Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Disruption of histidine and energy homeostasis in chronic obstructive pulmonary disease
Authors Diao W, Labaki WW , Han MK, Yeomans L , Sun Y , Smiley Z , Kim JH, McHugh C , Xiang P, Shen N, Sun X, Guo C, Lu M, Standiford TJ, He B, Stringer KA
Received 30 March 2019
Accepted for publication 1 August 2019
Published 3 September 2019 Volume 2019:14 Pages 2015—2025
DOI https://doi.org/10.2147/COPD.S210598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Wenqi Diao1, Wassim W Labaki2, MeiLan K Han2, Larisa Yeomans3, Yihan Sun4, Zyad Smiley4, Jae Hyun Kim3, Cora McHugh4, Pingchao Xiang5, Ning Shen6, Xiaoyan Sun6, Chenxia Guo6, Ming Lu6, Theodore J Standiford2, Bei He*,7, Kathleen A Stringer*,2,4
1Department of Respiratory Medicine, Peking University Third Hospital, Beijing, People’s Republic of China; 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, School of Medicine, University of Michigan, Ann Arbor, MI, USA; 3Biochemical Nuclear Magnetic Resonance Core, College of Pharmacy, University of Michigan, Ann Arbor, MI, USA; 4NMR Metabolomics Laboratory, Department of Clinical Pharmacy, College of Pharmacy, University of Michigan, Ann Arbor, MI, USA; 5Department of Respiratory and Critical Care Medicine, Shou-Gang Hospital Affiliated to Peking University, Beijing, People’s Republic of China; 6Department of Respiratory Medicine, Peking University Third Hospital, Beijing 100191, People’s Republic of China; 7Department of Respiratory Medicine, Peking University Health Sciences Center, Third Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kathleen A Stringer
College of Pharmacy, University of Michigan, Ann Arbor, MI 48104, USA
Tel +1 734 647 4775
Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) is a systemic condition that is too complex to be assessed by lung function alone. Metabolomics has the potential to help understand the mechanistic underpinnings that contribute to COPD pathogenesis. Since blood metabolomics may be affected by sex and body mass index (BMI), the aim of this study was to determine the metabolomic variability in male smokers with and without COPD who have a narrow BMI range.
Methods: We compared the quantitative proton nuclear magnetic resonance acquired serum metabolomics of a male Chinese Han population of non-smokers without COPD, and smokers with and without COPD. We also assessed the impact of smoking status on metabolite concentrations and the associations between metabolite concentrations and inflammatory markers such as serum interleukin-6 and histamine, and blood cell differential (%). Metabolomics data were log-transformed and auto-scaled for parametric statistical analysis. Mean normalized metabolite concentration values and continuous demographic variables were compared by Student’s t-test with Welch correction or ANOVA with post-hoc Tukey’s test, as applicable; t-test p-values for metabolomics data were corrected for false discovery rate (FDR). A Pearson association matrix was built to evaluate the relationship between metabolite concentrations, clinical parameters and markers of inflammation.
Results: Twenty-eight metabolites were identified and quantified. Creatine, glycine, histidine, and threonine concentrations were reduced in COPD patients compared to non-COPD smokers (FDR ≤15%). Concentrations of these metabolites were inversely correlated with interleukin-6 levels. COPD patients had overall dampening of metabolite concentrations including energy-related metabolic pathways such as creatine metabolism. They also had higher histamine levels and percent basophils compared to smokers without COPD.
Conclusion: COPD is associated with alterations in the serum metabolome, including a disruption in the histidine-histamine and creatine metabolic pathways. These findings support the use of metabolomics to understand the pathogenic mechanisms involved in COPD.
Trial registration www.clinicaltrials.gov, NCT03310177.
Keywords: China, metabolomics, histidine, energy homeostasis, inflammation, chronic obstructive pulmonary disease
Background
Chronic obstructive pulmonary disease (COPD) is an inflammatory lung condition with considerable mortality and morbidity worldwide.1 The grading of COPD severity using the GOLD (Global initiative for chronic Obstructive Lung Disease) classification is based on lung function, which does not accurately capture the breadth of COPD heterogeneity. As such, there is a large gap in our understanding of the mechanisms behind the various phenotypes and clinical manifestations associated with COPD, including emphysema, chronic bronchitis, systemic inflammation and muscle dysfunction.2 Disruptions in metabolism have been previously reported in patients with COPD,3–7 which supports the notion that metabolomics, the identification and measurement of small molecules in biological samples, may provide insight into potential links between metabolic features and pathological mechanisms in COPD.8 Furthermore, testing the associations between metabolite concentrations and other markers of COPD phenotypes, such as inflammatory cytokines (eg, interleukin-6) and blood cell differential, may further corroborate these links.
Previous COPD metabolomics studies have found metabolic changes related to sex and body mass index (BMI).4,5 BMI is frequently in the normal range and diet is most often regionally uniform among the Chinese Han population.9 A population-based study showed that COPD is two times more prevalent in men compared to women in China.10 This presents a unique opportunity to assess disease-related metabolic alterations with minimal bias from BMI and diet. To this end, we measured and compared serum metabolites using quantitative proton nuclear magnetic resonance (1H-NMR)-based metabolomics in male Chinese Han patients with COPD, smokers without COPD and non-smoker controls.
Methods
Subjects
The study (www.clinicaltrials.gov, NCT03310177) was approved by the Peking University (PUIRB) and the University of Michigan Institutional Review Boards (IRBMed). Written informed consent was acquired from all subjects before enrollment into the study in accordance with the principles of the Declaration of Helsinki.
Individuals with COPD were enrolled in the clinic of Peking University Third Hospital and Shougang Hospital in China from December 2015 to July 2017. The inclusion criteria for male COPD subjects have been previously described.11 Briefly, subjects had to: 1) be male of 40–80 years of age; 2) have a smoking history ≥10 pack-year; 3) carry the diagnosis of COPD according to the GOLD definition;1,12 4) not have experienced a respiratory exacerbation in the past 3 months; 5) be free of severe hepatic, cardiovascular, mental or renal dysfunction; and 6) not have other pulmonary diseases (eg, asthma). The control groups included male non-COPD smokers (NCS) and never smokers (NS) without COPD, who met the aforementioned 1st, 5th and 6th inclusion criteria of COPD patients. This paper represents a report of the NMR metabolomics portion and secondary outcomes of the study NCT protocol.
Spirometry and high-resolution computed tomography
Spirometry (SensorMedics, Yorba Linda, CA, USA) was performed in all subjects according to American Thoracic Society/European Respiratory Society guidelines.13 The predicted percent of forced expiratory volume in the first second (FEV1%pred) was used to evaluate lung function in order to categorize subjects as follows: GOLD I, FEV1%pred >80%; GOLD II, 80%> FEV1pred ≥50%; GOLD III, 50% > FEV1pred ≥30%; GOLD IV, FEV1pred <30%. Chest high resolution computed tomography (HRCT) with continuous slices of 0.625 mm was performed at the time of blood sample collection. Emphysema extent was assessed by calculating the percent of lung volume with a low attenuation area (LAA%) defined as less than −950 Hounsfield Units (HU) (AW 4.5 software, GE healthcare, Fairfield, CT, USA).14
Blood sample collection and management
Blood sample collection was conducted in the morning (9:00 AM to 12:00 PM) for which study participants were required to be fasting and to have stopped smoking for at least 12 hrs. Two tubes of blood were collected from each patient one for EDTA-preserved plasma and one for serum. The serum sample remained at room temperature (25 °C) for at least 30 mins until clotted. Both samples were centrifuged (3000× g, 4 °C,15 min) and serum or plasma was aliquoted (500 µL) and stored (−80 °C) until assay or the time of shipment to the University of Michigan (serum). At the time of shipment, serum samples were placed on dry ice and packaged in accordance with the requirements of World Courier (www.worldcourier.com). The dry ice supply was maintained during shipping so samples remained frozen during transit. Upon arrival to Ann Arbor, MI, they were inventoried and immediately stored (−80 °C) until the time of assay.
Quantitative 1-dimensional (D) proton (1H)-nuclear magnetic resonance (NMR)
At the time of the assay, samples were randomized and thawed in an ice-water bath. Pre-chilled methanol was added to the volume of each sample (2:1) to precipitate macromolecules as a modification of previously described protocols.15–18 The samples were dried on a FreeZone 4.5 −105 °C lyophilizer (Labconco Corporation, Kansas City, MO, USA) and ultrafiltered (3 kDa MWCO, Pall Nanosep, Westborough, MA, USA) prior to the addition of a known amount of formate that was used as an internal standard.
The 1-D 1H-NMR spectrum of each sample was acquired on a 500 MHz spectrometer (Agilent Inc., Santa Clara, CA) with host software VNMRJ 4.0. The resulting spectra were processed and profiled to identify and quantify metabolites using Chenomx NMR Suite 8.3 (Chenomx Inc., Edmonton, AB, Canada) and its reference library that contains 338 compounds. Details of the 1H-NMR protocol and pulse sequence can be found in the Supplemental materials.
Measurement of blood leukocytes, analytes and free hemoglobin concentrations
Blood leukocytes were counted and sorted via a routine laboratory protocol, and plasma fibrinogen concentration was measured by the Clauss method19 within 6 hrs of sample collection. Technical replicate serum samples were used to measure levels of histamine, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), compounds known to be associated with inflammation and COPD.20–22 Serum histamine was extracted with methanol (1:1) and was subsequently assayed with a commercial ELISA kit (Abcam, Cambridge, UK). Serum TNF-α and IL-6 were arrayed with Aimplex® Human Custom 9-plex kit (Quantobio, Beijing, China) on a NovoCyte D1040 flow cytometer (ACEA Biosciences, Hangzhou, China). Serum free hemoglobin was detected using a colorimetric kit (Cayman Chemical, Ann Arbor, MI, USA) on a 96-well plate reader (Molecular Devices, Sunnyvale, CA, USA) using residuals from the serum samples assayed by NMR at the University of Michigan. All assays were performed according to the manufacturers’ instructions.
Statistical analysis
Only metabolites present in at least 70% of the samples were considered for statistical analysis. Missing values were replaced with the half of minimum value of the data set. The metabolomics concentration data were log-transformed and auto-scaled to achieve a normal distribution. Mean normalized metabolite concentration values and continuous demographic variables were compared by unpaired Student’s t-test with Welch correction or ANOVA with post-hoc Tukey’s test, as applicable. Categorical variables were compared with an exact Chi-square test. For metabolomics data, t-test p-values were corrected for false discovery rate (FDR) using the modified Benjamini-Hochberg method reported by Storey.23 A Pearson association matrix was built to evaluate the relationship between metabolite concentrations, clinical parameters and markers of inflammation. In this preliminary study, for metabolite concentration comparisons, FDR-corrected p-values ≤0.15 were viewed as potentially differentiating. For other comparisons, p-values of less than 0.05 were considered statistically significant; all statistical analyses were performed in R v3.4.0.
Results
Subject demographics
A total of 166 subjects were enrolled in the study of which 9 were excluded; samples from the remaining 157 subjects were shipped to the University of Michigan for assay. Another 12 subjects were excluded due to insufficient sample volume, poor quality NMR spectra, or high hemoglobin concentrations,16 leaving metabolomics data from 145 subjects for final analysis (Figure S1). The demographic data of the final cohort are summarized in Table 1. Age and BMI were similar across all groups, and smoking history was not different between smokers with and without COPD. However, fibrinogen, IL-6, TNF-α, neutrophils and basophils were all significantly higher in patients with COPD compared to NCS (p<0.05, t-test).
 |
Table 1 Demographic and clinical data of analyzed study cohort |
Serum 1-D 1H-NMR metabolomics differentiates COPD patients from smokers without COPD
A total of 28 serum metabolites were identified and quantified (Table S1). The metabolomics data set and associated NMR spectra can be found at the NIH’s metabolomics repository (http://www.metabolomicsworkbench.org/). While there were differences in metabolite concentrations between NCS and COPD (Figure 1A), the data did not differentiate GOLD I/II versus GOLD III/IV in the COPD group (Figure S2A and B). No discriminating metabolites were observed between GOLD stage A-D. In general, mean normalized metabolite concentrations were lower in COPD subjects compared to NCS (Figure 1A). Specifically, concentrations of creatine, glycine, histidine, and threonine were reduced in COPD compared to NCS subjects (Figure 1B). Figure S3 illustrates the metabolic relationship between threonine, glycine and creatine.
Because 54.4% of the COPD patients were current smokers (Table 1), we investigated whether smoking status influenced the serum NMR-detected metabolome of COPD (Figure S4). The only metabolite concentration that was different (post-hoc Tukey’s test p<0.05) between current and former smokers with COPD was citrate. Additionally, we also investigated whether the serum metabolome was affected by BMI (Figure S5); glucose was the only metabolite that correlated with BMI (Pearson correlation test, p<0.05).
Metabolite concentrations are associated with pulmonary function, emphysema, and markers of inflammation
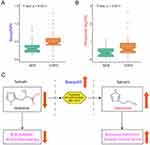
Creatine, histidine, threonine as well as lactate, proline and serine were positively correlated with FEV1 (%pred); creatine, histidine as well as 3-hydroxybutyrate, betaine, carnitine, glutamine, acetylcarnitine and valine were correlated with LAA (%); creatine, glycine, histidine as well as carnitine, lactate, lysine, phenylalanine, serine, tyrosine were correlated with IL-6 levels; histidine as well as betaine, glutamine, acetylcarnitine and valine were also negatively correlated with TNF-α levels (Figure 2). Given the relationship between histidine concentration and pulmonary function and inflammation, we assessed whether histamine (a product of histidine metabolism) levels differed between NCS and COPD subjects. Histamine concentration was higher in COPD (Figure 3A) as were basophils (%) (Figure 3B), cells known to produce histamine.22
Discussion
We identified a broad disruption in energy-related metabolism associated with COPD without BMI bias in a Chinese Han male population. In particular, components of creatine metabolism, including creatine, glycine, and threonine, had lower concentrations in patients with COPD compared with NCS. We also introduce the possibility that a disruption in histidine-histamine metabolism contributes to COPD pathogenesis.
Metabolic differences likely reflect COPD
Importantly, these metabolic changes are not likely explained by demographic differences in this study, since previous studies reported the metabolic alterations in COPD may be related to sex and BMI.4,5 Although we did not directly assess diet information from our study cohort, the Chinese diet is much more consistent and uniform compared to the Western diet.24 In addition, our study participants had an average BMI ~25 with low variance. Furthermore, smoking status had a minimal impact on the metabolome which implies that the differential metabolites of NCS and COPD were not attributable to current smoking status; this is consistent with previous studies.25,26 The absence of a smoking-induced change in the metabolome may have been aided by a 12 hr abstinence prior to the collection of blood for our metabolomics analysis. As such, our findings support the notion that the COPD metabolic derangements we found are likely attributable to the underlying disease.
Comparison with the previous studies
Two earlier studies that included males and females, also investigated the metabolic profiles of COPD using liquid chromatography-mass spectrometry (LC/MS) and 1H-NMR metabolomics, respectively.7,27 Since the LC/MS study was conducted using a different analytical platform than ours, it is difficult to make a direct comparison with our results.7 The earlier 1H-NMR study of 15 males and 17 females reported a COPD-induced reduction in BCAAs (leucine and isoleucine).27 We made a similar observation but it did not fall below our FDR cut-off of 15%. Additionally, an NMR metabolomics study of exhaled breath condensate (EBC) also distinguished COPD from controls but the metabolic profile of EBC was distinct from that of serum.28 In aggregate, these studies show how different biofluids and the metabolomics data that are acquired from different analytical platforms provide unique information about COPD.
Disorder of energy-related metabolic pathways in COPD
The differentiating metabolites we identified in our study share common metabolic pathways. Creatine, glycine and threonine are part of the glycine, serine, and threonine pathway (Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway: hsa00260). Notably, creatine, a non-proteogenic amino acid, is an important intermediate in the metabolism of tissues with high energy demand such as skeletal muscle.29,30 It also acts to maintain ATP levels and serves in an energy shuttle between the sites of ATP synthesis and utilization.30 Given the importance of creatine, glycine and essential amino acids (eg, threonine) for providing energy and preventing skeletal muscle atrophy,31,32 it is plausible that their reduced serum concentrations may either contribute to or be a result of exercising tissues dysfunction and increased ventilatory load in COPD patients. Notably, here and as has been previously shown, the extent of emphysema (LAA%) was inversely associated with creatine and histidine concentrations.33 This likely disruption of energy balance is further corroborated by the ketone body, 3-hydroxybutyrate, in our COPD subjects, which may be a compensatory signal for altered energy metabolism.
Disruption of histidine-histamine metabolism could contribute to a pro-inflammatory COPD phenotype
Histidine is an essential amino acid and is the precursor of histamine.34,35 In our COPD cohort, serum histidine was reduced with an associated increase in histamine and basophils (%), cells known to produce histamine.22 Histidine is known to be inversely associated with inflammation and oxidative stress in patients with metabolic syndrome and chronic kidney disease; in fact, histidine supplementation has been shown to suppress inflammatory processes.21,36 Moreover, in in-vitro experiments, supplemental histidine lowered the expression of IL-6, TNF-α and nuclear factor kappa-B (NF-κB) in adipocytes.21 We found the same relationship in our cohort as histidine was negatively correlated with serum IL-6 and TNF-α, implying the possibility that lower histidine concentrations may contribute to a pro-inflammatory state in COPD. In aggregate, these findings implicate disruption in histidine-histamine metabolism as contributing to COPD (Figure 3C).
We also observed higher serum levels of histamine, a metabolic product of histidine, in smokers with COPD compared to smokers without COPD. Histamine is harbored and released from the granules of mast cells and basophils, leading to tissue edema, bronchoconstriction and bronchial smooth muscle contraction via the H1 receptor.37–39 It has also been reported that increased histamine levels are associated with lung function decline and mortality in COPD,40–42 it is also widely recognized to play a pronounced role in asthma.43 Given our observed relationship between levels of histidine, histamine and basophil (%) on one hand and pulmonary function, LAA (%) and inflammation markers on the other, we suggest that the disruption of histidine-histamine metabolism could contribute to a pro-inflammatory COPD phenotype.
Limitations
We acknowledge that there are limitations to this study. Our findings are based on a single point in time measurement and we did not assess the influence of medications (eg, steroids) and non-pharmaceutical interventions such as exercise training. Longitudinal assessment of metabolic changes, as well as diet and medication use, will be important for future studies in order to fully assess the stability of the COPD metabolome. Also, given the higher prevalence of COPD in men in China,10 we limited this initial study to male patients. Given the known sex-related differences in the metabolome,5 a more in-depth assessment of such differences in regard to COPD phenotypes is needed. Finally, we acknowledge the preliminary nature of this study and that it will require validation in a larger, independent cohort.
Implications
We identified a broad disruption in energy-related and anti-inflammatory metabolism especially in creatine, glycine, threonine and histidine serum concentrations. This warrants further study and possible consideration that their supplement may improve dysfunction of exercising tissue and ventilatory load, as well as lower inflammation status in COPD patients.
Conclusion
In conclusion, we identified metabolic disturbances in the serum of Chinese male patients that may contribute to the inflammatory phenotype of COPD. In aggregate, these data corroborate previous findings of broad suppression of amino acid concentrations in COPD patients in a male Chinese Han population without BMI bias and they also extend them to introduce a potential mechanistic pathway involving a disruption in histidine-histamine homeostasis that could contribute to inflammatory processes. This could drive avenues of research including more expansive testing of amino acid supplementation in COPD, which has shown some promise44 as well as studies directed at increasing understanding of underlying mechanisms.
Abbreviations
COPD, chronic obstructive pulmonary disease; BMI, body mass index; NMR, nuclear magnetic resonance; FDR, false discovery rate; HRCT, high resolution computed tomography; LAA, low attenuation area; GOLD, global Initiative for chronic obstructive lung disease; NCS, non-COPD smokers; NS, Never smokers; FEV1, forced expiratory volume in the first second; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6.
Ethics approval and consent to participate
The study (www.clinicaltrials.gov, NCT03310177) was approved by the Peking University (PUIRB) and the University of Michigan Institutional Review Boards (IRBMed). Written informed consent was acquired from all subjects before enrollment into the study in accordance with the principles of the Declaration of Helsinki.
Data sharing statement
The dataset supporting the conclusions of this article is available in the NIH Metabolomics Workbench repository (http://www.metabolomicsworkbench.org/).
Acknowledgments
The present study was funded by the National Natural Science Foundation of China (Grant Nos: 81270097; 81470235; 81670034) and Beijing Medical University (Grant Nos: 20110176; 20160529) and the University of Michigan Health System-Peking University Health Science Center Joint Institute for Translational and Clinical Research. K. Stringer’s contribution was supported in part by a grant from the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS; GM111400). M. Han and W. Labaki’s contribution was supported by a grant from the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health (K24 HL138188). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS, NHLBI, or the National Institutes of Health.
Author contributions
W Diao directed the execution of the study, participated in the assaying of the metabolomics samples, conducted bioinformatic and statistical analyses and interpretation and wrote the manuscript; W Labaki, assisted with data analysis and interpretation and the writing of the manuscript; M Han, T Standiford, B He, N Shen and K Stringer were involved in study design; P Xiang, N Shen, Y Sun, C Guo and M Lu enrolled subjects; L Yeomans, Z Smiley, Y Sun, J Kim and C McHugh performed the metabolomics assays, free haemoglobin assays, NMR spectral analyses and generated the metabolomics data set; C Guo measured IL-6, TNF-alpha and fibrinogen levels. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr MeiLan Han reports personal fees from BI, GSK, AZ and research support from Novartis and Sunovion, outside of the submitted work. The authors report no other conflicts of interest in this work.
References
1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
2. Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur Respir J. 2008;31(4):869–873. doi:10.1183/09031936.00111707
3. Cebron Lipovec N, Beijers RJ, van den Borst B, Doehner W, Lainscak M, Schols AM. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: a systematic review. COPD. 2016;13(3):399–406. doi:10.3109/15412555.2016.1140732
4. Ubhi BK, Riley JH, Shaw PA, et al. Metabolic profiling detects biomarkers of protein degradation in COPD patients. Eur Respir J. 2012;40(2):345–355. doi:10.1183/09031936.00112411
5. Naz S, Kolmert J, Yang M, et al. Metabolomics analysis identifies sex-associated metabotypes of oxidative stress and the autotaxin-lysoPA axis in COPD. Eur Respir J. 2017;49:6. doi:10.1183/13993003.02322-2016
6. Wang L, Tang Y, Liu S, et al. Metabonomic profiling of serum and urine by 1H NMR-based spectroscopy discriminates patients with chronic obstructive pulmonary disease and healthy individuals. PLoS One. 2013;8(6):e65675. doi:10.1371/journal.pone.0065675
7. Chen Q, Deeb RS, Ma Y, Staudt MR, Crystal RG, Gross SS. Serum metabolite biomarkers discriminate healthy smokers from COPD smokers. PLoS One. 2015;10(12):e0143937. doi:10.1371/journal.pone.0143937
8. Serkova NJ, Standiford TJ, Stringer KA. The emerging field of quantitative blood metabolomics for biomarker discovery in critical illnesses. Am J Respir Crit Care Med. 2011;184(6):647–655. doi:10.1164/rccm.201103-0474CI
9. Keil U, Kuulasmaa K. WHO MONICA project: risk factors. Int J Epidemiol. 1989;18(3 Suppl 1):S46–S55.
10. Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430. doi:10.1016/S2213-2600(18)30103-6
11. Diao W, Shen N, Du Y, Qian K, He B. Characterization of throat microbial flora in smokers with or without COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1933–1946. doi:10.2147/COPD.S140243
12. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
13. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
14. Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653–657. doi:10.1164/ajrccm.152.2.7633722
15. Ambroggio L, Florin TA, Shah SS, et al. Emerging biomarkers of illness severity: urinary metabolites associated with sepsis and necrotizing methicillin-resistant staphylococcus aureus pneumonia. Pharmacotherapy. 2017;37(9):1033–1042. doi:10.1002/phar.1973
16. Stringer KA, Younger JG, McHugh C, et al. Whole blood reveals more metabolic detail of the human metabolome than serum as measured by 1H-NMR spectroscopy: implications for sepsis metabolomics. Shock. 2015;44(3):200–208. doi:10.1097/SHK.0000000000000406
17. Puskarich MA, Finkel MA, Karnovsky A, et al. Pharmacometabolomics of l-carnitine treatment response phenotypes in patients with septic shock. Ann Am Thorac Soc. 2015;12(1):46–56. doi:10.1513/AnnalsATS.201409-415OC
18. Nagana Gowda GA, Gowda YN, Raftery D. Expanding the limits of human blood metabolite quantitation using NMR spectroscopy. Anal Chem. 2015;87(1):706–715. doi:10.1021/ac503651e
19. Thyagarajan B, Jacobs DR, Apostol GG, Smith LJ, Lewis CE, Williams OD. Plasma fibrinogen and lung function: the CARDIA Study. Int J Epidemiol. 2006;35(4):1001–1008. doi:10.1093/ije/dyl049
20. Faner R, Tal-Singer R, Riley JH, et al. Lessons from ECLIPSE: a review of COPD biomarkers. Thorax. 2014;69(7):666–672. doi:10.1136/thoraxjnl-2013-204778
21. Feng RN, Niu YC, Sun XW, et al. Histidine supplementation improves insulin resistance through suppressed inflammation in obese women with the metabolic syndrome: a randomised controlled trial. Diabetologia. 2013;56(5):985–994. doi:10.1007/s00125-013-2839-7
22. MacGlashan D
23. Storey J. A direct approach to false discovery rates. J Royal Stat Soc B. 2002;64(3):479–498. doi:10.1111/rssb.2002.64.issue-3
24. Holmes E, Loo RL, Stamler J, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453(7193):396. doi:10.1038/nature06882
25. Xu T, Holzapfel C, Dong X, et al. Effects of smoking and smoking cessation on human serum metabolite profile: results from the KORA cohort study. BMC Med. 2013;11:60. doi:10.1186/1741-7015-11-60
26. Gu F, Derkach A, Freedman ND, et al. Cigarette smoking behaviour and blood metabolomics. Int J Epidemiol. 2016;45(5):1421–1432. doi:10.1093/ije/dyv330
27. Wang L, Tang Y, Liu S, et al. Metabonomic profiling of serum and urine by (1)H NMR-based spectroscopy discriminates patients with chronic obstructive pulmonary disease and healthy individuals. PLoS One. 2013;8(6):e65675–e65675. doi:10.1371/journal.pone.0065675
28. de Laurentiis G, Paris D, Melck D, et al. Separating smoking-related diseases using NMR-based metabolomics of exhaled breath condensate. J Proteome Res. 2013;12(3):1502–1511. doi:10.1021/pr301171p
29. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107–1213. doi:10.1152/physrev.2000.80.3.1107
30. Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr. 2007;27:241–261. doi:10.1146/annurev.nutr.27.061406.093621
31. Montesano A, Senesi P, Luzi L, Benedini S, Terruzzi I. Potential therapeutic role of L-carnitine in skeletal muscle oxidative stress and atrophy conditions. Oxid Med Cell Longev. 2015;2015:646171. doi:10.1155/2015/659750
32. Wächter S, Vogt M, Kreis R, et al. Long-term administration of L-carnitine to humans: effect on skeletal muscle carnitine content and physical performance. Clin Chim Acta. 2002;318(1–2):51–61. doi:10.1016/S0009-8981(01)00804-X
33. Celli BR, Locantore N, Tal-Singer R, et al. Emphysema and extrapulmonary tissue loss in COPD: a multi-organ loss of tissue phenotype. Eur Respir J. 2018;51(2):1702146. doi:10.1183/13993003.02146-2017
34. Young VR. Adult amino acid requirements: the case for a major revision in current recommendations. J Nutr. 1994;124(8 Suppl):1517s–1523s. doi:10.1093/jn/124.suppl_8.1517S
35. Andersen HH, Elberling J, Arendt-Nielsen L. Human surrogate models of histaminergic and non-histaminergic itch. Acta Derm Venereol. 2015;95(7):771–777. doi:10.2340/00015555-2146
36. Watanabe M, Suliman ME, Qureshi AR, et al. Consequences of low plasma histidine in chronic kidney disease patients: associations with inflammation, oxidative stress, and mortality. Am J Clin Nutr. 2008;87(6):1860–1866. doi:10.1093/ajcn/87.6.1860
37. Wishart DS, Feunang YD, Marcu A, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46(D1):D608–D617. doi:10.1093/nar/gkx1089
38. Panula P, Chazot PL, Cowart M, et al. International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol Rev. 2015;67(3):601–655. doi:10.1124/pr.114.010249
39. Brown RH, Zerhouni EA, Hirshman CA. Reversal of bronchoconstriction by inhaled nitric oxide. Histamine versus methacholine. Am J Respir Crit Care Med. 1994;150(1):233–237. doi:10.1164/ajrccm.150.1.8025755
40. Hospers JJ, Postma DS, Rijcken B, Weiss ST, Schouten JP. Histamine airway hyper-responsiveness and mortality from chronic obstructive pulmonary disease: a cohort study. Lancet. 2000;356(9238):1313–1317. doi:10.1016/S0140-6736(00)02815-4
41. Sparrow D, O’Connor G, Colton T, Barry CL, Weiss ST. The relationship of nonspecific bronchial responsiveness to the occurrence of respiratory symptoms and decreased levels of pulmonary function: the Normative Aging Study. Am Rev Respir Dis. 1987;135(6):1255–1260.
42. Postma D, Rijcken B. The role of atopy and hyperresponsiveness in the development of COPD. Eur Respir Rev. 1997;(7)159–162.
43. Hopp RJ, Townley RG, Biven RE, Bewtra AK, Nair NM. The presence of airway reactivity before the development of asthma. Am Rev Respir Dis. 1990;141(1):2–8. doi:10.1164/ajrccm/141.1.2
44. Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Effectiveness of essential amino acid supplementation in stimulating whole body net protein anabolism is comparable between COPD patients and healthy older adults. Metabolism. 2017;69:120–129. doi:10.1016/j.metabol.2016.12.010
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.