Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Disrupted Alpha-Ketoglutarate Homeostasis: Understanding Kidney Diseases from the View of Metabolism and Beyond
Authors Guo L, Chen S, Ou L, Li S, Ye ZN , Liu HF
Received 5 April 2022
Accepted for publication 17 June 2022
Published 27 June 2022 Volume 2022:15 Pages 1961—1974
DOI https://doi.org/10.2147/DMSO.S369090
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Lijing Guo,1,2,* Shihua Chen,1,2,* Liping Ou,1,2,* Shangmei Li,1 Zhen-Nan Ye,1,2 Hua-Feng Liu1,2
1Guangdong Provincial Key Laboratory of Autophagy and Major Chronic Non-Communicable Diseases, Affiliated Hospital of Guangdong Medical University, Zhanjiang, 524001, People’s Republic of China; 2Institute of Nephrology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, 524001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhen-Nan Ye; Hua-Feng Liu, Email [email protected]; [email protected]
Abstract: Alpha-ketoglutarate (AKG) is a key intermediate of various metabolic pathways including tricarboxylic acid (TCA) cycle, anabolic and catabolic reactions of amino acids, and collagen biosynthesis. Meanwhile, AKG also participates in multiple signaling pathways related to cellular redox regulation, epigenetic processes, and inflammation response. Emerging evidence has shown that kidney diseases like diabetic nephropathy and renal ischemia/reperfusion injury are associated with metabolic disorders. In consistence with metabolic role of AKG, further metabolomics study demonstrated a dysregulated AKG level in kidney diseases. Intriguingly, earlier studies during the years of 1980s and 1990s indicated that AKG may benefit wound healing and surgery recovery. Recently, interests on AKG are arising again due to its protective roles on healthy ageing, which may shed light on developing novel therapeutic strategies against age-related diseases including renal diseases. This review will summarize the physiological and pathological properties of AKG, as well as the underlying molecular mechanisms, with a special emphasis on kidney diseases.
Keywords: alpha-ketoglutarate, kidney diseases, metabolism, diabetic nephropathy, acute kidney injury
Introduction
Kidney diseases are a globally life-threatening burden and increase the incidence of end-stage renal disease (ESRD). Accumulating evidence has shown that metabolic reprogramming is not only a feature but also contributor to the progression and prognosis of kidney injury.1 Of these, energy metabolism attracts more attention. Alpha-ketoglutarate (AKG), as a rate-determining intermediate of tricarboxylic acid (TCA) cycle, its dysregulation has been found to be associated with declined renal function.2 Moreover, AKG has long been used as a key component of the histidine–tryptophan–ketoglutarate (HTK) preservation solution for kidney transplantation, although little is known about the mechanism.3 Particularly, recent studies have demonstrated that AKG benefits healthy ageing, fertilization capacity, and stemness maintaining.4 This sheds light on a clinical application. However, a systematic review on the advances of AKG, especially its association in kidney diseases is missing. Therefore, this review will delineate the biochemical properties of AKG, summarize pathological alterations of AKG levels in kidney diseases and the underlying molecular mechanisms, and discuss future directions.
Physiological Aspects on AKG
Production of AKG
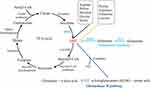
AKG can be generated via three major metabolic pathways (Figure 1). The first one is oxidative decarboxylation of isocitrate in the TCA cycle catalyzed by NAD-dependent isocitrate dehydrogenase 3 (IDH3) in mitochondrion.5,6 The second is called glutaminase I pathway, in which glutamine is deaminated by glutaminase (GLS) to generate glutamate, subsequently converted to AKG by glutamate dehydrogenase (GDH1) or one of the several transaminases, like alanine aminotransferase and aspartate aminotransferase. This cascade reaction is also a part of the glutaminolysis pathway.7,8 The third one is glutaminase II pathway, through which glutamine is transaminated to α-ketoglutaramate (KGM), subsequently deaminated to AKG by ω-amidase.9 It is noteworthy that the glutaminase I pathway is confined largely to mitochondria, whereas glutaminase II pathway occurs in both the cytosol and mitochondria.10
Besides, AKG is also produced via several other amino acid metabolic pathways, mainly arginine, proline, histidine, glycine, and serine.11,12 In serine synthesis pathway, glycolytic or gluconeogenic intermediate 3-phosphoglycerate is used for de novo synthesis with three steps involving enzymes of phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase 1 (PSAT1), and phosphoserine phosphatase (PSPH). Among these steps, AKG is generated by PSAT1 in a glutamate (Glu)-dependent transamination reaction.13
AKG is ubiquitously distributed in most of the tissues, whereas dominantly produced or uptook via kidney, intestine/gastrointestinal tract. In kidney, AKG mainly participates in the maintenance of acid–base balance,2 while in gastrointestinal tract, AKG mainly functions as a source of energy.14 Muscle is another tissue where AKG is enriched for conservation of amino acids and protein synthesis.15 In addition, AKG inhibited hepatic gluconeogenesis in liver and can serve as a biomarker for non-alcoholic fatty liver disease (NAFLD).16
Utilization of AKG
AKG is the main precursor of glutamine. It is first transaminated by glutamate dehydrogenase (GDH) to glutamate, and subsequently aminated by glutamine synthetase (GS) to generate glutamine.17 Meanwhile, AKG also participates in the synthesis of other nonessential amino acids, such as aspartate and proline. As a pre-source of aspartate, AKG is converted to oxaloacetate in TCA cycle, oxaloacetate can further be converted to aspartate by glutamic-oxaloacetic transaminase 2 (GOT2). Interestingly, a study in cancer cells found that addition of AKG in OXPHOS-incompetent cancer cells can exhaust aspartate and thus conferred cytotoxicity.18 Proline synthesis includes a first step utilizing pyrroline-5-carboxylate (P5C) synthase to convert glutamate to P5C, subsequently generating proline by P5C reductase.19
In TCA cycle, AKG is dominantly utilized by ketoglutarate dehydrogenase (α-KGDH) to converted to succinyl-CoA. Noteworthy, there is no net synthesis of AKG in TCA cycle, and these AKG cannot be utilized for the biosynthesis of amino acids or other cellular functions.20 The other way around, a reductive reaction can reverse AKG to citrate. In hypoxia and cells with defective mitochondria, AKG is reductively metabolized to citrate by IDH1 to provide precursors for macromolecular synthesis and replenish intermediates for TCA cycle.21 Noteworthy, this reaction undertakes both in mitochondrion and cytoplasm. While in cytoplasm, either exogenous AKG or mitochondrial citrate transported by SLC25A1 can further generate acetyl-coenzyme A, which consequently inhibits autophagy.22
Enzyme Regulation on AKG Metabolism
In line with the enzyme-catalyzed production and utilization of AKG, regulation of the enzyme activity can therefore affect AKG metabolism. IDH3 consists of the αβ and αγ heterodimers and using NAD(+) as the electron acceptor, and its activity can be regulated by conformational change.23 Glutaminase pathways can be regulated by post-translational modifications of the enzymes. For example, Ser314 phosphorylation site on glutaminase C by protein kinase C epsilon (PKCε) results in an upregulation of its activity.24 SUCLA2-coupled succinylation of glutaminase at Lys311 also promoted its activity.25 Moreover, glutaminolysis is a major endogenous source of AKG production; thus, modulation of glutaminolysis affects AKG metabolism.8
α-KGDH plays a key role not only in TCA cycle catalyzing AKG to succinyl-CoA but also functions as a hub between energy production and nitrogen metabolism. Its regulation involved ATP/ADP ratio, NADH/NAD+ ratio, calcium and the substrate availability in mitochondria.26
Cell Availability of AKG
Normally, AKG is considered as membrane non-permeable. Thus, membrane-permeable esters, such as dimethyl α-ketoglutarate (DM-KG) and octyl α-ketoglutarate (O-KG) are utilized, which is crucial to cultured cells. Although these derivatives will undergo hydrolyzation by esterases to generate cell impermeable AKG, a compound remaining trapped within the cell, a systematic study has shown that each of the precursor itself (or another yet-to-be-discovered degradation product) rather than AKG itself may lead to different biological functions.27
However, absorption of AKG seems to be cell-specific, since fibroblasts collected from human amniotic fluid demonstrated effective uptake.28 This may depend on whether cells harbor a transporter or receptor. The main transporter is called sodium-dicarboxylate cotransporters (also called SLC13 transporters), which utilize inward movement of Na+ to drive the uptake of AKG.29 The AKG receptor is denominated as OXGR1, which is also known as GPR99 or GPR80, and predominantly expressed in the renal distal tubules.30,31 In terms of absorption and transportation in vivo, AKG is freely filtered in the glomerulus and actively reabsorbed in the proximal tubule and Henle’s loop, as well as gut, especially the upper small intestine.2,32 A pharmacokinetic study showed that the in vivo half-life time of AKG is quite short, from several minutes to less than 1 hour, depending on the administration routes. Therefore, this suggests that a consecutive supplementation via drinking water is more reasonable for animal experiments.32 A caution should be taken is that AKG solution is acidic, which may also affect cell response. Thus, the pH should be adjusted to neutral prior to addition to culture medium.
AKG on Metabolism and Its Bioenergetics
As an inheritor of the glutamine carbon, as well as the most important nitrogen transporters (by transamination), AKG is broadly involved in cellular metabolism, including glucose metabolism, biosynthesis of amino acids and lipid metabolism. For instance, AKG could ameliorate lipid peroxidation and lipid accumulation in kidney induced by ammonium acetate.33 Moreover, administration of AKG has also been reported to reduce fat mass, decrease the levels of hypercholesterolaemic markers, and confer other anti-obesity effects.34 In parallel with these positive effects mentioned-above, AKG response to T2D-associated acidosis, accelerating gluconeogenesis via performing as a substrate for of this pathway.35
In terms of its bioenergetics, firstly, AKG is further catabolized to succinyl-CoA and subsequently succinate, accompanied with generation of GTP and NADH.36 NADH can facilitate ATP generation via electron transport chain (ETC).37 However, excessive AKG may inhibit ATP synthase.38 Besides, glutamate and aspartate also serve as fuel sources.39
Islet and Insulin Secretion
It has long been discovered that AKG can potentiate insulin secretion.40 AKG participates in TCA cycle, thus further increasing the ATP/ADP ratio and consequently promoting insulin secretion, especially in a sustained phase, rather than the ATP-sensitive K+ (KATP) channel-mediated acute influence.41,42 Intriguingly, it is also recently discovered that AKG may accelerate insulin secretion via a “counterclockwise” TCA cycle flux initiated by isocitrate dehydrogenase-2 (IDH2).5 Besides, AKG also shows a protective effect on pancreatic β-cell, which may also contribute to a benefit effect against diabetic kidney diseases.43
Gluconeogenesis and Glucose Homeostasis
Renal gluconeogenesis accounts for ∼40% of endogenous gluconeogenesis during fasting, occurring mainly in the kidney proximal tubule. While only few hints indicate that AKG is involved in renal gluconeogenesis, the role of AKG in hepatic gluconeogenesis provides implications.44 Yuan et al demonstrated that AKG treatment inhibits hepatic gluconeogenesis in diabetes.16 Miller and colleagues also found a reduction of AKG is accompanied with glucagon-triggered hepatic gluconeogenesis.45 Interestingly, a recent study showed that renal gluconeogenesis is impaired in chronic kidney disease, consequently resulting in decreased glucose, metabolic changes and aggravated renal prognosis.46
Acid-Base Homeostasis
Metabolic acidosis has been identified as an independent risk factor for progression of chronic and acute kidney injury.47,48 Interestingly, it has been shown that AKG is critical to maintain acid-base balance in kidney to ameliorate injury. During the onset of metabolic acidosis, the apical Na+/H+ antiporter activity is activated, stimulating the reabsorption of AKG, thus resulting in a drop in urinary output of AKG.2 AKG can be further catabolized to yield two bicarbonate ions, which partially compensate for the acidosis via transportation into the venous blood.49 Under base loading conditions, urinary excretion of AKG increased, which results in a loss of “potential HCO3–”, thus providing the advantage of minimizing bicarbonaturia under alkali load, and thereby reducing the formation of calcium–phosphate precipitates.2
Stemness Maintaining
It has also been discovered that AKG contributes to stem maintenance. Chaperone-mediated autophagy (CMA) is suppressed when the stem cells preserve a pluripotent state, whereas CMA-mediated IDH1/2 degradation is upregulated during differentiation. Thereby the intracellular AKG level is reduced and in turn pluripotency-associated epigenome is modified.50 However, in primed stem cells, AKG boosts the initial differentiation, which suggests that the pluripotent state (naive or primed) determines the effect of AKG on self-renewal or differentiation.51 Interestingly, considering that stem/progenitor cells may exist in the kidney, pluripotency acquisition and maintenance may also contribute to AKG-induced benefits against renal impairment.52,53
Collagen Metabolism and Fibrosis
Noteworthy, AKG also participates in collagen metabolism. The main mechanism is based on facilitating the endoplasmic reticulum-located prolyl-4-hydroxylase (P4H), which catalyzes the formation of 4-hydroxyproline and consequently produces mature collagen.54 Meanwhile, AKG can also increase the proline pool, which is a fundamental substrate for collagen biosynthesis.19 Additionally, AKG also reduces the degradation of collagen in fibroblasts.55 Although biosynthesis of collagen is a critical process for wound healing, its dysregulation results in fibrosis characterized by excessive accumulation of fibrous connective tissue.56 Therefore, the role of AKG in organ repair and fibrosis should be carefully evaluated.
Disrupted AKG Homeostasis in Kidney Diseases
In line with the pivotal role of AKG in physiology, extensive researches have shown a dysregulated AKG level in kidney diseases. Alterations of AKG in different kidney diseases are summarized in this section, and providing an insight that AKG may be regarded as a biomarker to predict the risk of disease progression.
AKG in Acute Kidney Injury
Acute kidney injury (AKI) is a major kidney disease in hospitalized patients with surgery, and increases the risk of developing chronic kidney disease (CKD). Major causes include a shortage of nutrition and oxygen supply, which further leads to metabolic disturbances.57 Therefore, metabolites, including AKG, have shown abnormal responses.
In detail, a lipopolysaccharide- (LPS-) treated AKI model showed that there is a net renal glutamine efflux, and activity of glutamine biosynthesis was increased and glutaminase remained unchanged, indicating that the concentration of AKG in kidney may be decreased.58 In line with this, beforehand supplementation of AKG attenuates LPS‑induced acute liver injury by counteracting oxidative stress and improving energy metabolism.59
Ischemia-Induced AKI
Ischemia/reperfusion injury (IRI) results in decreased cellular uptake of AKG due to a suppressed activity of Na+/dicarboxylate cotransporter 3.60 In vitro research on HK-2 cells suggested that AKG has protective effect on hypoxia-induced AKI.61,62 As indicated in other model, this is possibly involved in the modulation of NF-kB-mediated inflammation and macrophage polarization.63 However, another further in vitro model demonstrated that supplementation with AKG/malate did not show benefits in mice undergoing AKI; this possibly results from cardiovascular depressive effects, such as a reduction in mean arterial blood pressure and heart rate.64 Moreover, as mentioned above, ischemia results in a reduction of AKG, thus leading to accumulation of FOXO3, thereby stimulating autophagy and antioxidative response.65
Cyanogens-Induced AKI
Uptake of cyanogens results in an increased oxidative stress by depleting GSH. Post-treatment supplementation of AKG not only reacts directly with cyanogens but also exerts antioxidant effect, thus further ameliorating kidney injury.66,67
AKG in Chronic Kidney Diseases (CKD)
Diabetic Kidney Diseases (DKD)
Both glycolysis and level of AKG have been frequently reported to be aberrantly regulated in diabetic kidney, thus further contributing to kidney fibrosis as indicated in other organs.55,68 While glycolysis is usually enhanced, alterations of AKG concentration in DKD remain controversial. In a diabetic mice model (db/m vs db/db), AKG concentration in kidney cortex is elevated in 12-week-old diabetic versus control mice.69 A follow-up research by the same group further found that at 24 weeks of age, AKG in kidney cortex significantly increased as well, while there was no significant difference between the two groups in either plasma or urine.70
In contrast, Liang et al reported that AKG concentration in plasma of diabetic patients with disordered bone metabolism declined compared with healthy volunteers.71 Besides, two published research based on Joslin Medalist cohort showed different trends, the Medalist with CKD (patients with diabetes and stage 3b CKD) had lower plasma AKG compared with Medalist protected from CKD (diabetes, without CKD).72 Medalist with DN (class IIb–III) retained higher plasma AKG, compared with Medalist protected from DN (class 0-I).73 These controversies may be partially due to disease background and progression stage, and sample source. On the other hand, it also indicates that the benefits of AKG complement may attribute to molecular regulation, rather than simply compensating AKG concentration.
Besides, urinary excretion of AKG in diabetic nephropathy was also inconsistent in different reports. An increase in 28-week-old Akita mice was reported,74 while another clinical study reported that AKG was increased in a discovery study, but a further validation study showed no significance.75
Unilateral Ureteral Obstruction (UUO)
In a complete unilateral ureteral obstruction (CUUO) model established in rabbit, urine specimens of operated groups were drawn from hydronephrosis of the obstructed kidney, while control group (sham operated) was only obtained from the bladder under asepsis. NMR-based metabolomic analysis revealed that urinary AKG was reduced in relieved obstructed side of CUUO 1 week postrelief (P1) in comparison with obstructed side of CUUO for 2 weeks (O2), although AKG in P1 remained higher than control. As a key intermediate of the TCA cycle, abnormal of AKG may indicate a mitochondrial dysfunction, and its reduction in P1 (versus O2) suggests a metabolic recovery.76
Kidney Ageing
In 2014, AKG was first reported to extend the lifespan of adult Caenorhabditis elegans.38 Thereafter, this beneficial effect has been further discovered in Drosophila,77 and mice.78 Consistently, it has been reported that circulatory AKG concentration was reduced in ageing mice.79 This reduction was also found in ageing human beings.80 Recently, the anti-ageing effect of AKG is emerging; a translational research has been carried out and shown that a compound formulation with AKG and vitamins reduces an average 8-year reduction in biological aging measured by epigenetic clock.81
AKG in Other Kidney Diseases
Renal Cancer
AKG also participated in cancer development. The cancer microenvironment usually undergoes nutrient deprivation, which further results in reduced intracellular AKG levels due to GDH1 degradation.82 In line with this, supplementation of AKG in p53-deficient cancer cells restored a p53-like chromatin and transcriptional profile, thereby suppressing tumor.83 Similarly, decreased levels of AKG have been frequently found in other cancers, while AKG supplementation restricted tumor growth and extending survival.84,85 Besides, abnormal metabolism of AKG due to mutation of the IDH1, or loss-of-function of D-2-hydroxyglutarate dehydrogenase (D2HGDH), leads to the accumulation of D-2-hydroxyglutarate (D-2HG), a widely recognized oncometabolite, and resulting in cancers including Wilm’s tumor.86 The underlying mechanism involved in competitive inhibition of enzymes using AKG as a co-substrate, including histone demethylases and DNA demethylases, since the chemical structure of D-2HG is similar to AKG.87
Cystinosis
Nephropathic cystinosis is a lysosomal storage disease characterized by lysosomal cystine accumulation. Both cellular and plasma levels of AKG increased in cystinosis patients. In contrast to its usually considered antioxidant effect, upregulated AKG bridged cystinosin loss to increased oxidative stress, stimulation of autophagy and proximal tubule injury in cystinosis.88
Chronic Renal Failure (CRF)
A CRF model induced by 5/6 nephrectomy showed that oxidation of AKG was reduced. This is involved in a secondary hyperparathyroidism, which further impaired the activity of α-KGDH.89 Consistently, administration of calcium-AKG in CRF patients undergoing hemodialysis improved kidney functions, including increased plasma concentrations of arginine, proline and histidine, as well as decreased inorganic phosphate and urea.90
Lupus Nephritis (LN) and Immune System
In murine LN, the kidney becomes hypoxic, and consequently the renal-infiltrating T cells showed an increased concentration of AKG to support glycolysis, which further resulted in an enhanced T cell function and promoted kidney injury.91 Immune homeostasis is also regulated by AKG via metabolic reprogramming, and epigenetic alterations. This has been extensively studied in different immune responses, such as macrophage polarization,92 mucosal-associated invariant T (MAIT) cell effector response,93 B cell and T cell proliferation and differentiation94 and other immune context.95
Molecular Mechanisms
AMPK and mTOR Pathway
AMPK and mTOR signaling are involved in the beneficial effects of AKG promoting lysosomal translocation and activation of mTORC1; consequently, autophagy is blocked.96 Conversely, it is also reported that AKG suppressed ATP synthase and TOR in nematodes, thereby inducing autophagy and extending the lifespan.38,97 In line with this, phospho-Akt is inhibited after supplementation of AKG.98 Following research in Drosophila also found that activation of AMPK was also involved in the anti-ageing effect.77 The same mechanism was also reported in mouse model, in which they found that level of AKG declined with aging in the follicle fluids of human, while supplementation of AKG to mice can delay fertility ageing by down-regulation of mTOR and activation of AMPK signaling not only in the ovary but also in the kidney.99 The binding of AKG to calcium/calmodulin-dependent protein kinase kinase 2 (CamKK2), an upstream kinase of AMPK, may be involved.100
FOXO Signaling
AKG increased the mRNA expression of FOXO in Drosophila [60]. Moreover, FOXO maintains the cytosolic levels of α-ketoglutarate by promoting IDH1 expression.101 Besides, AKG is a co-substrate of prolyl hydroxylase domain protein (PHD), which catalyzes proline hydroxylation and further facilitates FOXO3 degradation. Consistently, reduction of AKG in hypoxic tubules results in stabilization of FOXO3, thus leading to FoxO3 accumulation and activation, and promoting autophagy. This may explain the renal protective effect of FOXO on attenuating chronic kidney disease development.65
Sirtuins
Sirtuins (SIRTs) belong to class III histone deacetylases (HDACs) that participate in diverse cellular functions including mitochondrial energy homeostasis, antioxidant activity, cell proliferation and DNA repair. Mammals express seven different SIRTs (SIRT1-7).102 A large body of literature describes that SIRTs perform renal protective effects against acute kidney injury, diabetic kidney disease, renal fibrosis, and kidney aging. Of these, SIRT1, SIRT3 and SIRT6 are the most studied ones in kidney, although the molecular mechanisms may be quite different, due to their distinct intracellular localization and a broad range of target proteins.103 For example, SIRT3 localizes in the mitochondrial matrix and boosts ATP generation via direct binding to and regulating complex I, succinate dehydrogenase A of complex II, and ATP synthase (complex V). SIRT3 is attenuated after tubular cell injury.104 The underlying mechanism may be involved in AKG accumulation via SIRT3-regulated glutaminolysis.105
Transcription Factors
Transcription factors are associated with the onset or progression of kidney disease, and AKG regulates transcriptional activity by serving as a co-factor of epigenetic modulation. Nuclear factor erythroid 2-related factor 2 (Nrf2) is known as a master regulator against oxidative stress, and AKG could upregulate its expression.106 Hypoxia-inducible factor (HIF)-1 plays a pivotal role in response to low oxygen concentrations or hypoxia, and AKG facilitates its degradation in the presence of oxygen.107 In addition, a large number of other transcription factors such as mitochondrial transcription factor A (TFAM),108 transcriptional intermediary factor 1 gamma (tif1γ)109 and CCCTC-binding factor (CTCF)110 can be regulated by AKG as well.
Other Signaling Pathways
Other involved signaling pathways include JNK, NF-κB, Ras-ERK, and glucocorticoid receptor (GR)-mediated signaling. For instance, AKG significantly phosphorylate JNK to trigger apoptosis, while the AKG receptor GPR99 was not activated.111,112 The other way around, activity of JNK pathway may also regulate the production of AKG via modulating glutaminase (GLS) activity.113 Besides, it is recently defined that AKG can directly bind with IKKβ to activate NF-κB signaling. Interestingly, in vivo study demonstrated that the AKG is from on-site production, thus requiring an interaction between IKKβ with GDH1.114 Meanwhile, in oxidative stress-induced AKI, treatment with tert-butyl hydroperoxide (TBHP), which is a well-recognized compound to induce oxidant injury in in vitro models of TEC, resulted in the activation of Raf-MEK1/2-ERK1/2 pathway, while AKG can reverse this activation and ameliorated mitochondrial function and cell apoptosis.115 However, another research showed that ERK1/2 pathway was inhibited after AKG treatment.111 In addition, a recent study showed that glucocorticoid (GC) promoted TCA cycle flow rate via accelerating glutamine to AKG.116 Intriguingly, another investigation reported that GC decreased the concentration of AKG in acute lymphoblastic leukemia cells.117
Last but not least, other signaling pathways that are frequently reported to be associated with kidney diseases, such as TGFβ signaling,118 Wnt signaling,85 Sonic hedgehog (Shh),119 JAK/STAT can also be regulated by AKG, and mostly showing inhibitory effect.
DNA Damage
Interestingly, AKG also participates in repairing DNA alkylation damage. DNA alkylation refers to the introduce of alkyl carbon groups to specific bases, resulting in alkylation products such as O6‐methylguanine, O6‐ethylguanine, O2‐alkylthymine and O4‐alkylthymine, which cause DNA mutations.120 A recent research in E. coli found that AKG contributes to DNA damage repair via its role as a cofactor of DNA repair enzyme AlkB.121,122 At least eight homologs of AlkB in human have been found, namely hABH1-8.123 Since aberrant alkylation can induce kidney tumor, it is reasonable to hypothesize that the anticancer effect of AKG at least partially due to facilitating DNA damage repair.124,125 Besides, an evolutionary analysis suggested that cytosine DNA methyltransferase activity can result in DNA alkylation damage as a byproduct.126 Meanwhile, abnormal DNA methylation was reported in DKD.127 Therefore, it is worthy to investigate whether increased nucleic acids alkylation exists in DKD. This may shed light on the utilization of AKG in DKD treatment.
Epigenetic Modifications
Moreover, AKG is a cofactor of several demodification enzymes. One of the largest enzyme families is called Fe2+/AKG-dependent hydroxylases, which utilize a mononuclear Fe2+ site to react with oxygen by coupling oxidative decomposition of AKG (forming CO2 and succinate) to the hydroxylation of the primary substrate, such as polynucleotides, proteins, lipids, and a wide array of small molecules.128 Several main subfamilies are discussed as follows:
AlkB dioxygenase. Alkbh5 is an RNA demethylase, which controls the epigenetic mark of N6-methyladenosine (m6A) by modulating transcription, translation, and cellular localization of RNA.129 AKG is indispensable for the oxidative demethylation of N6-methyladenosine (m6A).
Tet dioxygenase. Ten-eleven translocation enzymes (Tet1-3) catalyze the oxidation of 5-methylcytosine in DNA to 5-hydroxymethylcytosine (5hmC), which is an intermediate product generated during cytosine demethylation.130
Fat mass and obesity-associated protein (FTO). FTO is another AKG-dependent dioxygenase, which functions as m6A demethylase that reduces m6A methylation and contributes to inflammation in obesity. A genetic association study demonstrated that FTO polymorphism is a risk factor for mortality in chronic kidney disease of various severity.131
Prolyl hydroxylases. AKG is a substrate of cytoplasmic prolyl hydroxylases (PHD) 1–3 that decrease cellular levels of the hypoxia-inducible factor 1α (HIF-1α) in a ubiquitin-mediated degradation manner.107 Thus, supplementation of AKG ameliorates hypoxia injury or pseudohypoxia in cancer.132,133 Meanwhile, there is also a crosstalk between PHD and Akt, thus AKG supplementation could accelerate PHD2 activity, subsequently degraded Akt.98
Histone modification. AKG decreases the accumulations of histone-3-lysine-9 trimethylation (H3K9me3) and H3K27me3, which are mostly associated with regions of transcriptionally silenced chromatin. In addition, JHDM1 (JmjC domain-containing histone demethylase 1) depends on the presence of α-ketoglutarate and Fe(II) to specifically demethylates H3K36, which is associates transcriptionally active regions.134,135 Moreover, via promoting histone/DNA demethylation, including H3K9me3, H3K27me3 and ten-eleven translocation (Tet)-dependent DNA demethylation, AKG contributes to maintaining pluripotency and self-renewal.136
Considering that AKG is mainly generated in mitochondria, it is poorly understood that how it is available in the nucleus to exert an epigenetic modification function. The finding of on-site production of AKG in nucleus is a reasonable explanation, Traube et al found that the glutamate dehydrogenase in neurons, which catalyzes glutamate into AKG, translocates to the nucleus by Tet3, suggesting an on-site production of AKG.137
AKG and Reactive Oxygen Species (ROS)
AKG is mostly considered as an antioxidant reagent. On one hand, AKG can is a precursor of glutamine, which exerts antioxidative function as well. On the other hand, AKG improved the enzyme activities of SOD, CAT, and glutathione peroxidase. Furthermore, other antioxidant mechanism included H2O2 decomposition via a nonenzymatic oxidative decarboxylation, clearing damaged mitochondria by promoting mitophagy.138,139 Besides, AKG can also protect kidney from the cytotoxicity of cyanide by counteracting ROS and lipid peroxidation.140 Noteworthy, a recent study also showed that AKG can induce ROS.141 Thus, autophagy induction by AKG may also be involved in ROS elevation.
Off-Target Effect of AKG
Notably, in contrast to prevailing assumptions that AKG esters can permeabilize cell membrane, a recent research found that esters perform a spurious metabolic effect, because they undergo a rapid hydrolysis in aqueous solution like cell culture medium. Consequently, alpha-ketoglutaric acid and alcohol are released, which may result in off-target effect, considering the acidified microenvironments and unappreciated contributions to other cellular responses.142
The off-target effect also leads to another question, namely, among the oral versus intravenous and bolus versus continuous administration, as well as the mode of release (controlled versus rapid), which is a more effective route in humans? Further studies are yet to be conducted.
Targeting AKG as Therapeutic Strategy
Since AKG is normally considered safe and well bioavailable, oral or intravenous infusion supplementation of AKG is an effective therapeutic strategy. A study showed that infusion of AKG enhanced renal blood flow and benefited renal function after cardiac surgical procedures.143 Meanwhile, it has also been found that addition of AKG can ameliorate energy deficit and rescue kidney injured by ischemia and related insults.61 Besides, the proximal tubular Na+-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin, which is a new antihyperglycemic drug with protective effects on the kidney in type 2 diabetes, was recently reported to increase renal levels of AKG, indicating that the therapeutic effect is at least partially mediated by AKG.144
Future Perspectives and Concluding Remarks
Although AKG is emerging as a promising therapeutic intervention for preventing or control metabolic diseases, the exact role and concentration change in these diseases are complex and controversial. Future investigation is needed to clarify details of different diseases and stages of disease development. Meanwhile, the underlying mechanisms are yet to be further studied. Moreover, clinical trials are urgently needed to conduct, and different esters such as dimethyl-ketoglutarate (DMKG), trifluoromethylbenzyl AKG (TFMKG), octyl AKG, and ornithine AKG should be evaluated.27,145,146 Besides, the physiological concentration of AKG in the plasma ranges around tens of micromolar, which is much lower than the typically utilized concentrations (in millimolar range) in published studies.147 Whether this high concentration contributes to the above-mentioned spurious effect is unknown, and a comparative study between the high and physiological concentrations should be explored. In addition, epithelial mesenchymal transition (EMT) and endothelial mesenchymal transition (EndMT) programs have been involved in the progression of fibrosis in the kidney, and preliminary hints indicate that AKG may play a role in this pathology.148,149 Thus, this aspect also deserves further investigation.
In summary, as a rate-determining metabolic intermediate and co-substrate of multiple enzymes, AKG regulates kidney function via the metabolism–epigenetics axis. Meanwhile, its chemical properties such as acid–base balance and ion transportation may also participate in renal homeostasis. Since AKG has long been used as a nutritional supplement in athletes to increase the size and strength of their muscles, its safety has been proved. This makes it a promising therapeutic agent. However, dysregulated AKG is disease- and progression stage-dependent. Therefore, a comprehensive understanding of the role and mechanisms of AKG can accelerate novel insights into the pathogenesis of kidney diseases, which consequently facilitates the translation to clinical therapeutic strategies.
Acknowledgments
The authors would like to thank the anonymous reviewers for their valuable comments and suggestions for improving the quality of the paper. This work was funded by the National Natural Science Foundation of China (No. 82100769), and Science and Technology Planning Project of Zhanjiang City (2021A05064).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hewitson TD, Smith ER. A Metabolic reprogramming of glycolysis and glutamine metabolism is a requisite for renal fibrogenesis—why and how? Front Physiol. 2021;12:645857. doi:10.3389/fphys.2021.645857
2. Tokonami N, Morla L, Centeno G, et al. Alpha-ketoglutarate regulates acid-base balance through an intrarenal paracrine mechanism. J Clin Invest. 2013;123(7):3166–3171. doi:10.1172/JCI67562
3. Legeai C, Durand L, Savoye E, Macher MA, Bastien O. Effect of preservation solutions for static cold storage on kidney transplantation outcomes: a national registry study. Am J Transplant. 2020;20(12):3426–3442. doi:10.1111/ajt.15995
4. Gyanwali B, Lim ZX, Soh J, et al. Alpha-ketoglutarate dietary supplementation to improve health in humans. Trends Endocrinol Metab. 2022;33(2):136–146. doi:10.1016/j.tem.2021.11.003
5. Zhang GF, Jensen MV, Gray SM, et al. Reductive TCA cycle metabolism fuels glutamine- and glucose-stimulated insulin secretion. Cell Metab. 2021;33(4):804–817 e805. doi:10.1016/j.cmet.2020.11.020
6. Al-Khallaf H. Isocitrate dehydrogenases in physiology and cancer: biochemical and molecular insight. Cell Biosci. 2017;7:37. doi:10.1186/s13578-017-0165-3
7. Dorai T, Dorai B, Pinto JT, Grasso M, Cooper AJL. High levels of glutaminase ii pathway enzymes in normal and cancerous prostate suggest a role in ‘glutamine addiction’. Biomolecules. 2019;10(1):548. doi:10.3390/biom10010002
8. Yang L, Venneti S, Nagrath D. Glutaminolysis: a hallmark of cancer metabolism. Annu Rev Biomed Eng. 2017;19:163–194. doi:10.1146/annurev-bioeng-071516-044546
9. Dorai T, Pinto JT, Denton TT, Krasnikov BF, Cooper AJL. The metabolic importance of the glutaminase II pathway in normal and cancerous cells. Anal Biochem. 2020;1:114083. doi:10.1016/j.ab.2020.114083
10. Dorai T, Pinto JT, Denton TT, Krasnikov BF, Cooper AJL. The metabolic importance of the glutaminase II pathway in normal and cancerous cells. Anal Biochem. 2022;644:114083. doi:10.1016/j.ab.2020.114083
11. Rinaldi G, Pranzini E, Van Elsen J, et al. In vivo evidence for serine biosynthesis-defined sensitivity of lung metastasis, but not of primary breast tumors, to mtorc1 inhibition. Mol Cell. 2021;81(2):386–397e387. doi:10.1016/j.molcel.2020.11.027
12. Cheng ZX, Guo C, Chen ZG, et al. Glycine, serine and threonine metabolism confounds efficacy of complement-mediated killing. Nat Commun. 2019;10(1):3325. doi:10.1038/s41467-019-11129-5
13. Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16(10):650–662. doi:10.1038/nrc.2016.81
14. Hou Y, Wang L, Ding B, et al. Alpha-ketoglutarate and intestinal function. Front Biosci. 2011;16(3):1186–1196. doi:10.2741/3783
15. Wernerman J, Hammarqvist F, Vinnars E. Alpha-ketoglutarate and postoperative muscle catabolism. Lancet. 1990;335(8691):701–703. doi:10.1016/0140-6736(90)90811-i
16. Yuan Y, Zhu C, Wang Y, et al. Alpha-ketoglutaric acid ameliorates hyperglycemia in diabetes by inhibiting hepatic gluconeogenesis via serpina1e signaling. Sci Adv. 2022;8(18):eabn2879. doi:10.1126/sciadv.abn2879
17. Xiao D, Zeng L, Yao K, Kong X, Wu G, Yin Y. The glutamine-alpha-ketoglutarate (akg) metabolism and its nutritional implications. Amino Acids. 2016;48(9):2067–2080. doi:10.1007/s00726-016-2254-8
18. Madala HR, Helenius IT, Zhou W, et al. Nitrogen trapping as a therapeutic strategy in tumors with mitochondrial dysfunction. Cancer Res. 2020;80(17):3492–3506. doi:10.1158/0008-5472.CAN-20-0246
19. Albaugh VL, Mukherjee K, Barbul A. Proline precursors and collagen synthesis: biochemical challenges of nutrient supplementation and wound healing. J Nutr. 2017;147(11):2011–2017. doi:10.3945/jn.117.256404
20. Wu N, Yang M, Gaur U, Xu H, Yao Y, Li D. Alpha-ketoglutarate: physiological functions and applications. Biomol Ther (Seoul). 2016;24(1):1–8. doi:10.4062/biomolther.2015.078
21. Mullen AR, Wheaton WW, Jin ES, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011;481(7381):385–388. doi:10.1038/nature10642
22. Marino G, Pietrocola F, Kong Y, et al. Dimethyl alpha-ketoglutarate inhibits maladaptive autophagy in pressure overload-induced cardiomyopathy. Autophagy. 2014;10(5):930–932. doi:10.4161/auto.28235
23. Sun P, Liu Y, Ma T, Ding J. Structure and allosteric regulation of human nad-dependent isocitrate dehydrogenase. Cell Discov. 2020;6(1):94. doi:10.1038/s41421-020-00220-7
24. Han T, Zhan W, Gan M, et al. Phosphorylation of glutaminase by pkcepsilon is essential for its enzymatic activity and critically contributes to tumorigenesis. Cell Res. 2018;28(6):655–669. doi:10.1038/s41422-018-0021-y
25. Tong Y, Guo D, Lin SH, et al. Sucla2-coupled regulation of gls succinylation and activity counteracts oxidative stress in tumor cells. Mol Cell. 2021;81(11):2303–2316e2308. doi:10.1016/j.molcel.2021.04.002
26. Tretter L, Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2335–2345. doi:10.1098/rstb.2005.1764
27. Baracco EE, Castoldi F, Durand S, et al. Alpha-ketoglutarate inhibits autophagy. Aging. 2019;11(11):3418–3431. doi:10.18632/aging.102001
28. Aussel C, Coudray-Lucas C, Lasnier E, Cynober L, Ekindjian OG. Alpha-ketoglutarate uptake in human fibroblasts. Cell Biol Int. 1996;20(5):359–363. doi:10.1006/cbir.1996.0042
29. Dantzler WH. Renal organic anion transport: a comparative and cellular perspective. Biochim Biophys Acta. 2002;1566(1–2):169–181. doi:10.1016/s0005-2736(02)00599-0
30. He W, Miao FJ, Lin DC, et al. Citric acid cycle intermediates as ligands for orphan g-protein-coupled receptors. Nature. 2004;429(6988):188–193. doi:10.1038/nature02488
31. Qi AD, Harden TK, Nicholas RA. Gpr80/99, proposed to be the p2y(15) receptor activated by adenosine and amp, is not a p2y receptor. Purinergic Signal. 2004;1(1):67–74. doi:10.1007/s11302-004-5069-0
32. Dabek M, Kruszewska D, Filip R, et al. Alpha-ketoglutarate (akg) absorption from pig intestine and plasma pharmacokinetics. J Anim Physiol Anim Nutr (Berl). 2005;89(11–12):419–426. doi:10.1111/j.1439-0396.2005.00566.x
33. Velvizhi S, Dakshayani KB, Subramanian P. Effects of alpha-ketoglutarate on antioxidants and lipid peroxidation products in rats treated with ammonium acetate. Nutrition. 2002;18(9):747–750. doi:10.1016/s0899-9007(02)00825-0
34. Zheng J, Xiao H, Duan Y, et al. Roles of amino acid derivatives in the regulation of obesity. Food Funct. 2021;12(14):6214–6225. doi:10.1039/d1fo00780g
35. Aljaylani A, Fluitt M, Piselli A, Shepard BD, Tiwari S, Ecelbarger CM. Acid loading unmasks glucose homeostatic instability in proximal-tubule-targeted insulin/insulin-like-growth-factor-1 receptor dual knockout mice. Cell Physiol Biochem. 2020;54(4):682–695. doi:10.33594/000000248
36. Hou Y, Yao K, Wang L, et al. Effects of alpha-ketoglutarate on energy status in the intestinal mucosa of weaned piglets chronically challenged with lipopolysaccharide. Br J Nutr. 2011;106(3):357–363. doi:10.1017/S0007114511000249
37. Vatrinet R, Leone G, De Luise M, et al. The alpha-ketoglutarate dehydrogenase complex in cancer metabolic plasticity. Cancer Metab. 2017;5:3. doi:10.1186/s40170-017-0165-0
38. Chin RM, Fu X, Pai MY, et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and tor. Nature. 2014;510(7505):397–401. doi:10.1038/nature13264
39. Yang Y, He P, Hou Y, Liu Z, Zhang X, Li N. Osmundacetone modulates mitochondrial metabolism in non-small cell lung cancer cells by hijacking the glutamine/glutamate/alpha-kg metabolic axis. Phytomedicine. 2022;100:154075. doi:10.1016/j.phymed.2022.154075
40. Lenzen S, Schmidt W, Rustenbeck I, Panten U. 2-ketoglutarate generation in pancreatic b-cell mitochondria regulates insulin secretory action of amino acids and 2-keto acids. Biosci Rep. 1986;6(2):163–169. doi:10.1007/BF01115002
41. Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9(1):25–53.
42. Hoang M, Joseph JW. The role of alpha-ketoglutarate and the hypoxia sensing pathway in the regulation of pancreatic beta-cell function. Islets. 2020;12(5):108–119. doi:10.1080/19382014.2020.1802183
43. Song J, Ma D, Xing Y, et al. Alpha-ketoglutarate promotes pancreatic progenitor-like cell proliferation. Int J Mol Sci. 2018;19:4. doi:10.3390/ijms19040943
44. Merlin ME, Campello AP, Kluppel ML. Enalapril maleate affects 2-oxoglutarate metabolism in mitochondria from the rat kidney cortex. Cell Biochem Funct. 1994;12(1):21–28. doi:10.1002/cbf.290120104
45. Miller RA, Shi Y, Lu W, et al. Targeting hepatic glutaminase activity to ameliorate hyperglycemia. Nat Med. 2018;24(4):518–524. doi:10.1038/nm.4514
46. Verissimo T, Faivre A, Rinaldi A, et al. Decreased renal gluconeogenesis is a hallmark of chronic kidney disease. J Am Soc Nephrol. 2022;33(4):810–827. doi:10.1681/ASN.2021050680
47. Wesson DE, Buysse JM, Bushinsky DA. Mechanisms of metabolic acidosis-induced kidney injury in chronic kidney disease. J Am Soc Nephrol. 2020;31(3):469–482. doi:10.1681/ASN.2019070677
48. Bugarski M, Ghazi S, Polesel M, Martins JR, Hall AM. Changes in NAD and lipid metabolism drive acidosis-induced acute kidney injury. J Am Soc Nephrol. 2021;32(2):342–356. doi:10.1681/ASN.2020071003
49. Curthoys NP, Gstraunthaler G. Mechanism of increased renal gene expression during metabolic acidosis. Am J Physiol Renal Physiol. 2001;281(3):F381–390. doi:10.1152/ajprenal.2001.281.3.F381
50. Xu Y, Zhang Y, Garcia-Canaveras JC, et al. Chaperone-mediated autophagy regulates the pluripotency of embryonic stem cells. Science. 2020;369(6502):397–403. doi:10.1126/science.abb4467
51. TeSlaa T, Chaikovsky AC, Lipchina I, et al. Alpha-ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab. 2016;24(3):485–493. doi:10.1016/j.cmet.2016.07.002
52. Lazzeri E, Angelotti ML, Peired A, et al. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat Commun. 2018;9(1):1344. doi:10.1038/s41467-018-03753-4
53. Humphreys BD, Valerius MT, Kobayashi A, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2(3):284–291. doi:10.1016/j.stem.2008.01.014
54. Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003;22(1):15–24. doi:10.1016/s0945-053x(03)00006-4
55. Ge J, Cui H, Xie N, et al. Glutaminolysis promotes collagen translation and stability via alpha-ketoglutarate-mediated mtor activation and proline hydroxylation. Am J Respir Cell Mol Biol. 2018;58(3):378–390. doi:10.1165/rcmb.2017-0238OC
56. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi:10.1038/nm.2807
57. Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365(9457):417–430. doi:10.1016/S0140-6736(05)17831-3
58. Austgen TR, Chen MK, Moore W, Souba WW. Endotoxin and renal glutamine metabolism. Arch Surg. 1991;126(1):23–27. doi:10.1001/archsurg.1991.01410250027003
59. Wang L, Hou Y, Yi D, et al. Dietary supplementation with glutamate precursor alpha-ketoglutarate attenuates lipopolysaccharide-induced liver injury in young pigs. Amino Acids. 2015;47(7):1309–1318. doi:10.1007/s00726-015-1966-5
60. Matsuzaki T, Watanabe H, Yoshitome K, et al. Downregulation of organic anion transporters in rat kidney under ischemia/reperfusion-induced acute [corrected] renal failure. Kidney Int. 2007;71(6):539–547. doi:10.1038/sj.ki.5002104
61. Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci U S A. 2000;97(6):2826–2831. doi:10.1073/pnas.97.6.2826
62. Weinberg JM, Venkatachalam MA, Roeser NF, et al. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Renal Physiol. 2000;279(5):F927–943. doi:10.1152/ajprenal.2000.279.5.F927
63. Cheng MX, Cao D, Chen Y, Li JZ, Tu B, Gong JP. Alpha-ketoglutarate attenuates ischemia-reperfusion injury of liver graft in rats. Biomed Pharmacother. 2019;111:1141–1146. doi:10.1016/j.biopha.2018.12.149
64. Bienholz A, Petrat F, Wenzel P, et al. Adverse effects of alpha-ketoglutarate/malate in a rat model of acute kidney injury. Am J Physiol Renal Physiol. 2012;303(1):F56–63. doi:10.1152/ajprenal.00070.2012
65. Li L, Kang H, Zhang Q, D’Agati VD, Al-Awqati Q, Lin F. Foxo3 activation in hypoxic tubules prevents chronic kidney disease. J Clin Invest. 2019;129(6):2374–2389. doi:10.1172/JCI122256
66. Bhattacharya R, Satpute RM, Hariharakrishnan J, Tripathi H, Saxena PB. Acute toxicity of some synthetic cyanogens in rats and their response to oral treatment with alpha-ketoglutarate. Food Chem Toxicol. 2009;47(9):2314–2320. doi:10.1016/j.fct.2009.06.020
67. Bhattacharya R, Rao P, Singh P, et al. Biochemical, oxidative and histological changes caused by sub-acute oral exposure of some synthetic cyanogens in rats: ameliorative effect of alpha-ketoglutarate. Food Chem Toxicol. 2014;67:201–211. doi:10.1016/j.fct.2014.02.038
68. Liu H, Takagaki Y, Kumagai A, Kanasaki K, Koya D. The pkm2 activator tepp-46 suppresses kidney fibrosis via inhibition of the emt program and aberrant glycolysis associated with suppression of hif-1alpha accumulation. J Diabetes Investig. 2021;12(5):697–709. doi:10.1111/jdi.13478
69. Sas KM, Kayampilly P, Byun J, et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight. 2016;1(15):e86976. doi:10.1172/jci.insight.86976
70. Baek J, Pennathur S. Urinary 2-hydroxyglutarate enantiomers are markedly elevated in a murine model of type 2 diabetic kidney disease. Metabolites. 2021;11:8. doi:10.3390/metabo11080469
71. Liang WD, Huang PJ, Xiong LH, et al. Metabolomics and its application in the mechanism analysis on diabetic bone metabolic abnormality. Eur Rev Med Pharmacol Sci. 2020;24(18):9591–9600. doi:10.26355/eurrev_202009_23047
72. Gordin D, Shah H, Shinjo T, et al. Characterization of glycolytic enzymes and pyruvate kinase m2 in type 1 and 2 diabetic nephropathy. Diabetes Care. 2019;42(7):1263–1273. doi:10.2337/dc18-2585
73. Qi W, Keenan HA, Li Q, et al. Pyruvate kinase m2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med. 2017;23(6):753–762. doi:10.1038/nm.4328
74. You YH, Quach T, Saito R, Pham J, Sharma K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J Am Soc Nephrol. 2016;27(2):466–481. doi:10.1681/ASN.2015030302
75. Liu JJ, Liu S, Gurung RL, et al. Urine tricarboxylic acid cycle metabolites predict progressive chronic kidney disease in type 2 diabetes. J Clin Endocrinol Metab. 2018;103(12):4357–4364. doi:10.1210/jc.2018-00947
76. Wang Z, Xu R, Shen G, Feng J. Metabolic response in rabbit urine to occurrence and relief of unilateral ureteral obstruction. J Proteome Res. 2018;17(9):3184–3194. doi:10.1021/acs.jproteome.8b00304
77. Su Y, Wang T, Wu N, et al. Alpha-ketoglutarate extends drosophila lifespan by inhibiting mtor and activating AMPK. Aging. 2019;11(12):4183–4197. doi:10.18632/aging.102045
78. Asadi Shahmirzadi A, Edgar D, Liao CY, et al. Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab. 2020;32(3):447–456 e446. doi:10.1016/j.cmet.2020.08.004
79. Tian Q, Zhao J, Yang Q, et al. Dietary alpha-ketoglutarate promotes beige adipogenesis and prevents obesity in middle-aged mice. Aging Cell. 2020;19(1):e13059. doi:10.1111/acel.13059
80. Harrison AP, Pierzynowski SG. Biological effects of 2-oxoglutarate with particular emphasis on the regulation of protein, mineral and lipid absorption/metabolism, muscle performance, kidney function, bone formation and cancerogenesis, all viewed from a healthy ageing perspective state of the art–review article. J Physiol Pharmacol. 2008;59:91–106.
81. Demidenko O, Barardo D, Budovskii V, et al. Rejuvant®, a potential life-extending compound formulation with alpha-ketoglutarate and vitamins, conferred an average 8 year reduction in biological aging, after an average of 7 months of use, in the truage DNA methylation test. Aging. 2021;13(22):24485–24499. doi:10.18632/aging.203736
82. Shao J, Shi T, Yu H, et al. Cytosolic gdh1 degradation restricts protein synthesis to sustain tumor cell survival following amino acid deprivation. EMBO J. 2022;41(2):e110306. doi:10.15252/embj.2021110306
83. Morris J, Yashinskie JJ, Koche R, et al. Alpha-ketoglutarate links p53 to cell fate during tumour suppression. Nature. 2019;573(7775):595–599. doi:10.1038/s41586-019-1577-5
84. Mao L, Chen J, Lu X, et al. Proteomic analysis of lung cancer cells reveals a critical role of bcat1 in cancer cell metastasis. Theranostics. 2021;11(19):9705–9720. doi:10.7150/thno.61731
85. Tran TQ, Hanse EA, Habowski AN, et al. Alpha-ketoglutarate attenuates wnt signaling and drives differentiation in colorectal cancer. Nat Cancer. 2020;1(3):345–358. doi:10.1038/s43018-020-0035-5
86. Yong C, Stewart GD, Frezza C. Oncometabolites in renal cancer. Nat Rev Nephrol. 2020;16(3):156–172. doi:10.1038/s41581-019-0210-z
87. Liu Y, Yang C. Oncometabolites in cancer: current understanding and challenges. Cancer Res. 2021;81(11):2820–2823. doi:10.1158/0008-5472.CAN-20-3730
88. Jamalpoor A, van Gelder CA, Yousef Yengej FA, et al. Cysteamine-bicalutamide combination therapy corrects proximal tubule phenotype in cystinosis. EMBO Mol Med. 2021;13(7):e13067. doi:10.15252/emmm.202013067
89. Perna AF, Zayed MA, Massry SG. Impaired activity of alpha-ketoglutarate dehydrogenase of heart mitochondria in chronic renal failure: role of secondary hyperparathyroidism. Nephron. 1991;59(2):221–225. doi:10.1159/000186554
90. Riedel E, Hampl H, Steudle V, Nundel M. Calcium alpha-ketoglutarate administration to malnourished hemodialysis patients improves plasma arginine concentrations. Miner Electrolyte Metab. 1996;22(1–3):119–122.
91. Chen PM, Wilson PC, Shyer JA, et al. Kidney tissue hypoxia dictates t cell-mediated injury in murine lupus nephritis. Sci Transl Med. 2020;12:538. doi:10.1126/scitranslmed.aay1620
92. Liu PS, Wang H, Li X, et al. Alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18(9):985–994. doi:10.1038/ni.3796
93. Howson LJ, Li J, von Borstel A, et al. Mucosal-associated invariant t cell effector function is an intrinsic cell property that can be augmented by the metabolic cofactor alpha-ketoglutarate. J Immunol. 2021;206(7):1425–1435. doi:10.4049/jimmunol.2001048
94. Jiang Y, Li C, Wu Q, et al. Iron-dependent histone 3 lysine 9 demethylation controls b cell proliferation and humoral immune responses. Nat Commun. 2019;10(1):2935. doi:10.1038/s41467-019-11002-5
95. Zaslona Z, O’Neill LAJ. Cytokine-like roles for metabolites in immunity. Mol Cell. 2020;78(5):814–823. doi:10.1016/j.molcel.2020.04.002
96. Duran RV, Oppliger W, Robitaille AM, et al. Glutaminolysis activates rag-mtorc1 signaling. Mol Cell. 2012;47(3):349–358. doi:10.1016/j.molcel.2012.05.043
97. Chai M, Jiang M, Vergnes L, et al. Stimulation of hair growth by small molecules that activate autophagy. Cell Rep. 2019;27(12):3413–3421 e3413. doi:10.1016/j.celrep.2019.05.070
98. Shrimali NM, Agarwal S, Kaur S, et al. Alpha-ketoglutarate inhibits thrombosis and inflammation by prolyl hydroxylase-2 mediated inactivation of phospho-akt. EBioMedicine. 2021;73:103672. doi:10.1016/j.ebiom.2021.103672
99. Zhang Z, He C, Gao Y, et al. Alpha-ketoglutarate delays age-related fertility decline in mammals. Aging Cell. 2021;20(2):e13291. doi:10.1111/acel.13291
100. Jin L, Chun J, Pan C, et al. The plag1-gdh1 axis promotes anoikis resistance and tumor metastasis through camkk2-AMPK signaling in lkb1-deficient lung cancer. Mol Cell. 2018;69(1):87–99e87. doi:10.1016/j.molcel.2017.11.025
101. Charitou P, Rodriguez-Colman M, Gerrits J, et al. Foxos support the metabolic requirements of normal and tumor cells by promoting idh1 expression. EMBO Rep. 2015;16(4):456–466. doi:10.15252/embr.201439096
102. Hong YA, Kim JE, Jo M, Ko GJ. The role of sirtuins in kidney diseases. Int J Mol Sci. 2020;21:18. doi:10.3390/ijms21186686
103. Morigi M, Perico L, Benigni A. Sirtuins in renal health and disease. J Am Soc Nephrol. 2018;29(7):1799–1809. doi:10.1681/ASN.2017111218
104. Wang Q, Xu J, Li X, et al. Sirt3 modulate renal ischemia-reperfusion injury through enhancing mitochondrial fusion and activating the erk-opa1 signaling pathway. J Cell Physiol. 2019;234(12):23495–23506. doi:10.1002/jcp.28918
105. Zhou W, Hu G, He J, et al. Senp1-sirt3 signaling promotes alpha-ketoglutarate production during m2 macrophage polarization. Cell Rep. 2022;39(2):110660. doi:10.1016/j.celrep.2022.110660
106. He L, Wu J, Tang W, et al. Prevention of oxidative stress by alpha-ketoglutarate via activation of car signaling and modulation of the expression of key antioxidant-associated targets in vivo and in vitro. J Agric Food Chem. 2018;66(43):11273–11283. doi:10.1021/acs.jafc.8b04470
107. Hagos Y, Schley G, Schodel J, et al. Alpha-ketoglutarate-related inhibitors of hif prolyl hydroxylases are substrates of renal organic anion transporters 1 (OAT1) and 4 (OAT4). Pflugers Arch. 2012;464(4):367–374. doi:10.1007/s00424-012-1140-9
108. Wen YA, Xiong X, Scott T, et al. The mitochondrial retrograde signaling regulates wnt signaling to promote tumorigenesis in colon cancer. Cell Death Differ. 2019;26(10):1955–1969. doi:10.1038/s41418-018-0265-6
109. Rossmann MP, Hoi K, Chan V, et al. Cell-specific transcriptional control of mitochondrial metabolism by tif1gamma drives erythropoiesis. Science. 2021;372(6543):716–721. doi:10.1126/science.aaz2740
110. Chisolm DA, Savic D, Moore AJ, et al. Ccctc-binding factor translates interleukin 2- and alpha-ketoglutarate-sensitive metabolic changes in t cells into context-dependent gene programs. Immunity. 2017;47(2):251–267e257. doi:10.1016/j.immuni.2017.07.015
111. Kalawaj K, Slawinska-Brych A, Mizerska-Kowalska M, et al. Alpha ketoglutarate exerts in vitro anti-osteosarcoma effects through inhibition of cell proliferation, induction of apoptosis via the jnk and caspase 9-dependent mechanism, and suppression of tgf-beta and VEGF production and metastatic potential of cells. Int J Mol Sci. 2020;21:24. doi:10.3390/ijms21249406
112. Zurek A, Mizerska-Kowalska M, Slawinska-Brych A, et al. Alpha ketoglutarate exerts a pro-osteogenic effect in osteoblast cell lines through activation of jnk and mtor/s6k1/s6 signaling pathways. Toxicol Appl Pharmacol. 2019;374:53–64. doi:10.1016/j.taap.2019.04.024
113. Lukey MJ, Greene KS, Erickson JW, Wilson KF, Cerione RA. The oncogenic transcription factor c-Jun regulates glutaminase expression and sensitizes cells to glutaminase-targeted therapy. Nat Commun. 2016;7:11321. doi:10.1038/ncomms11321
114. Wang X, Liu R, Qu X, et al. Alpha-ketoglutarate-activated nf-kappab signaling promotes compensatory glucose uptake and brain tumor development. Mol Cell. 2019;76(1):148–162 e147. doi:10.1016/j.molcel.2019.07.007
115. Nowak G, Clifton GL, Godwin ML, Bakajsova D. Activation of erk1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am J Physiol Renal Physiol. 2006;291(4):F840–855. doi:10.1152/ajprenal.00219.2005
116. Stifel U, Wolfschmitt EM, Vogt J, et al. Glucocorticoids coordinate macrophage metabolism through the regulation of the tricarboxylic acid cycle. Mol Metab. 2022;57:101424. doi:10.1016/j.molmet.2021.101424
117. Dyczynski M, Vesterlund M, Bjorklund AC, et al. Metabolic reprogramming of acute lymphoblastic leukemia cells in response to glucocorticoid treatment. Cell Death Dis. 2018;9(9):846. doi:10.1038/s41419-018-0625-7
118. Xu L, Yang TY, Zhou YW, et al. Bmal1 inhibits phenotypic transformation of hepatic stellate cells in liver fibrosis via idh1/alpha-kg-mediated glycolysis. Acta Pharmacol Sin. 2022;43(2):316–329. doi:10.1038/s41401-021-00658-9
119. Cao Y, Lin SH, Wang Y, Chin YE, Kang L, Mi J. Glutamic pyruvate transaminase gpt2 promotes tumorigenesis of breast cancer cells by activating sonic hedgehog signaling. Theranostics. 2017;7(12):3021–3033. doi:10.7150/thno.18992
120. Peng Y, Pei H. DNA alkylation lesion repair: outcomes and implications in cancer chemotherapy. J Zhejiang Univ Sci B. 2021;22(1):47–62. doi:10.1631/jzus.B2000344
121. Silas Y, Singer E, Das K, Lehming N, Pines O. A combination of class-I fumarases and metabolites (alpha-ketoglutarate and fumarate) signal the DNA damage response in Escherichia coli. Proc Natl Acad Sci U S A. 2021;118:23. doi:10.1073/pnas.2026595118
122. Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. Oxidative demethylation by Escherichia coli alkb directly reverts DNA base damage. Nature. 2002;419(6903):174–178. doi:10.1038/nature00908
123. Kurowski MA, Bhagwat AS, Papaj G, Bujnicki JM. Phylogenomic identification of five new human homologs of the DNA repair enzyme alkb. BMC Genomics. 2003;4(1):48. doi:10.1186/1471-2164-4-48
124. Swann PF, Kaufman DG, Magee PN, Mace R. Induction of kidney tumours by a single dose of dimethylnitrosamine: dose response and influence of diet and benzo(a)pyrene pretreatment. Br J Cancer. 1980;41(2):285–294. doi:10.1038/bjc.1980.41
125. Tran TQ, Ishak Gabra MB, Lowman XH, et al. Glutamine deficiency induces DNA alkylation damage and sensitizes cancer cells to alkylating agents through inhibition of alkbh enzymes. PLoS Biol. 2017;15(11):e2002810. doi:10.1371/journal.pbio.2002810
126. Rosic S, Amouroux R, Requena CE, et al. Evolutionary analysis indicates that DNA alkylation damage is a byproduct of cytosine DNA methyltransferase activity. Nat Genet. 2018;50(3):452–459. doi:10.1038/s41588-018-0061-8
127. Marumo T, Yagi S, Kawarazaki W, et al. Diabetes induces aberrant DNA methylation in the proximal tubules of the kidney. J Am Soc Nephrol. 2015;26(10):2388–2397. doi:10.1681/ASN.2014070665
128. Hausinger RP. Feii/alpha-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol. 2004;39(1):21–68. doi:10.1080/10409230490440541
129. Purslow JA, Nguyen TT, Khatiwada B, Singh A, Venditti V. N (6)-methyladenosine binding induces a metal-centered rearrangement that activates the human RNA demethylase alkbh5. Sci Adv. 2021;7:34. doi:10.1126/sciadv.abi8215
130. Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by mll partner tet1. Science. 2009;324(5929):930–935. doi:10.1126/science.1170116
131. Spoto B, Mattace-Raso F, Sijbrands E, et al. The fat-mass and obesity-associated gene (fto) predicts mortality in chronic kidney disease of various severity. Nephrol Dial Transplant. 2012;27(Suppl 4):iv58–62. doi:10.1093/ndt/gfs550
132. Stegen S, Laperre K, Eelen G, et al. Hif-1alpha metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565(7740):511–515. doi:10.1038/s41586-019-0874-3
133. Brinkley G, Nam H, Shim E, et al. Teleological role of l-2-hydroxyglutarate dehydrogenase in the kidney. Dis Model Mech. 2020;13:11. doi:10.1242/dmm.045898
134. Tsukada Y, Fang J, Erdjument-Bromage H, et al. Histone demethylation by a family of jmjc domain-containing proteins. Nature. 2006;439(7078):811–816. doi:10.1038/nature04433
135. Klose RJ, Kallin EM, Zhang Y. Jmjc-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7(9):715–727. doi:10.1038/nrg1945
136. Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518(7539):413–416. doi:10.1038/nature13981
137. Traube FR, Ozdemir D, Sahin H, et al. Redirected nuclear glutamate dehydrogenase supplies tet3 with alpha-ketoglutarate in neurons. Nat Commun. 2021;12(1):4100. doi:10.1038/s41467-021-24353-9
138. Liu S, He L, Yao K. The antioxidative function of alpha-ketoglutarate and its applications. Biomed Res Int. 2018;2018:3408467. doi:10.1155/2018/3408467
139. An D, Zeng Q, Zhang P, et al. Alpha-ketoglutarate ameliorates pressure overload-induced chronic cardiac dysfunction in mice. Redox Biol. 2021;46:102088. doi:10.1016/j.redox.2021.102088
140. Hariharakrishnan J, Satpute RM, Prasad GB, Bhattacharya R. Oxidative stress mediated cytotoxicity of cyanide in LLC-mk2 cells and its attenuation by alpha-ketoglutarate and n-acetyl cysteine. Toxicol Lett. 2009;185(2):132–141. doi:10.1016/j.toxlet.2008.12.011
141. Zhang JY, Zhou B, Sun RY, et al. The metabolite alpha-kg induces gsdmc-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 2021;31(9):980–997. doi:10.1038/s41422-021-00506-9
142. Parker SJ, Encarnacion-Rosado J, Hollinshead KER, et al. Spontaneous hydrolysis and spurious metabolic properties of alpha-ketoglutarate esters. Nat Commun. 2021;12(1):4905. doi:10.1038/s41467-021-25228-9
143. Jeppsson A, Ekroth R, Friberg P, et al. Renal effects of alpha-ketoglutarate early after coronary operations. Ann Thorac Surg. 1998;65(3):684–690. doi:10.1016/s0003-4975(97)01337-4
144. Onishi A, Fu Y, Patel R, et al. A role for tubular na(+)/h(+) exchanger nhe3 in the natriuretic effect of the sglt2 inhibitor empagliflozin. Am J Physiol Renal Physiol. 2020;319(4):F712–F728. doi:10.1152/ajprenal.00264.2020
145. Cynober L, Lasnier E, Le Boucher J, Jardel A, Coudray-Lucas C. Effect of ornithine alpha-ketoglutarate on glutamine pools in burn injury: evidence of component interaction. Intensive Care Med. 2007;33(3):538–541. doi:10.1007/s00134-006-0511-0
146. Deen PM, Robben JH. Succinate receptors in the kidney. J Am Soc Nephrol. 2011;22(8):1416–1422. doi:10.1681/ASN.2010050481
147. Wagner BM, Donnarumma F, Wintersteiger R, Windischhofer W, Leis HJ. Simultaneous quantitative determination of alpha-ketoglutaric acid and 5-hydroxymethylfurfural in human plasma by gas chromatography-mass spectrometry. Anal Bioanal Chem. 2010;396(7):2629–2637. doi:10.1007/s00216-010-3479-0
148. Srivastava SP, Koya D, Kanasaki K. MicroRNAs in kidney fibrosis and diabetic nephropathy: roles on emt and endmt. Biomed Res Int. 2013;2013:125469. doi:10.1155/2013/125469
149. Sciacovelli M, Goncalves E, Johnson TI, et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature. 2016;537(7621):544–547. doi:10.1038/nature19353
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.