010;11(5):461–468. doi:10.3109/17482960903552488
57. Simmons Z, Bremer BA, Robbins RA, Walsh SM, Fischer S. Quality of life in ALS depends on factors other than strength and physical function. Neurology. 2000;55(3):388–392. doi:10.1212/wnl.55.3.388
58. Fisk JD. A comparison of health utility measures for the evaluation of multiple sclerosis treatments. J Neurol Neurosurg Psychiatry. 2005;76(1):58–63. doi:10.1136/jnnp.2003.017897
59. Pohar SL, Allyson Jones C. The burden of Parkinson disease (PD) and concomitant comorbidities. Arch Gerontol Geriatr. 2009;49(2):317–321. doi:10.1016/j.archger.2008.11.006
60. Edwards JD, Koehoorn M, Boyd LA, Levy AR. Is health-related quality of life improving after stroke?: a comparison of health utilities indices among Canadians with stroke between 1996 and 2005. Stroke. 2010;41(5):996–1000. doi:10.1161/STROKEAHA.109.576678
61. Feeny D, Furlong W, Boyle M, Torrance GW. Multi-attribute health status classification systems: health utilities index. PharmacoEconomics. 1995;7(6):490–502. doi:10.2165/00019053-199507060-00004
62. Andresen EM, Rothenberg BM, Kaplan RM. Performance of a self-administered mailed version of the quality of well-being (QWB-SA) questionnaire among older adults. Med Care. 1998;36(9):1349–1360. doi:10.1097/00005650-199809000-00007
63. Smith PS, Crossley B, Greenberg J, Wilder C, Carroll B. Agreement among three quality of life measures in patients with ALS. Amyotroph Lateral Scler Mot Neuron Disord off Publ World Fed Neurol Res Group Mot Neuron Dis. 2000;1(4):269–275.
64. Ilse B, Prell T, Walther M, et al. Relationships between disease severity, social support and health-related quality of life in patients with amyotrophic lateral sclerosis. Soc Indic Res. 2015;120(3):871–882. doi:10.1007/s11205-014-0621-y
65. Winter Y, Schepelmann K, Spottke AE, et al. Health-related quality of life in ALS, myasthenia gravis and facioscapulohumeral muscular dystrophy. J Neurol. 2010;257(9):1473–1481. doi:10.1007/s00415-010-5549-9
66. Jones AR, Jivraj N, Balendra R, et al. Health utility decreases with increasing clinical stage in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(3–4):285–291. doi:10.3109/21678421.2013.872149
67. Green C, Kiebert G, Murphy C, et al. Patients’ health-related quality-of-life and health state values for motor neurone disease/amyotrophic lateral sclerosis. Qual Life Res. 2003;12(5):565–574. doi:10.1023/A:1025052609818
68. Kiebert G, Green C, Murphy C, et al. Patients’ health-related quality of life and utilities associated with different stages of amyotrophic lateral sclerosis. J Neurol Sci. 2001;191(1–2):87–93.
69. Longinetti E, Fang F. Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr Opin Neurol. 2019;32(5):771–776. doi:10.1097/WCO.0000000000000730
70. Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7(11):639–649. doi:10.1038/nrneurol.2011.153
71. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–955. doi:10.1016/S0140-6736(10)61156-7
72. Labra J, Menon P, Byth K, Morrison S, Vucic S. Rate of disease progression: a prognostic biomarker in ALS. J Neurol Neurosurg Psychiatry. 2016;87(6):628–632. doi:10.1136/jnnp-2015-310998
73. Chio A, Calvo A, Moglia C, Mazzini L, Mora G, PARALS study group. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2011;82(7):740–746. doi:10.1136/jnnp.2010.235952
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



