Back to Journals » Drug Design, Development and Therapy » Volume 16
Disease Modifying Therapies for the Management of Children with Spinal Muscular Atrophy (5q SMA): An Update on the Emerging Evidence
Authors Hjartarson HT, Nathorst-Böös K, Sejersen T
Received 7 January 2022
Accepted for publication 1 June 2022
Published 16 June 2022 Volume 2022:16 Pages 1865—1883
DOI https://doi.org/10.2147/DDDT.S214174
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Helgi Thor Hjartarson,1 Kristofer Nathorst-Böös,1 Thomas Sejersen1,2
1Department of Neuropediatrics, Astrid Lindgren Children´s Hospital, Karolinska University Hospital, Stockholm, Sweden; 2Department of Women’s and Children’s Health, Karolinska Institute, Stockholm, Sweden
Correspondence: Thomas Sejersen, Department of Women’s and Children’s Health, Karolinska Institute, Karolinska Vägen 37A, Stockholm, 171 76, Sweden, Tel +46 8 51777342, Email [email protected]
Abstract: SMA (5q SMA) is an autosomal recessive neuromuscular disease with an estimated incidence of approximately 1 in 11,000 live births, characterized by progressive degeneration and loss of α-motor neurons in the spinal cord and brain stem, resulting in progressive muscle weakness. The disease spectrum is wide, from a serious congenital to a mild adult-onset disease. SMA is caused by biallelic mutations in the SMN1 gene and disease severity is modified primarily by SMN2 copy number. Before the advent of specific disease altering treatments, SMA was the second most common fatal autosomal recessive disorder after cystic fibrosis and the most common genetic cause of infant mortality. Nusinersen, risdiplam, and onasemnogene abeparvovec are presently the only approved disease modifying therapies for SMA, and the aim of this review is to discuss their mode of action, effects, safety concerns, and results from real-world experience. All exert their action by increasing the level of SMN protein in lower motor neuron. Nusinersen and risdiplam by modifying the SMN2 gene product, and onasemnogene abeparvovec by delivering SMN1 gene copies into cells. All have an established clinical efficacy. An important feature shared by all three is that early intervention is associated with a better treatment outcome, such that in cases where treatment is initiated in an early pre-symptomatic period, it may result in normal – or almost normal – motor development. Thus, early diagnosis followed by swift initiation of treatment is fundamental for the treatment response and consequently long-term prognosis in SMA type 1, and probably SMA type 2. The same principle similarly applies to the milder phenotypes. All three therapies are relatively novel, with risdiplam being the latest addition. Except for nusinersen, real-world data are still scarce, and long-term data are quite naturally lacking.
Keywords: spinal muscular atrophy, treatment, disease-modifying, gene therapy
Background
Spinal muscular atrophies (SMAs) are a group of genetic diseases caused by progressive degeneration and loss of α-motor neurons (also known as lower motor neurons) in the spinal cord and brain stem, resulting in progressive muscle weakness.1 The topic of this review is the most common form, which is classic proximal or 5q SMA, hereafter referred to as SMA. SMA is an autosomal recessive neuromuscular disease with an estimated incidence of approximately 1 in 11,000 live births.2,3 Data from newborn screening programs suggest that this number may be lower.4 Before the advent of specific disease altering treatments, SMA was the second most common fatal autosomal recessive disorder after cystic fibrosis5 and the most common genetic cause of infant mortality.1,6
SMA is a disease spectrum, from a serious congenital to a mild adult-onset disease. The current universal classification, established in 1992,7 denotes three clinical types according to age at symptom onset and maximum motor milestones achieved (Box 1). It preceded the discovery of the genetic cause by a few years. The causative SMN1-gene localized to 5q11.2-13.3 (at that time called SMN gene, short for Survival of Motor Neuron) was identified and characterized in 1995.8 Initially presumed to be present in one telomeric copy, SMN1, and up to several centromeric copies, SMN2, it soon was suggested that SMN2 copy number might influence SMA disease severity.9 In 1997, it was revealed that a C to T base change within exon 7 of the SMN2 gene is detrimental for its transcription, by causing exclusion of exon 7 in most of the transcripts, resulting in low levels of SMN protein produced.10 A few years later it was unequivocally proven that there indeed is an inverse relationship between SMN2 copy number and clinical SMA phenotype.11 There are outliers. Most patients with 4 SMN2 copies will develop SMA type 3, but 7–10% will develop SMA type 2, and some may even develop an SMA type 1 phenotype.12 Some classifications also encompass SMA type 0, representing prenatal-onset disease, and SMA type 4, in cases of very mild adult-onset disease (Figure 1). Of infants affected by SMA, type 1 accounts for approximately 60%. SMA type 4 is rare, at just over 2% of the total number of SMA cases, with a median age at onset of 46 years.13 Specific types can be further divided into subtypes, eg, 1A, 1B, 1C, 3A and 3B, et cetera, based on differing severity. A decimal system was proposed early on, but never gained widespread use.14
 |
Box 1 Characteristics of SMA Type 1, 2, and 3 |
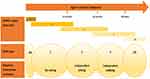 |
Figure 1 Schematic illustration of SMA types, maximal milestones achieved within each type, as well as the most typical SMN2 copy numbers for respective type.104 Notes: Adapted from Schorling DC, Pechmann A, Kirschner J. Advances in treatment of spinal muscular atrophy - new phenotypes, new challenges, new implications for care. J Neuromuscul Dis. 2020;7(1):1–13. © 2020 – IOS Press and the authors. All rights reservedThis article is published online with Open Access and distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC 4.0). |
All SMA types are progressive, differing in the rate of progression and the point at which clinical deterioration begins.13,15–18
The role of the SMN protein is incompletely understood and SMA has traditionally been seen as a pure α-motor neuron disease. There is increasing evidence that the SMN protein is important also in other tissues.19–21 Consequently, it may be advantageous increasing its levels in tissues outside the central nervous system (CNS).6
The natural history of SMA type 1 is well established,15,16 such that data from natural history cohorts can be used for comparison to treatment cohorts. Mortality is approximately 95% by age 18 months, usually related to respiratory complications. The natural history of SMA types 2 and 3 is fairly well established.17,22–24 Survival is somewhat shortened in SMA type 2A, but virtually normal in types 2B, 3, and 4.24 The less severe types display great clinical heterogeneity and consequently uncertain prognosis in individual cases. Further natural history studies, at least in SMA types 1 and 2, are ethically unimaginable in countries where disease modifying therapies now are available.
Until recently, SMA treatment was solely supportive. International consensus guidelines pertaining to standard of care in SMA were first published in 2007,25 with a revised update published a decade later.3,26 They provide a detailed framework for work-up and follow-up of patients with the different SMA types, and are organ system-specific, thus aiding clinicians from different specialties caring for these often very complex patients.
Clinical assessment of patients with SMA involves the usage of motor scales.27 Table 1 summarizes the ones most frequently used.
 |
Table 1 Functional Motor Scales Most Often Used in SMA |
SMN Modifying Therapies
Two pivotal publications on two novel treatments in SMA type 1 were published in 2017.28,29 The first disease modifying therapy ensued, signaling a new era in SMA treatment.30 Recently, a third novel treatment has entered the scene.31 The therapeutic landscape has changed dramatically, ie, in countries where the new treatments are available.
In this review, we discuss these three treatment options, specifically nusinersen (brand name Spinraza, company Biogen) previously ISIS-SMNRX, and later IONIS-SMNRX; onasemnogene abeparvovec (brand name Zolgensma, company Novartis) previously AVXS-101; and risdiplam (brand name Evrysdi, company Roche) previously RG7916. Figure 2 illustrates their mode of action. Nusinersen and risdiplam both modify the SMN2 gene product, and both require continuous usage. Onasemnogene abeparvovec is a one-time somatic gene therapy whereby a viral vector delivers functioning copies of the SMN1 gene into cells.
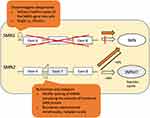 |
Figure 2 Simplified illustration of the mechanism of action of the three disease modifying therapies for SMA: Onasemnogene abeparvovec, nusinersen and risdiplam. Abbreviations: SMN∆7, SMN protein lacking exon 7. Notes: Adapted from Schorling DC, Pechmann A, Kirschner J. Advances in treatment of spinal muscular atrophy - new phenotypes, new challenges, new implications for care. J Neuromuscul Dis. 2020;7(1):1–13. © 2020 – IOS Press and the authors. All rights reservedThis article is published online with Open Access and distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC 4.0).104 |
We will not discuss possible future therapies, some of which are currently under investigation.
Nusinersen
Mechanism of Action and Administration
Nusinersen is an antisense oligonucleotide (ASO) belonging to phosphorothioate (PS) oligodeoxynucleotides. Under normal circumstances ASOs do not cross the blood-brain barrier, necessitating intrathecal (IT) administration in order to exert effect within the CNS.32 For patients with complex spine anatomy due to advanced scoliosis or after spinal fusion, x-ray guided transforaminal delivery or the use of a reservoir may be a feasible option.33–35 Nusinersen has a long half-life of approximately five months, and it is administered initially as four loading doses over a 2-month period, followed by maintenance doses every 4 months. The dose is the same in all age groups.
Pharmacokinetic studies indicate that nusinersen is cleared from the cerebrospinal fluid (CSF) into the systemic circulation, and it has also been identified postmortem in peripheral tissues such as liver and kidney from treated infants.36 Whether this “leakage” translates to effects outside the CNS is unknown. Nusinersen exerts its effect via gene-product modification. The SMN2 gene also encodes for SMN protein but differs by 11 nucleotides from SMN1, resulting in skipping of exon 7 in 80–90% of the mature RNA transcripts and production of a truncated non-functional protein, SMN∆7.37 Nusinersen targets a heterogenous nuclear ribonucleoprotein A1-dependent splicing silencer within the SMN2 pre-messenger RNA, downstream of exon 7. This adjusts the splicing process leading to increased synthesis of transcripts containing exon 7.38–40
Results from Clinical Trials with Nusinersen on Symptomatic SMA
The first human clinical Phase 1 and 2 studies of nusinersen in children with SMA types 2 and 3,38 and type 1 (CS3a study)36 were published in 2016. Encouraging results laid the grounds for three Phase 3 studies: ENDEAR, CHERISH, and NURTURE. The results of the first two were the basis on which nusinersen became approved as the first available disease modifying treatment for SMA.40
ENDEAR (CS3B) was a randomized, double-blind, sham-controlled, international multicenter study in infants with SMA type 1 and two copies of the SMN2 gene, <7 months of age at inclusion. A total of 121 symptomatic infants underwent the study procedure, 80 in the nusinersen group and 41 in the control (=no drug) group.29 The study was terminated early, as a prespecified interim analysis found a significantly higher percentage of motor-milestone responders on HINE-2 in the treatment group than the control group, 41% vs 0% (P < 0.001). In the final analysis, the percentage in the treatment group was 51%, while the control group remained at 0%. The corresponding CHOP INTEND response, as a secondary end point, was 71% and 3% (P < 0.001). Some participants achieved clinically meaningful motor milestones, eg, head control, ability to roll over, independent sitting. The treatment group had a significantly higher overall survival for the study period (P = 0.004) as well as a prolonged time to need for permanent assisted ventilation. Infants with a shorter disease duration fared better compared with those with a longer disease duration, suggesting that early initiation of treatment is important in SMA type 1.
CHERISH (CS4) was a randomized, double-blind, sham-controlled, international multicenter study in children with SMA type 2, aged 2–12 years. A total of 126 symptomatic children were enrolled, 84 in the nusinersen group and 42 in the control group. They were also stratified according to age at screening (<6 years vs ≥6 years).41 Only 16% were older than 6 years. All could sit independently and had relatively high baseline scores on both HFMSE and RULM. Children with severe contractures or scoliosis, respiratory insufficiency, or a gastric tube were not eligible. This trial was also terminated early for ethical reasons, on account of an interim analysis. By then, the least-squares mean difference in HFMSE score from baseline was 5.9 points (P < 0.001). In the final analysis, the difference was 4.9 points, with a mean gain of 3.9 points in the nusinersen group, and a mean loss of 1.0 points in the control group. An improvement of ≥3 points on HFMSE is proposed as being clinically meaningful42 and a higher percentage in the treatment group met this end points (57% vs 26%). The change from baseline in RULM score between the groups, was 3.7 points (+4.2 vs +0.5) points, and the improvements were greatest in younger children with a short disease duration.
In December 2016, the US Food & Drug Administration (FDA) approved nusinersen under the brand name Spinraza® as the first drug for the treatment of SMA. The European Medicines Agency (EMA) followed suit 6 months later. As of August 2021, nusinersen is available in 22 European countries; in 14 without restrictions, in 7 only to specific SMA types and/or with restrictions, and in 1 through an early access program.43 Nusinersen is formally approved for use in all SMA types, age groups, and disease stages. According to Biogen, as of March 2021 more than 11,000 patients worldwide had received treatment with nusinersen.44
Long-term results of patients with SMA types 2 and 3, aged 2–15 years at enrollment, who participated in the earlier CS2 Phase 1/2 study followed by the CS12 Phase 2, open-label extension study, confirmed improvements in motor function and stabilization of disease activity throughout approximately three years of follow-up.45 Likewise, final analysis of the CS3A study46 demonstrates a durable clinical response in a substantial proportion of the SMA type 1 treatment cohort, comparable with results from the ENDEAR study, over a median follow-up of 36.2 months. At study closure, 75% of the participants were alive. Lastly, EMBRACE was a randomized, double-blind, sham-controlled Phase 2 study of 21 symptomatic infants and children who did not meet the eligibility criteria of the ENDEAR or CHERISH studies. The blinded 14-month part was shortened on account of ENDEAR interim results. Part 2 recruited 20 participants, was open label, with a study period of 28 months. All but one of the participants treated with nusinersen in both parts of the study met HINE-2 motor-milestone response criteria, independent of age at SMA onset.47
All participants in the studies mentioned above, had the opportunity to enroll in SHINE (NCT02594124), an on-going open-label extension study, investigating long-term efficacy and safety of nusinersen in the different phenotypes. According to Biogen, SHINE has enrolled 292 participants and will be completed in August 2023.
Pre-Symptomatic Treatment with Nusinersen
NURTURE (CS5) is an on-going Phase 2, open-label, single-arm, multinational study in 25 pre-symptomatic infants with either 2 (N = 15) or 3 (N = 10) SMN2 gene copies, ie, without treatment they most likely would develop SMA type 1 or type 2, respectively.48 All were ≤6 weeks of age at first dose. As of this writing, only results of an interim analysis have been published.48 With no withdrawals, participants’ median age was 38.4 months, and all had therefore passed the age of expected symptom onset. 2/25 (both with 2 SMN2 copies) utilized respiratory intervention, for 2 and 10 hours per day, respectively. 3/25 (also all with 2 SMN2 copies) had developed feeding difficulties, necessitating placement of gastrostomy tubes. 25/25 had acquired independent sitting, and 22/25 independent walking, the majority within the established WHO window for healthy children. The proportion of participants with clinically manifested SMA differed between the groups with 2 or 3 SMN2 copies; being higher in the former. At age 13 months, they were 67% and 20%. At age 24 months, interestingly, they were lower, at 47% and 0%, respectively.
Real-World Data on Nusinersen Treatment
Real-world data on nusinersen treatment in all SMA types and age-groups is growing. To date, most published studies lack a control group, and the study duration is short. Treatment cohorts are often small and subgroups smaller still, causing broad confidence intervals. Methodological differences complicate comparison between studies and interpretation of results in a larger context.
Coratti et al’s recent critical real-world review and meta-analysis on motor function in patients with SMA types 2 and 3 treated with nusinersen includes all relevant publications until January 2021, reviewing also natural history studies on similar patients using the same measures as a means of establishing eventual differences between treated and untreated cohorts. Clinical trials, case reports and data concerning to treatment of pre-symptomatic patients were excluded.49 Key results concerning HFMSE, RULM, and 6MWT from their review are presented in Table 2.
 |
Table 2 Meta-Analysis Results on Motor Function in Patients with SMA Types 2 and 3 Treated with Nusinersen |
Positive HFMSE change was reported in all studies and a multivariate meta-regression analysis confirms association with nusinersen treatment, irrespective of SMA type or age group. Less impressive change was reported on RULM, where pooled mean change across treated and untreated did not reach statistical significance. Untreated ambulant patients tend to have high scores on RULM, which may mask eventual positive effects of treatment (“ceiling effect”). Subgroup analysis demonstrated a bigger increase in RULM scores in non-ambulant vs ambulant, SMA type 2 vs type 3, and in pediatric vs adult patients. A significant increase was demonstrated on the 6MWT in pediatric and adult patients.
Table 3 provides an overview of more recently published real-world studies containing also SMA type 2 and type 3 and/or older patients.
 |
Table 3 Key Findings from Real-World Studies in Mixed Groups and in Older Patients |
Real-world motor results regarding SMA type 1 and nusinersen are summarized in Table 4. Treatment duration varies, from 6 months to 2 years. Some use CHOP INTEND, some HINE-2, some both. Age-group subdivisions differ and Audic et al50 incorporate patients with SMA type 2 into the <2 years of age group, without specifying eventual differences in outcome between the two. All differ from the ENDEAR study in that they also include older patients with a more advanced disease, requiring ventilatory and nutritional support at baseline. Across the board, the results are promising.
 |
Table 4 Real-World Data on Key Motor Function Results in SMA Type 1 Following Treatment with Nusinersen |
Safety Profile/Adverse Events (AEs)
The ENDEAR and CHERISH trials demonstrated similar frequency of AEs between treatment and placebo groups.29,41 The incidence of serious AEs was higher in the placebo group in both trials, consistent with disease progression rather than being related to nusinersen. Other publications have confirmed these findings, in different SMA types and age groups.36,38,45,48,50–59 Hydrocephalus coupled to nusinersen treatment became a concern soon after approval, but a recent retrospective study reveals that SMA patients had an approximately fourfold increased risk of hydrocephalus compared with non-SMA controls in the pre-nusinersen era.60 Hemorrhage near the thecal space has been reported in the setting of multiple lumbar puncture attempts.48 The treatment itself is invasive and for many patients entails general anesthesia. The most common treatment-related AEs are headache, post-puncture syndrome, back pain, nausea, vomiting, rash, and pyrexia. Meningitis is rare. Significant changes in laboratory values seem to be rare. Still, it is prudent to monitor for coagulation disorders prior administration, as well as for infection and kidney function.
Onasemnogene Abeparvovec
Mechanism of Action and Administration
Onasemnogene abeparvovec (OAV101, Zolgensma®, formerly AVXS-101, Novartis Gene Therapies) is somatic intravenously administered gene therapy for SMA developed by Avexis.61 It is based on a self-complementary adeno-associated virus serotype 9 (AAV9) vector carrying a human SMN1 gene under the control of a constitutive hybrid CMV enhancer/chicken-b-actin promoter expected to give a continuous SMN1 expression. AAV9 crosses the blood-brain barrier and target neurons in the CNS. It is not known to cause disease in humans. Preclinical studies in mice and nonhuman primates demonstrated correction of the SMA phenotype after AAV9-based SMN1 gene therapy.61–63 Subsequently, safety and efficacy were demonstrated in a prospective study (CL101, START) of infants with SMA type 1 receiving a single dose of onasemnogene abeparvovec gene transfer administered as intravenous (IV) infusion.28 In contrast to gene therapies based on lentivirus or retrovirus, AAV-based onasemnogene abeparvovec is not integrated into the genome, but persists in the cell nucleus predominantly as extrachromosomal episomes, thus reducing oncogenic potential.64
Before administration of onasemnogene abeparvovec, baseline laboratory testing is required, including AAV9 antibody testing, liver function test (transaminases, bilirubin), creatinine, complete blood count, and troponin-I. Following gene therapy, close monitoring of liver function, platelet count and troponin-I is mandatory. Risk for immune-mediated adverse events warrants prophylactic corticosteroid therapy. A minority of infants have antibodies against AAV9, precluding treatment with onasemnogene abeparvovec. In newborns, positive titers often reflect passive transfer of maternal antibodies. In such cases, re-testing after 1–2 months (during which time treatment with nusinersen is possible) is reasonable.
Results from Clinical Trials with Onasemnogene Abeparvovec on Symptomatic SMA
The first clinical study, evaluating the effect of a single dose of onasemnogene abeparvovec in SMA, denoted START, was performed in USA, on 15 infants (mean 6.3 months of age) with SMA type 1 and having 2 copies of SMN2 (12 receiving high dose onasemnogene abeparvovec, 3 receiving low dose onasemnogene abeparvovec) as a Phase 1 trial comparing with a historic cohort. At 20 months following gene transfer, 11 of the 12 children receiving high dose onasemnogene abeparvovec could sit unassisted and fed unassisted. The treatment overall resulted in survival and achievement of motor milestones and motor function incompatible with the natural course of the disease.28
Subsequently, an open-label Phase 3 study, CL-303 (STR1VE-US), was carried out in USA on 22 patients younger than 6 months with SMA type 1 and 2 SMN2 copies, as a single-arm, 18-month, single-dose study of IV administration of onasemnogene abeparvovec at the dose 1.1×1014 vg/kg. At 14 months of age, 20/22 patients were alive and free of permanent ventilation, and 18/22 completely without ventilatory support.65 Fifty-nine percent of the study participants achieved independent sitting (≥30 seconds) at 18 months, compared to 0% in a natural history control group, 68% (15/22) did not require feeding support, and their CHOP INTEND scores had increased 1-month post treatment, with a mean change of 6.9 points from baseline.65 STR1VE-EU (CL-302) was conducted in Europe for patients with SMA type 1 and 1 or 2 SMN2 copies, enrolling 32 patients with a more severe phenotype at baseline compared with the START and STR1VE-US trials. Patient survival was similar compared to STRIVE-US with 97% (31/32) surviving free of permanent ventilatory support at 14 months of age,66 and 39% without any daily ventilatory support. Forty-four percent achieved independent sitting (≥10 seconds) at the completion of the study. This effect is lower than that observed in the STR1VE-US trial, most likely reflecting initial differences in the study populations. However, a similar increase in CHOP INTEND scores was demonstrated, with a mean change of 6.0 points from baseline.66
Onasemnogene abeparvovec was approved by the FDA in May, 2019 and received conditional approval by EMA in May, 2020. The FDA approval covers children with SMA <2 years of age, whereas the EMA indication denotes children with bi-allelic SMN1 gene mutations and the clinical diagnosis SMA type 1 or ≤3 SMN2 gene copies. According to Novartis, as of Q4 2021 more than 1800 patients have been treated with onasemnogene abeparvovec.67
Presymptomatic Treatment with Onasemnogene Abeparvovec
A global, Phase 3, open-label, single-arm, single dose, multicenter study (SPR1NT, CL-304) on the effect of onasemnogene abeparvovec in 29 presymptomatic infants up to 6 weeks of age with 2 or 3 SMN2 copies is ongoing. Preliminary results show that all presymptomatic study participants treated with onasemnogene abeparvovec maintained respiratory and nutritional independence, that most treated children achieved age-appropriate milestones (eg, sitting, standing, walking). The latter was achieved by 64% (9/14) of participants with 2 SMN2 copies, and by 100% (15/15) of participants with 3 SMN2 copies. All participants with 2 SMN2 copies achieved a CHOP INTEND score of ≥58 points, a score not seen in the natural history of SMA type 1.
Real-World Data on Onasemnogene Abeparvovec Treatment
The largest cohort followed to date contains data from 76 children with SMA from Germany and Austria. They were aged 21 days–5 years, weighed between 4 and 15 kg and the follow-up period was at least 6 months. Fifty-eight children had received treatment with nusinersen prior to the onasemnogene abeparvovec.68 In 60 patients for which data were available, significant improvements were observed in motor function outcome measures in CHOP INTEND (≥4 points) and HFMSE scores (≥3 points). The improvement was most marked in children <8 months at the time for onasemnogene abeparvovec treatment, with a mean CHOP INTEND increase of 13.8 points. For the age group 8–24 months, the corresponding figure was 7.7 points. For children >24 months of age at the time of treatment, there was no statistically significant effect of onasemnogene abeparvovec treatment measured by CHOP INTEND.
Serious AEs occurred in 11% (8 children). Importantly, 8% (6 children) developed acute liver dysfunction. The side effects on liver function were dependent on age and nusinersen pre-treatment and showed a biphasic course in children ≥8 months of age. Mostly based on the liver enzyme elevations, prednisolone treatment was significantly prolonged to a mean duration of 15.7 weeks. Other observed side effects were fever (62%), vomiting or loss of appetite (54%), and thrombocytopenia (78%), whereas cardiac adverse events (abnormal echocardiography) were rare (2.6%).
The authors conclude that gene therapy with onasemnogene abeparvovec is safe and effective, provided close monitoring, for patients with SMA up to a weight of 15 kg and up to 24 months of age.68 Similar results have been obtained from real-world experiences reported from Australia.69
Safety Concerns for Onasemnogene Abeparvovec
Safety concerns include liver dysfunction, thrombocytopenia, thrombotic microangiopathy, and elevated troponin-I.70,71 Thrombotic microangiopathy is a rare, acute, and life-threatening condition, characterized by thrombocytopenia and microangiopathic hemolytic anemia with thrombocytopenia as a key feature. On account of the risk for liver injury and other immune mediated adverse events following AAV-based gene therapy, prophylactic systemic corticosteroids are recommended before and after administration of onasemnogene abeparvovec.70 IV administration of onasemnogene abeparvovec results in potential affection of a multitude of cell types, and adverse events in several cell types and organ systems have been reported, including blood (thrombocytes), liver, kidneys, and heart.28,70,71 Common side effects include vomiting and elevated liver enzymes.70,71 Although usually transient and of no clinical significance, it is imperative to be aware of the risk of liver failure, speculated to be caused by hepatotoxicity secondary to a hyperinflammatory reaction,72 and liver enzymes should be monitored for at least 3 months following onasemnogene abeparvovec infusion.70,71 CNS-related adverse events have not been observed in humans, however dorsal root ganglion toxicity has been observed with intrathecal administration in nonhuman primates, indicating the importance to monitor this as a potential adverse event. This and other immune mediated adverse events may require post treatment hospitalization and IV steroids and other immunosuppressants.
As onasemnogene abeparvovec contains a genetically modified organism, special precautions should be taken for preparation, handling, accidental exposure, and disposal. Also, temporary onasemnogene abeparvovec shedding occurs from the recipient after administration, primarily through bodily waste. For this reason, instructions for the proper handling of patient stools should be in place.
Risdiplam
Mechanism of Action and Administration
Risdiplam (Evrysdi®, Roche) was developed in collaboration between Roche, PTC Therapeutics and the SMA Foundation. It is the first orally administered drug developed to treat SMA. Risdiplam, which belongs to a group of drugs called small molecules, is a pyridazine derivative that modifies splicing of pre-mRNA from the SMN2 gene, promoting inclusion of exon 7. This increases the expression of full-length mRNA, resulting in higher levels of functional SMN protein.73,74 Risdiplam is administered orally once daily and the dose is determined by the patients’ age and weight, to a maximal dose of 5 mg in patients ≥2 years and ≥20 kg.75 Risdiplam may be administered through a feeding tube. Dosage studies have been conducted in both healthy adults as well as SMA patients.76 Risdiplam has been shown to distribute to both CNS and peripheral tissues in study animals31 and steady state is reached in 7–14 days.
Results from Clinical Trials with Risdiplam on Symptomatic SMA
FIREFISH is an on-going, two-part, open-label multicenter Phase 2/3 trial in infants with SMA type 1 and 2 SMN2 copies. Part 1, which is completed, was an exploratory dose finding one in which primary outcomes were determination of dosage, evaluation of safety, and pharmacokinetics and -dynamics.76 Twenty-one infants aged 1–7 months with an early onset disease (median 2 months) were enrolled. All had low baseline scores on CHOP INTEND and HINE-2, and none were able to sit without support. All experienced AEs during the study period, most often coupled to compromised respiratory function due to the underlying condition, rather than the study drug, ie, respiratory tract infections, acute respiratory failure, and respiratory distress. Four participants died, all from complications related to SMA. Pharmacokinetic data, confirming 2.1 times the baseline level of SMN protein in blood, 4 weeks after start in the high-dose group, laid the basis for selecting 0.2 mg per kilogram as the treatment dose for the second part. Post hox exploratory efficacy outcomes indicated a clinical treatment response to risdiplam. At 12 months, 41% (7/17) of the participants could sit without support for ≥5 seconds.
Part 2 of FIREFISH, on risdiplam efficacy and safety, recruited 41 infants whose clinical baseline characteristics were very similar to the ones in part 1. At 12 months, 29% (12/41) could sit without support for ≥5 seconds, a significant difference compared to natural history studies (P < 0.001). Ninety percent (37/41) had a CHOP INTEND increase of ≥4 points from baseline, 78% (32/41) were classified as HINE-2 motor milestone responders, and 85% (35/41) were event-free, ie, alive and without respiratory support for ≥16 hours per day. All three significant (P < 0.001) compared with natural history in SMA type 1.77 Recent pooled 24-month data from parts 1 and 2 (N = 58) reveal that 84% are event-free, 60% can sit without support for ≥5 seconds, and 40% for ≥30 seconds. Median change in CHOP INTEND score has continued to improve, from +20 points at 12 months, to +27 points at 24 months. Furthermore, serious AEs have been less frequent during the second 24 months, there have been no additional deaths in the study group, and the majority has maintained their ability to swallow.78
SUNFISH is also a two-part trial, where part 1 (N = 51) was the dose-finding part, and the second part pertains to the efficacy and safety of risdiplam in a broad population of individuals with SMA types 2 and 3. The parts had different patient cohorts and part 2 is currently on-going. Part 1 exploratory outcomes demonstrated significant improvement in motor function, compared to a natural history cohort. The part 2 study design is that of a Phase 3, randomized, double-blind, 12-month placebo-controlled (2 risdiplam: 1 placebo) period, followed by further 12 months open-label where all participants receive risdiplam. Thereafter, all will be eligible for a 3-year open-label extension.79 The second part recruited 180 participants (71% SMA type 2, 29% SMA type 3), aged 2–25 years, non-ambulant but with independent sitting for ≥5 seconds. Of note, there were no exclusion criteria related to scoliosis, contractures, or nutritional or respiratory support.
At 12 months, there was a significantly different change in MFM32 score (∆=1.55 points, P = 0.016) from baseline between the treatment (+1.36 points, N = 115) and placebo (−0.19 points, N = 59).79 Younger patients experienced the largest improvements, whereas in older patients risdiplam more seemed to lead to stabilization. A larger percentage of individuals in the risdiplam group improved by ≥3 points between baseline and 12 months (P = 0.047), and a significant treatment difference was observed between the groups in the change from baseline in RULM total score. Preliminary 24-month data demonstrate that the increase in MFM32 total score has been maintained between months 12 and 24 in the risdiplam treatment arm. Furthermore, MFM32 change from adjusted baseline was stable between months 12 and 24 in participants from the initial placebo arm, who then switched to risdiplam.80
JEWELFISH is an on-going, open-label, multicenter trial in patients aged 6 months–60 years, previously treated with other SMA disease modifying agents, including nusinersen and onasemnogene abeparvovec, some of which have received more than one. It has recruited 174 participants. Nine percent have SMA type 1, 62% SMA type 2, and 29% SMA type 3. Thirty-four percent are non-sitters, 57% sitters, and 9% are walkers. Recent interim exploratory data indicate overall stabilization in motor function, a sustained increase in median SMN protein levels (in blood) irrespective of previous treatment, as well as low discontinuation rate.81
Risdiplam was approved by the FDA in August, 2020, and by EMA in March, 2021. It is approved for patients with SMA types 1, 2, and 3, aged 2 months and upwards.
Pre-Symptomatic Treatment with Risdiplam
RAINBOWFISH is an on-going, open-label, single-arm, multicenter study on efficacy, safety, pharmacokinetics, and pharmacodynamics of risdiplam in 25 presymptomatic infants ≤6 weeks old.
Real-World Data on Risdiplam Treatment
As of this writing, no real-world data on risdiplam have been published.
Safety Concerns for Risdiplam
As with nusinersen and onasemnogene abeparvovec, most AEs and serious AEs are seemingly associated with progression of or complications related to the underlying condition, rather than the drug itself. Consequently, serious AEs are more common in SMA type 1 than types 2 or 3. The most reported risdiplam-related AEs in FIREFISH and SUNFISH are fever, diarrhea, mouth and aphthous ulcers, arthralgia, urinary tract infection, constipation, and skin rash. Skin-related events that may be related to the risdiplam, have resolved spontaneously.77,79
Early studies on risdiplam in cynomolgus monkeys (at high exposures) revealed retinal toxicity after 5–6 months of treatment.73 Pooled data on 464 patients, treated for up to 46 months, has not revealed such findings in humans.82
Animal studies have also revealed potential embryonal toxicity as well as sperm degeneration and reduced numbers. Concurrent use of contraceptive measures is recommended to female patients of reproductive age and potential effects on fertility should be discussed prior to initiating treatment in males.73,75 In vitro, risdiplam and its main metabolite, M1, significantly inhibit multidrug and toxin extrusion protein (MATE) 1 and MATE2-K. In vivo, this may translate to increased plasma levels of drugs eliminated via MATE1 and MATE2-K, eg, metformin.83
Discussion
Nusinersen, onasemnogene abeparvovec, and risdiplam are disease modifying therapies that all have an established short-term efficacy in SMA. Since the α–motor neuron loss in SMA is irreparable, focus must be on saving them. Current evidence supports the notion that the best response to treatment is to be expected in patient who initiate treatment early in their disease course and that it is best in the presymptomatic group.84,85 This is demonstrable for all three by comparing outcome in symptomatic vs presymptomatic individuals treated with the same drug, ie, most infants who initiate treatment in early presymptomatic period, seem to display a normal or near normal motor development. However, a non-negligible proportion of infants treated even in the first 2–3 weeks of life do end up with significant neurological deficits, including some who never walk independently. Altogether, the strong correlation between timing of intervention and outcome highlights the importance of early intervention, and these findings have laid the foundation for including SMA in newborn screening (NBS) programs in several countries.86 Diagnostic delay is also of relevance. In SMA type 1 there is on average a substantial, and now prognostically relevant, diagnostic delay with 3.5 months between symptom debut and confirmed diagnosis.87
Several important questions remain to be answered. Is any one of the presently available therapies superior? Most effective? Has least side-effects? Which is most cost-effective? All are extremely costly.88 At which time is it too late to initiate treatment, ie, at what point has an individual patient´s disease progressed to a point where hope of a clinically meaningful response becomes nonexistent? Are all SMA types amenable to treatment? Can, or indeed should, treatments be combined. If one fails, is initiating one of the others reasonable?
The first question is, in our opinion, presently not possible to answer, due firstly to differences in study designs and populations, and secondly to the nonexistence of long-term data for any of the therapies. A recent review pointed out that clinically important measures such as respiratory and nutritional support were poorly reported in published studies.89 Regarding combining two (or more) disease modifying therapies, publications are scarce.90 No yet published data present evidence in support of combination therapy, and a recent consensus statement advises against it.91 Changing from nusinersen to risdiplam or vice versa is a simpler issue. Likewise, changing from nusinersen or risdiplam to onasemnogene abeparvovec may be sensible when possible. On the other hand, continuing with nusinersen or risdiplam (or both) after treatment with somatic gene therapy is in our opinion, presently only reasonable in the context of a treatment study. Three actual studies are worth mentioning in this respect, DEVOTE (NTC04089566), ASCEND (NTC05067790), and RESPOND (NTC04488133). The first two aim to evaluate the effect of high dose nusinersen, ASCEND in patients previously treated with risdiplam. RESPOND is set to investigate clinical outcome following treatment with nusinersen in patients previously treated with onasemnogene abeparvovec. These as well as future studies will contribute to the growing body of knowledge and help with decision-making in the coming years.
Defining treatment failure is difficult, the flip side of which is reasonable treatment goals. Not all participants in any of the treatment groups in the published studies responded to treatment, and all displayed significant mortality. Furthermore, an increase of 1 point on any motor scale, albeit perhaps being statistically significant and not compatible with the natural progress in an untreated cohort, does not necessarily translate into meaningful, purposeful voluntary motor function. On that note, the definition of response with respect to motor scales like HINE-2 and CHOP INTEND in the context of SMA is selected not primarily to detect clinically meaningful responses to treatment, but rather deviations from the expected trajectory of motor-function decline in respective phenotypes, established in natural-history studies.29 The mechanism of functional improvement observed in many study participants has not been wholly explained. It may represent a) improved function of previously unwell remaining α-motor neurons, b) neuronal sprouting and re-innervation, leading possibly to larger motor units, or c) improved neuromuscular transmission.36 It may indeed be a combination of those factors. If motor unit enlargement plays a part, the risk of possible late complications analogous to post-polio is a concern. The reasonable aim of treatment in symptomatic individuals, is long-term stabilization. Standardizing patient evaluation will be crucial to long-term follow-up and to enable comparison between different orphan treatments. Treatment trials in orphan diseases, such as SMA, usually are of relatively short duration and contain a small sample size that may also be heavily selected. Subsequent drug approval can hence rest on relatively weak short-term evidence, without any long-term data. The use of different motor scales in the many trials is a problem, with respect to comparing study results and data. SMArtCARE recommendations for standardizing the evaluation of patients with SMA are shown in Table 5.92 Biomarkers are a hot topic since there is great need for tools to objectively monitor disease activity and progression as well as eventual treatment response. Two promising types of biomarkers in SMA are blood or CSF neurofilament (NF) levels,93 and neurophysiological investigations, such as compound muscle action potential (CMAP) and motor unit number estimation (MUNE).94,95 The latter are promising but less readily available, time-consuming, require special investigator training and may not be a practical routine investigative method except in a clinical trial setting. Determination of NF levels, on the other hand, is more readily available. NFs are intermediate filaments particularly abundant in, and essential for growth and function of, axons.96 Neurofilaments have been shown to be nonspecific biomarkers for neuroaxonal injury within the CNS, reflecting ongoing axonal injury.97–100 Levels of phosphorylated neurofilament heavy chain (pNFH) have been demonstrated to be a potential biomarker for disease activity and, subsequently, treatment response in SMA in children.101 This held true in the ENDEAR study.101 Olsson et al reported extremely high baseline CSF levels of NFL in 12 patients with SMA type 1 compared with healthy controls, normalizing between the fourth and fifth doses of nusinersen.102 Further studies are needed to decide whether this holds true in patients with long-standing disease.103
 |
Table 5 SMArtCARE Recommendations for Evaluation of SMA Patients |
New disease altering treatment options can dramatically change the course of individual SMA phenotypes104 translating to a significant difference in outcomes. This holds true especially in countries where NBS has been implemented. With later treatment, SMA type 1 may more resemble what is typical for SMA type 2 or SMA type 3, either entirely or partially. Likewise, with treatment a young individual with SMA type 2 may adapt an SMA type 3 trajectory. For older individuals with semi-advanced SMA type 2 or SMA type 3, a reasonable long-term treatment goal is stabilization. Owing to increased survival in symptomatic patients, new problems may arise, which previously have been irrelevant, eg, cardiac defects,104 early development of spinal deformities,105 and possible overrepresentation of cognitive impairment in SMA type 1.106
Since none of the treatments offers a cure for symptomatic individuals and as already vanished neurons cannot be replaced, early diagnosis by NBS, followed by swift initiation of treatment is fundamental for the prognosis in SMA type 1, and we would argue, SMA type 2 as well. The same principle similarly applies to the milder phenotypes. Transforming a fatal condition to a chronic one necessitates expanding relevant clinics and services to care for “new survivors”. We are presently in the middle of a paradigm shift regarding the standards of care for SMA. The immense positive is that the therapeutic landscape of SMA is radically changing. The yet unresolved medical and ethical issues will clarify in the coming years.
Disclosure
Thomas Sejersen: Recipient of honoraria received for lectures or consultancy from Biogen, Novartis, PTC Therapeutics, Sarepta Therapeutics, Roche, Hansa Biopharma and Sanofi Genzyme. The authors report no other conflicts of interest in this work.
References
1. Swoboda KJ, Prior TW, Scott CB, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57(5):704–712. doi:10.1002/ana.20473
2. Darras BT. Spinal muscular atrophies. Pediatr Clin North Am. 2015;62(3):743–766. doi:10.1016/j.pcl.2015.03.010
3. Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103–115. doi:10.1016/j.nmd.2017.11.005
4. Kay DM, Stevens CF, Parker A, et al. Implementation of population-based newborn screening reveals low incidence of spinal muscular atrophy. Genet Med. 2020;22(8):1296–1302. doi:10.1038/s41436-020-0824-3
5. D’Amico A, Mercuri E, Tiziano FD, et al. Spinal muscular atrophy. Orphanet J Rare Dis. 2011;6:71. doi:10.1186/1750-1172-6-71
6. Waldrop MA, Kolb SJ. Current treatment options in neurology-SMA therapeutics. Curr Treat Options Neurol. 2019;21(6):25. doi:10.1007/s11940-019-0568-z
7. Munsat TL, Davies KE. International SMA consortium meeting. (26–28 June 1992, Bonn, Germany). Neuromuscul Disord. 1992;2(5–6):423–428. doi:10.1016/S0960-8966(06)80015-5
8. Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–165. doi:10.1016/0092-8674(95)90460-3
9. Gavrilov DK, Shi X, Das K, et al. Differential SMN2 expression associated with SMA severity. Nat Genet. 1998;20(3):230–231. doi:10.1038/3030
10. Monani UR, Lorson CL, Parsons DW, et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Mol Genet. 1999;8(7):1177–1183. doi:10.1093/hmg/8.7.1177
11. Feldkotter M, Schwarzer V, Wirth R, et al. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70(2):358–368. doi:10.1086/338627
12. Darras BT, De Vivo DC. Precious SMA natural history data: a benchmark to measure future treatment successes. Neurology. 2018;91(8):337–339. doi:10.1212/WNL.0000000000006026
13. Zerres K, Rudnik-Schoneborn S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol. 1995;52(5):518–523. doi:10.1001/archneur.1995.00540290108025
14. Dubowitz V. Chaos in the classification of SMA: a possible resolution. Neuromuscul Disord. 1995;5(1):3–5. doi:10.1016/0960-8966(94)00075-K
15. Kolb SJ, Coffey CS, Yankey JW, et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883–891. doi:10.1002/ana.25101
16. Mercuri E, Lucibello S, Perulli M, et al. Longitudinal natural history of type I spinal muscular atrophy: a critical review. Orphanet J Rare Dis. 2020;15(1):84. doi:10.1186/s13023-020-01356-1
17. Zerres K, Rudnik-Schöneborn S, Forrest E, et al. A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. J Neurol Sci. 1997;146(1):67–72. doi:10.1016/S0022-510X(96)00284-5
18. Russman BS, Melchreit R, Drennan JC. Spinal muscular atrophy: the natural course of disease. Muscle Nerve. 1983;6(3):179–181. doi:10.1002/mus.880060302
19. Farrar MA, Park SB, Vucic S, et al. Emerging therapies and challenges in spinal muscular atrophy. Ann Neurol. 2017;81(3):355–368. doi:10.1002/ana.24864
20. Hamilton G, Gillingwater TH. Spinal muscular atrophy: going beyond the motor neuron. Trends Mol Med. 2013;19(1):40–50. doi:10.1016/j.molmed.2012.11.002
21. Yeo CJJ, Darras BT. Overturning the paradigm of spinal muscular atrophy as just a motor neuron disease. Pediatr Neurol. 2020;109:12–19. doi:10.1016/j.pediatrneurol.2020.01.003
22. Coratti G, Lucibello S, Pera MC, et al. Gain and loss of abilities in type II SMA: a 12-month natural history study. Neuromuscul Disord. 2020;30(9):765–771. doi:10.1016/j.nmd.2020.07.004
23. Mercuri E, Finkel R, Montes J, et al. Patterns of disease progression in type 2 and 3 SMA: implications for clinical trials. Neuromuscul Disord. 2016;26(2):126–131. doi:10.1016/j.nmd.2015.10.006
24. Wijngaarde CA, Stam M, Otto LAM, et al. Population-based analysis of survival in spinal muscular atrophy. Neurology. 2020;94(15):e1634–e1644. doi:10.1212/WNL.0000000000009248
25. Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22(8):1027–1049. doi:10.1177/0883073807305788
26. Finkel RS, Mercuri E, Meyer OH, et al. Diagnosis and management of spinal muscular atrophy: part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018;28(3):197–207. doi:10.1016/j.nmd.2017.11.004
27. Pierzchlewicz K, Kępa I, Podogrodzki J, et al. Spinal muscular atrophy: the use of functional motor scales in the era of disease-modifying treatment. Child Neurol Open. 2021;8:2329048X211008725. doi:10.1177/2329048X211008725
28. Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713–1722. doi:10.1056/NEJMoa1706198
29. Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723–1732. doi:10.1056/NEJMoa1702752
30. Mercuri E. Neuromuscular disorders: 2017, a year to remember. Lancet Neurol. 2018;17(1):12–13. doi:10.1016/S1474-4422(17)30418-0
31. Dhillon S. Risdiplam: first approval. Drugs. 2020;80(17):1853–1858. doi:10.1007/s40265-020-01410-z
32. Geary RS, Yu RZ, Levin AA. Pharmacokinetics of phosphorothioate antisense oligodeoxynucleotides. Curr Opin Investig Drugs. 2001;2(4):562–573.
33. Weaver JJ, Natarajan N, Shaw DWW, et al. Transforaminal intrathecal delivery of nusinersen using cone-beam computed tomography for children with spinal muscular atrophy and extensive surgical instrumentation: early results of technical success and safety. Pediatr Radiol. 2018;48(3):392–397. doi:10.1007/s00247-017-4031-6
34. Shashi KK, Stone SSD, Berde CB, et al. Intrathecal catheter and port placement for nusinersen infusion in children with spinal muscular atrophy and spinal fusion. Pediatr Radiol. 2021;51(13):2588–2595. doi:10.1007/s00247-021-05126-4
35. Carson VJ, Young M, Brigatti KW, et al. Nusinersen by subcutaneous intrathecal catheter for symptomatic spinal muscular atrophy patients with complex spine anatomy. Muscle Nerve. 2022;65(1):51–59. doi:10.1002/mus.27425
36. Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a Phase 2, open-label, dose-escalation study. Lancet. 2016;388(10063):3017–3026. doi:10.1016/S0140-6736(16)31408-8
37. Cartegni L, Krainer AR. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat Genet. 2002;30(4):377–384. doi:10.1038/ng854
38. Chiriboga CA, Swoboda KJ, Darras BT, et al. Results from a Phase 1 study of nusinersen (ISIS-SMN Rx) in children with spinal muscular atrophy. Neurology. 2016;86(10):890–897. doi:10.1212/WNL.0000000000002445
39. Meylemans A, De Bleecker J. Current evidence for treatment with nusinersen for spinal muscular atrophy: a systematic review. Acta Neurol Belg. 2019;119(4):523–533. doi:10.1007/s13760-019-01199-z
40. Hoy SM. Nusinersen: first global approval. Drugs. 2017;77(4):473–479. doi:10.1007/s40265-017-0711-7
41. Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus Sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625–635. doi:10.1056/NEJMoa1710504
42. Swoboda KJ, Scott CB, Crawford TO, et al. SMA CARNI-VAL trial part I: double-blind, randomized, placebo-controlled trial of L-carnitine and valproic acid in spinal muscular atrophy. PLoS One. 2010;5(8):e12140. doi:10.1371/journal.pone.0012140
43. Charles River Associates. Assessing the policy and access environment across European countries for SMA patients. 2021.
44. New data at cure SMA 2021 highlight the long-term efficacy of SPINRAZA® (nusinersen) and Biogen’s commitment to innovation in SMA therapy. 2021. https://investors.biogen.com/news-releases/news-release-details/new-data-cure-sma-2021-highlight-long-term-efficacy-spinrazar
45. Darras BT, Chiriboga CA, Iannaccone ST, et al. Nusinersen in later-onset spinal muscular atrophy: long-term results from the Phase 1/2 studies. Neurology. 2019;92:e2492–e2506. doi:10.1212/WNL.0000000000007527
46. Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: final report of a Phase 2, open-label, multicentre, dose-escalation study. Lancet Child Adolesc Health. 2021;5(7):491–500. doi:10.1016/S2352-4642(21)00100-0
47. Acsadi G, Crawford TO, Müller‐Felber W, et al. Safety and efficacy of nusinersen in spinal muscular atrophy: the EMBRACE study. Muscle Nerve. 2021;63(5):668–677. doi:10.1002/mus.27187
48. De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842–856. doi:10.1016/j.nmd.2019.09.007
49. Coratti G, Cutrona C, Pera MC, et al. Motor function in type 2 and 3 SMA patients treated with Nusinersen: a critical review and meta-analysis. Orphanet J Rare Dis. 2021;16(1):430. doi:10.1186/s13023-021-02065-z
50. Audic F, De la banda MGG, Bernoux D, et al. Effects of nusinersen after one year of treatment in 123 children with SMA type 1 or 2: a French real-life observational study. Orphanet J Rare Dis. 2020;15(1):148. doi:10.1186/s13023-020-01414-8
51. Walter MC, Wenninger S, Thiele S, et al. Safety and treatment effects of nusinersen in longstanding adult 5q-SMA type 3 - a prospective observational study. J Neuromuscul Dis. 2019;6(4):453–465. doi:10.3233/JND-190416
52. Pera MC, Coratti G, Bovis F, et al. Nusinersen in pediatric and adult patients with type III spinal muscular atrophy. Ann Clin Transl Neurol. 2021;8(8):1622–1634. doi:10.1002/acn3.51411
53. Hagenacker T, Wurster CD, Günther R, et al. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020;19(4):317–325. doi:10.1016/S1474-4422(20)30037-5
54. Konersman CG, Ewing E, Yaszay B, et al. Nusinersen treatment of older children and adults with spinal muscular atrophy. Neuromuscul Disord. 2021;31(3):183–193. doi:10.1016/j.nmd.2020.12.006
55. Szabo L, Gergely A, Jakus R, et al. Efficacy of nusinersen in type 1, 2 and 3 spinal muscular atrophy: real world data from Hungarian patients. Eur J Paediatr Neurol. 2020;27:37–42. doi:10.1016/j.ejpn.2020.05.002
56. Pechmann A, Langer T, Schorling D, et al. Evaluation of children with SMA type 1 under treatment with nusinersen within the expanded access program in Germany. J Neuromuscul Dis. 2018;5(2):135–143. doi:10.3233/JND-180315
57. Pane M, Coratti G, Sansone VA, et al. Nusinersen in type 1 spinal muscular atrophy: twelve-month real-world data. Ann Neurol. 2019;86(3):443–451. doi:10.1002/ana.25533
58. Aragon-Gawinska K, Seferian AM, Daron A, et al. Nusinersen in patients older than 7 months with spinal muscular atrophy type 1: a cohort study. Neurology. 2018;91(14):e1312–e1318. doi:10.1212/WNL.0000000000006281
59. Pechmann A, Langer T, Wider S, et al. Single-center experience with intrathecal administration of Nusinersen in children with spinal muscular atrophy type 1. Eur J Paediatr Neurol. 2018;22(1):122–127. doi:10.1016/j.ejpn.2017.11.001
60. Viscidi E, Wang N, Juneja M, et al. The incidence of hydrocephalus among patients with and without spinal muscular atrophy (SMA): results from a US electronic health records study. Orphanet J Rare Dis. 2021;16(1):207. doi:10.1186/s13023-021-01822-4
61. Foust KD, Wang X, McGovern VL, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28(3):271–274. doi:10.1038/nbt.1610
62. Duque SI, Arnold WD, Odermatt P, et al. A large animal model of spinal muscular atrophy and correction of phenotype. Ann Neurol. 2015;77(3):399–414. doi:10.1002/ana.24332
63. Meyer K, Ferraiuolo L, Schmelzer L, et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol Ther. 2015;23(3):477–487. doi:10.1038/mt.2014.210
64. Colella P, Ronzitti G, Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol Ther Methods Clin Dev. 2018;8:87–104. doi:10.1016/j.omtm.2017.11.007
65. Day JW, Finkel RS, Chiriboga CA, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, Phase 3 trial. Lancet Neurol. 2021;20(4):284–293. doi:10.1016/S1474-4422(21)00001-6
66. Mercuri E, Muntoni F, Baranello G, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): an open-label, single-arm, multicentre, Phase 3 trial. Lancet Neurol. 2021;20(10):832–841. doi:10.1016/S1474-4422(21)00251-9
67. Novartis. Q4 2021 results investor presentation. 2022:28.
68. Weiss C, Ziegler A, Becker -L-L, et al. Gene replacement therapy with onasemnogene abeparvovec in children with spinal muscular atrophy aged 24 months or younger and bodyweight up to 15 kg: an observational cohort study. Lancet Child Adolesc Health. 2022;6(1):17–27. doi:10.1016/S2352-4642(21)00287-X
69. D’Silva AM, Holland S, Kariyawasam D, et al. Onasemnogene abeparvovec in spinal muscular atrophy: an Australian experience of safety and efficacy. Ann Clin Transl Neurol. 2022;9(3):339–350. doi:10.1002/acn3.51519
70. European Medicines Agency. Zolgensma: EPAR - Product information. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/zolgensma.
71. US Food & Drug Administration. ZOLGENSMA Product Information. 2021. https://www.fda.gov/vaccines-blood-biologics/zolgensma
72. Feldman AG, Parsons JA, Dutmer CM, et al. Subacute liver failure following gene replacement therapy for spinal muscular atrophy type 1. J Pediatr. 2020;225:252–258 e1. doi:10.1016/j.jpeds.2020.05.044
73. Ratni H, Ebeling M, Baird J, et al. Discovery of risdiplam, a selective survival of motor neuron-2 (SMN2) gene splicing modifier for the treatment of Spinal Muscular Atrophy (SMA). J Med Chem. 2018;61(15):6501–6517. doi:10.1021/acs.jmedchem.8b00741
74. Poirier A, Weetall M, Heinig K, et al. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol Res Perspect. 2018;6(6):e00447. doi:10.1002/prp2.447
75. European Medicines Agency. Evrysdi: EPAR - Product Information. European Medicines Agency; 2018.
76. Baranello G, Darras BT, Day JW, et al. Risdiplam in type 1 spinal muscular atrophy. N Engl J Med. 2021;384:915–923. doi:10.1056/NEJMoa2009965
77. Darras BT, Masson R, Mazurkiewicz-Bełdzińska M, et al. Risdiplam-treated infants with type 1 spinal muscular atrophy versus historical controls. N Engl J Med. 2021;385(5):427–435. doi:10.1056/NEJMoa2102047
78. Masson R, Boespflug-Tanguy O, Darras BT, et al. FIREFISH Parts 1 and 2: 24-month safety and efficacy of risdiplam in Type 1 spinal muscular atrophy (SMA).
79. Mercuri E, Deconinck N, Mazzone ES, et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): a Phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022;21(1):42–52. doi:10.1016/S1474-4422(21)00367-7
80. Oskoui M, Day JW, Deconinck N, et al. SUNFISH Part 2: 24-month efficacy and safety of risdiplam in patients with Type 2 or non-ambulant Type 3 spinal muscular atrophy (SMA).
81. Chiriboga CA, Bruno C, Duong T, et al. JEWELFISH: safety and pharmacodynamic data in non-naïve patients with spinal muscular atrophy (SMA) receiving treatment with risdiplam.
82. Servais L, Baranello G, Bertini E, et al. Pooled safety data from the risdiplam clinical trial development program.
83. Fowler S, Brink A, Cleary Y, et al. Addressing today’s Absorption, Distribution, Metabolism, and Excretion (ADME) challenges in the translation of in vitro ADME characteristics to humans: a case study of the SMN2 mRNA splicing modifier risdiplam. Drug Metab Dispos. 2022;50(1):65–75. doi:10.1124/dmd.121.000563
84. Lowes LP, Alfano LN, Arnold WD, et al. Impact of age and motor function in a Phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Pediatr Neurol. 2019;98:39–45. doi:10.1016/j.pediatrneurol.2019.05.005
85. Dangouloff T, Servais L. Clinical evidence supporting early treatment of patients with spinal muscular atrophy: current perspectives. Ther Clin Risk Manag. 2019;15:1153–1161. doi:10.2147/TCRM.S172291
86. Dangouloff T, Vrščaj E, Servais L, et al. Newborn screening programs for spinal muscular atrophy worldwide: where we stand and where to go. Neuromuscul Disord. 2021;31(6):574–582. doi:10.1016/j.nmd.2021.03.007
87. Lin CW, Kalb SJ, Yeh WS. Delay in diagnosis of spinal muscular atrophy: a systematic literature review. Pediatr Neurol. 2015;53(4):293–300. doi:10.1016/j.pediatrneurol.2015.06.002
88. Chaytow H, Faller KME, Huang Y-T, et al. Spinal muscular atrophy: from approved therapies to future therapeutic targets for personalized medicine. Cell Rep Med. 2021;2(7):100346. doi:10.1016/j.xcrm.2021.100346
89. Erdos J, Wild C. Mid- and long-term (at least 12 months) follow-up of patients with spinal muscular atrophy (SMA) treated with nusinersen, onasemnogene abeparvovec, risdiplam or combination therapies: a systematic review of real-world study data. Eur J Paediatr Neurol. 2022;39:1–10. doi:10.1016/j.ejpn.2022.04.006
90. Harada Y, Rao VK, Arya K, et al. Combination molecular therapies for type 1 spinal muscular atrophy. Muscle Nerve. 2020;62(4):550–554. doi:10.1002/mus.27034
91. Kirschner J, Butoianu N, Goemans N, et al. European ad-hoc consensus statement on gene replacement therapy for spinal muscular atrophy. Eur J Paediatr Neurol. 2020;28:38–43. doi:10.1016/j.ejpn.2020.07.001
92. Pechmann A, König K, Bernert G, et al. SMArtCARE - A platform to collect real-life outcome data of patients with spinal muscular atrophy. Orphanet J Rare Dis. 2019;14(1):18. doi:10.1186/s13023-019-0998-4
93. Zetterberg H. Neurofilament light: a dynamic cross-disease fluid biomarker for neurodegeneration. Neuron. 2016;91(1):1–3. doi:10.1016/j.neuron.2016.06.030
94. Kariyawasam D, D’Silva A, Howells J, et al. Motor unit changes in children with symptomatic spinal muscular atrophy treated with nusinersen. J Neurol Neurosurg Psychiatry. 2020;92(1):78–85. doi:10.1136/jnnp-2020-324254
95. Sleutjes B, Wijngaarde CA, Wadman RI, et al. Assessment of motor unit loss in patients with spinal muscular atrophy. Clin Neurophysiol. 2020;131(6):1280–1286. doi:10.1016/j.clinph.2020.01.018
96. Yuan A, Rao MV, Nixon RA, et al. Neurofilaments at a glance. J Cell Sci. 2012;125(Pt 14):3257–3263. doi:10.1242/jcs.104729
97. Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci. 2005;233(1–2):183–198. doi:10.1016/j.jns.2005.03.015
98. Gunnarsson M, Malmeström C, Axelsson M, et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol. 2011;69(1):83–89. doi:10.1002/ana.22247
99. Bacioglu M, Maia L, Preische O, et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron. 2016;91(1):56–66. doi:10.1016/j.neuron.2016.05.018
100. Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 2019;76(9):1035–1048. doi:10.1001/jamaneurol.2019.1534
101. Darras BT, Crawford TO, Finkel RS, et al. Neurofilament as a potential biomarker for spinal muscular atrophy. Ann Clin Transl Neurol. 2019;6(5):932–944. doi:10.1002/acn3.779
102. Olsson B, Alberg L, Cullen NC, et al. NFL is a marker of treatment response in children with SMA treated with nusinersen. J Neurol. 2019;266(9):2129–2136. doi:10.1007/s00415-019-09389-8
103. Totzeck A, Stolte B, Kizina K, et al. Neurofilament heavy chain and tau protein are not elevated in cerebrospinal fluid of adult patients with spinal muscular atrophy during loading with nusinersen. Int J Mol Sci. 2019;20(21):5397. doi:10.3390/ijms20215397
104. Schorling DC, Pechmann A, Kirschner J. Advances in treatment of spinal muscular atrophy - new phenotypes, new challenges, new implications for care. J Neuromuscul Dis. 2020;7(1):1–13. doi:10.3233/JND-190424
105. Lavie M, Diamant N, Cahal M, et al. Non-respiratory complications of nusinersen-treated spinal muscular atrophy type 1. Pediatr Pulmonol. 2021;56. doi:10.1002/ppul.25140
106. Polido GJ, Miranda MMVD, Carvas Junior N, et al. Cognitive performance of children with spinal muscular atrophy: a systematic review. Dement Neuropsychol. 2019;13(4):436–443. doi:10.1590/1980-57642018dn13-040011
107. Glanzman AM, Mazzone E, Main M, et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord. 2010;20(3):155–161. doi:10.1016/j.nmd.2009.11.014
108. O’Hagen JM, Glanzman AM, McDermott MP, et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord. 2007;17(9–10):693–697. doi:10.1016/j.nmd.2007.05.009
109. Haataja L, Mercuri E, Regev R, et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr. 1999;135(2 Pt 1):153–161. doi:10.1016/S0022-3476(99)70016-8
110. Bishop KM, Montes J, Finkel RS. Motor milestone assessment of infants with spinal muscular atrophy using the Hammersmith infant neurological Exam-Part 2: experience from a nusinersen clinical study. Muscle Nerve. 2018;57(1):142–146. doi:10.1002/mus.25705
111. Mazzone ES, Mayhew A, Montes J, et al. Revised upper limb module for spinal muscular atrophy: development of a new module. Muscle Nerve. 2017;55(6):869–874. doi:10.1002/mus.25430
112. Dunaway Young S, Montes J, Kramer SS, et al. Six-minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve. 2016;54(5):836–842. doi:10.1002/mus.25120
113. Osredkar D, Jílková M, Butenko T, et al. Children and young adults with spinal muscular atrophy treated with nusinersen. Eur J Paediatr Neurol. 2021;30:1–8. doi:10.1016/j.ejpn.2020.11.004
114. Wataya T, Takasaki S, Hoshino M, et al. Real-world safety of nusinersen in Japan: results from an interim analysis of a post-marketing surveillance and safety database. Int J Neurosci. 2021;1–13. doi:10.1080/00207454.2021.1995382
115. Lefeuvre C, Brisset M, Sarlon M, et al. Nusinersen treatment in adults with severe spinal muscular atrophy: a real-life retrospective observational cohort study. Rev Neurol. 2022;178(3):234–240. doi:10.1016/j.neurol.2021.10.010
116. Tscherter A, Rüsch CT, Baumann D, et al. Evaluation of real-life outcome data of patients with spinal muscular atrophy treated with nusinersen in Switzerland. Neuromuscul Disord. 2022;32:399–409. doi:10.1016/j.nmd.2022.02.001
117. Pane M, Coratti G, Pera MC, et al. Nusinersen efficacy data for 24-month in type 2 and 3 spinal muscular atrophy. Ann Clin Transl Neurol. 2022;9(3):404–409. doi:10.1002/acn3.51514
118. Chan SH, Chae J-H, Chien Y-H, et al. Nusinersen in spinal muscular atrophy type 1 from neonates to young adult: 1-year data from three Asia-Pacific regions. J Neurol Neurosurg Psychiatry. 2021;92(11):1244–1246. doi:10.1136/jnnp-2020-324532
119. Pane M, Coratti G, Sansone VA, et al. Type I SMA “new natural history”: long-term data in nusinersen-treated patients. Ann Clin Transl Neurol. 2021;8(3):548–557. doi:10.1002/acn3.51276
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
