Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Dietary Supplementations and Depression
Authors Thurfah JN, Christine, Bagaskhara PP, Alfian SD , Puspitasari IM
Received 26 January 2022
Accepted for publication 2 May 2022
Published 17 May 2022 Volume 2022:15 Pages 1121—1141
DOI https://doi.org/10.2147/JMDH.S360029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jihan Nurul Thurfah,1,2,* Christine,1,2,* Petrus Putra Bagaskhara,1,2,* Sofa Dewi Alfian,1,3 Irma Melyani Puspitasari1,3
1Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, West Java, Indonesia; 2Pharmacist Professional Program, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, West Java, Indonesia; 3Center of Excellence in Higher Education for Pharmaceutical Care Innovation, Universitas Padjadjaran, Sumedang, West Java, Indonesia
*These authors contributed equally to this work
Correspondence: Irma Melyani Puspitasari, Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, West Java, Indonesia, Tel +62 22 84288888; Ext: 3510, Email [email protected]
Abstract: Depression is a mood disturbance condition that occurs for more than two weeks in a row, leading to suicide. Due to adverse effects of depression, antidepressants and adjunctive therapies, such as dietary supplementation, are used for treatment. Therefore, this review explored and summarized dietary supplements’ types, dosages, and effectiveness in preventing and treating depression. A literature search of the PubMed database was conducted in August 2021 to identify studies assessing depression, after which scale measurements based on dietary supplements were identified. From the obtained 221 studies, we selected 63 papers. Results showed PUFA (EPA and DHA combination), vitamin D, and probiotics as the most common supplementation used in clinical studies to reduce depressive symptoms. We also observed that although the total daily PUFA dosage that exhibited beneficial effects was in the range of 0.7– 2 g EPA and 0.4– 0.8 g DHA daily, with an administration period of three weeks to four months, positive vitamin D-based supplementation effects were observed after administering doses of 2000 IU/day or 50,000 IU/week between 8 weeks and 24 months. Alternatively, microbes from the genus Lactobacillus and Bifidobacterium in the probiotic group with a minimum dose of 108 CFU in various dose forms effectively treated depression. Besides, a depression scale was helpful to assess the effect of an intervention on depression. Hence, PUFA, vitamin D, and probiotics were proposed as adjunctive therapies for depression treatment based on the results from this study.
Keywords: depression, dietary supplementation, clinical studies, PUFA, vitamin D, probiotics
Introduction
Depression is a mood disturbance condition that lasts for more than two weeks in a row. As per the United States Department of Health and Human Services, the prevalence of depression is 8.7% in individuals aged between 18 and 25 years.1 Approximately, 264 million people worldwide suffer from this disorder.2 It is characterized by sadness, feelings of worthlessness or guilt, appetite changes, sleep problems, fatigue, loss of interest, aimless physical activities or slowed movements, thinking challenges, and worst of all, suicidal thoughts.3 Depression can also lead to reduced activities levels, hampering the ability to work and study. Suicide is the worst impact of depression, causing the deaths of; >700,000 people every year. Suicide is the fourth leading cause of death among 15–29-year-olds.4
Depression has several types, including depressive disorder (clinical depression), bipolar disorder (manic depression), persistent depressive disorder (dysthymia), and seasonal affective disorder (SAD).5 A potential cause of major depressive disorder (MDD) is an inflammatory reaction in the hippocampus.6 MDD can also occur due to bad life experiences. Child abuse, neglect, and loss increase the risk of MDD in adulthood.7 The exact cause of bipolar disorder (manic depression) is unknown, but several factors may contribute such as biological differences and genetics. Biological differences include circadian dysregulation of mood disorders, Circadian Locomotor Output Cycles Kaput (CLOCK) protein and Brain and Muscle ARNT-like Protein 1 (BMAL1), and pharmacological triggers like tricyclic antidepressants (TCAs).8 The existence of complex interactions between neurotransmitters and receptors that affect the brain’s chemical moods play a role in persistent depressive disorder. Neurotransmitters that affect mood include dopamine, epinephrine, norepinephrine, GABA, and glutamate,9 which are the neurotransmitters in question. The exact cause of SAD is unclear, but it may be due to chemical changes in the brain attributable to inadequate sunlight and shorter days. Additionally, melatonin may also be associated with SAD.10
Depression can be treated with antidepressants that are categorized into five groups, including tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), serotonin-norepinephrine reuptake inhibitors, and non-TCA antidepressants.11 These treatments are effective;12,13 however, meta-analytical studies have found low remission rates,14 and antidepressants and placebo drugs only provide clinically significant differences in patients with upper-end severe depression. The reason for the skepticism with regard to the prescription of antidepressant drugs is their addictive effect. A study revealed that female patients and patients from ethnic minorities often choose complementary and alternative medicine (CAM) as the first step in treating depression.14 This skepticism refers to the trend of using CAM. One type of CAM approach includes natural products like dietary supplements (vitamins, minerals, and probiotics).15
Dietary supplements are orally consumed products, such as vitamins, herbs, minerals, amino acids, enzymes, metabolites, probiotics, and other substances for supplementing typical diets.16 Several dietary supplements have also been commonly used for reducing depression symptoms. For example, Kazemi et al17 explained that dietary supplementation, such as prebiotic use, significantly decreased the Beck Depression Inventory (BDI) scores compared with the placebo group, after eight weeks of intervention. Other dietary supplementations, such as polyunsaturated fatty acid (PUFA), vitamin D,18 B12,19 magnesium, zinc,20 and curcumin,21 have also been studied for their efficacy and effects as add-on supplements for depression treatment. In a review conducted by Hoffman et al,18 vitamin D and PUFA supplementation had complementarily positive effects on depressive symptoms, anxiety, and pain. Therefore, this review explored and summarized clinical study updates on dietary supplements’ types, dosages, and effectiveness to reduce symptoms of depression.
Methods
Search Strategy and Selection Criteria
The search of the PubMed database was conducted in August 2021, using the terms “(dietary supplementation) AND (depression)” on the advanced query box. Afterward, some criteria were put in place to choose relevant articles, including only articles published in English, article types reporting clinical studies or randomized controlled trials, and those with a maximum publication date of 10 years.
The Review Process
Extracted article references were manually searched and screened for relevance. Then, we collected the following information: country of study, type of supplementation, the dosage used, the scale of depression, and results.
Clinical Studies on Dietary Supplementations for Treating Depression
Although we finally identified 221 article records from the advanced search data, 137 articles were excluded because the studies did not focus on depression or the journal had incomplete titles and abstracts. Likewise, 21 articles were excluded due to ineligibility (Figure 1). Therefore, we included 63 articles and further grouped these studies into five broad groups based on the number of supplements used in the research. The five groups were PUFA, vitamin D, probiotics, a combination of vitamins and nutrients, and other supplements.
 |
Figure 1 Literature search results. |
Table 1 summarizes the study characteristics of the 63 selected articles. We observed that the most common supplementation used was that of the PUFA group and its combination (n = 17, 26.9%), followed by vitamin D (n = 15, 23.8%), probiotics (n = 8, 12.7%), then combination of vitamins and nutrients (n = 6, 9.5%). Furthermore, most clinical studies were conducted in Iran (41.3%), with adult participants aged above 18 (98.4%). From all studies, the most commonly used scale was the BDI and its respective version (47.6%).
 |
Table 1 Study Characteristics |
The Polyunsaturated Fatty Acid (PUFA) Group
This review found that the most commonly used supplementation in treating depression was the PUFA group, which was a combination of Docosahexaenoic acid (DHA) and Eicosapentaenoic acid (EPA). Table 2 shows that from the twelve clinical studies that used PUFA supplementation for depression, eight studies showed positive effects in improving depression symptoms and reducing the scale of depression. However, the other four had no significant impact on the scale of depression, meaning that most of the studies agreed that PUFA was beneficial as a treatment for depression.
 |
Table 2 Effects of Using PUFA Supplements for Treating Depression |
From the eight studies that showed PUFA’s beneficial effects on depression, we observed that the range of the total daily dosage of the EPA ranged from 0.7 g to 2 g and DHA was from 0.4 g to 0.8 g. Moreover, the frequency of PUFA supplement intake varied from one to nine times daily, within a 3-week to 4-month administration period. Most studies showed beneficial effects in a total daily dose of PUFA supplementation and a total daily dose of 180 mg of EPA and 120 mg of DHA, with an intake frequency of six times daily. However, the studies with no significant impact used a higher dosage with higher study durations of up to three years. However, we identified one study by McNamara et al22 that stated that PUFA had a beneficial effect on depression, with a total daily dosage that was higher than the other studies, more specifically, at 16.2 grams daily (10.8-g EPA + 5.4 g DHA, divided to 2 tablespoons/day). This dosing was based on a previous study that reported supplementation’s efficacy and safety data in patients with attention deficit hyperactivity disorder. Moreover, only one study of the eight,23 wherein the subjects were pregnant women at various stages of pregnancy, also showed beneficial effects of PUFA’s supplementation on pregnancy depression. A review conducted by Hsu et al24 also supported this result, which explained that in addition to preventing pregnancy-related depression, sufficient consumption of omega-3 fatty acid supplements such as EPA during pregnancy was important, because they served as the critical building blocks of the fetal brain and retina. They also determine the pregnancy length.
Omega-3 fatty acids are substrates that affect the formation of neurotransmitters and prostaglandins. Therefore, they have extensively functions with regard to the maintenance and regulation of a healthy brain. Furthermore, it has been reported that depression rates can increase with lower levels of membrane-related omega-3 fatty acids.25 The main pathway target of omega-3, which is also linked to the inflammatory hypothesis of depression,26 is the arachidonic acid inflammatory pathway.27 Two possible pathways exist in the reduced synthesis of eicosanoids from arachidonic acid by DHA and EPA, as explained by Wani et al.25 First, they combine with arachidonic acid for amalgamation into membrane-based phospholipids, thereby reducing both cellular and plasma concentrations of arachidonic acid. DHA and EPA, which hinder the release of pro-inflammatory cytokines (interferon-γ, tumor necrosis factor [TNF]-α, interleukin [IL]-1β, IL-2, and IL-6), are determined by the eicosanoid discharge. The other possible way is that of the cyclooxygenase enzyme system. Here, EPA can compete with arachidonic acid to block the pro-inflammatory eicosanoid synthesis from arachidonic acid, prostaglandin E2, and thromboxanes B225 by inhibiting the action of phospholipase A2, as shown in Figure 2.26 Additionally, Lotrich et al28 reported that the elevated ratio of arachidonic acid to omega-3 fatty acid was linked to depression. The observation from other intervention studies also indicated that administering omega-3 fatty acids can decrease this ratio and improve depressive symptoms.
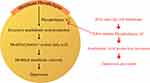 |
Figure 2 The proposed mechanism of EPA in inhibiting phospholipase A2 and preventing depression. |
Additionally, in the pathophysiology of depression, the upregulation of 5-HT2A/C receptors is proposed to play a significant role. From the previous study, it was observed that for an individual with chronic stage omega-3 fatty acid deficiencies, changes in the concentration of brain fatty acids can result in an altered serotonergic or dopaminergic neurotransmission, increase in 5-HT2,27 and decrease in serotonin and dopamine in the prefrontal cortex,29 thereby indicating the importance of adequate PUFA intake. A few studies we obtained also investigated the efficacy of PUFA combined with other dietary supplements, such as 5-aminolevulinic acid (ALA), curcumin, vitamin E, and vitamin B on depression (Table 3). From the five studies obtained, the overall result was that those combinations showed no significant effect. From these studies, only one study showed beneficial supplement effects on depression, which was the combination of PUFA and vitamin E.30 The study used 1000-mg PUFA with 400 IU vitamin E daily for twelve weeks, with the subjects being women with polycystic ovary syndrome (PCOS). Jamilian et al stated that compared to a single supplementation, combining supplements gave better and more significant treatment results, including improved mental health parameters. Alternatively, the other four studies obtained showed statistically non-significant results with the use of PUFA combined with other supplements in treating depression, thereby proposing that PUFA use during depression conditions was preferred without any supplement combination.
 |
Table 3 Effects of Combined PUFA Supplementations for Treating Depression |
Vitamin D
Table 4 shows that nine of the 15 vitamin D studies reported beneficial effects on improving depression symptoms. These studies showed that vitamin D supplementation at 2000 IU/day–50,000 IU/week for eight weeks (two months) to 24 months of supplementation positively affected depression. However, although the exact dosage was used in one study, no significant impact on depression was observed since the study included a dialysis patients. Furthermore, the duration of the study was from eight weeks (2 months) to 24 months of supplementation before a positive result. Two studies were also observed in addition to a single supplementation, which combined vitamin D supplementation with probiotics31 and zinc.32 The findings of both studies showed that the combined supplementation had positive effects on depression. Therefore, since probiotics and zinc influence depression, its proposed action mechanism is discussed below. Notably, seven of 15 findings mentioned that vitamin D supplementation had no significant effect on depression, which was attributed to the low intervention impact, compared to efficient treatment standards like antidepressants.33
 |
Table 4 Effects of Vitamin D Supplementation on Depression |
The potential function of vitamin D is to regulate calcium-phosphate homeostasis.34 Its mechanism is through the vitamin D receptor, which affects most physiological systems, including the brain.35 Some studies have mentioned that lower serum levels of 25(OH) D3 were associated with depression.36 Furthermore, depression results from some pathways, including chronic inflammation, formation of serotonin, expression of mitochondrial proteins, Ca2+ homeostasis, and antioxidant gene expression,37 which are indirectly related to vitamin D. Chronic inflammation accounts for depression in the presence of inflammatory cytokine expression, which can be reduced with vitamin D supplementation.38–40
The lack of vitamin D in the serum can also lead to depression through mitochondrial function and serotonin formation pathways. In the serotonin formation pathway, vitamin D fails to induce the serotonin-synthesized gene of tryptophan hydroxylase 2 (TPH-2) while repressing the expression of TPH-1.41 Its consequence is a decline in plasma tryptophan levels; tryptophan cannot be transferred into the brain. This inhibition results in low serotonin levels in the brain, causing depression.42 Additionally, mitochondrial function will be impaired in the presence of low serum vitamin D levels,43 resulting in elevated reactive oxygen species (ROS) and reduced ATP formation, impacting Ca2+ and antioxidant gene (GSH) homeostasis. Significant antioxidants in neurons are GSH or glutathione. They are depleted when ROS is high,44 resulting in depression. Both the elevation of ROS and glutamate, which leads to higher Ca2+ levels, causes a decline in the number and size of GABAergic neurons. This decline accounts for the imbalance in excitatory and inhibitory neurons that leads to depression.37 Studies showed that vitamin D supplementation normalized vitamin D levels in the serum to prevent depression. Figure 3 depicts the proposed vitamin D pathway to prevent depression. Toxic doses of vitamin D are been considered to be >50 × 103 IU/day45 and are detailed in Table 5.
 |
Table 5 Dietary Supplementation Permissible Levels |
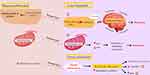 |
Figure 3 The proposed vitamin D pathway to prevent depression. |
Probiotics
Other supplementations studied for reducing depression are probiotics. Probiotics are live microorganisms that can improve gut health. Lately, they have been used to manage and treat various diseases, one of which is depression.46 Table 6 shows eight studies reporting the usage of probiotic supplementation in depression. While five of these studies showed beneficial effects on depression symptoms and measured improvements using a depression scale, the other three showed no significant impact on depression.
 |
Table 6 Effects of Probiotic Supplementation for Treating Depression |
The microbe species included in the studies with positive results comprised various bacteria from the genus Lactobacillus (L. acidophilus, L. casei, L. fermentum, L.rhamnosus, and L. bulgaricus) and the genus Bifidobacterium (Bifidobacterium bifidum and Bifidobacterium lactis) with various dosage ranges above 108 CFU in the form of yogurt, capsules, and sachets. The positive effect of using probiotic supplementations in depression was also supported by a meta-analysis conducted by Chao et al.47 The study explained that probiotics were adjunct therapies that can improve depressive symptoms in patients diagnosed with depression. Yong et al48 explained another advantage of probiotics as an antidepressive treatment. According to his study, although probiotics are primarily safe to be consumed and socially help patients, they are proposed not to receive the negative stigma associated with standard antidepressants.
In studies with negative results, Louzada et al49 explained that compared to previous studies wherein probiotics simultaneously had significant effects on depression parameter scores, the negative outcome could be attributed to the characteristics of participants included in their research, which are proposed to not have been clinically diagnosed as depressed. Moreover, other uncontrolled events could have interfered with the study result.49 It was also explained by Kazemi et al17 that probiotics were only beneficial when used as an additive to regular antidepressants as compared to probiotics that were used as primary therapy for depression.17
Although an in-depth mechanism explaining probiotics’ effect on depression is not fully understood yet,46 a possible pathway is connected to the microbiota–gut–brain (MGB) axis in the pathophysiology of depression. The MGB axis allows two-way communication between the gut microbiota and the human brain. Depression is therefore developed when the MGB axis becomes maladaptive, adversely affecting the host physiology, which eventually leads to depression. Hence, probiotics also help alleviate depression by modulating the MGB axis. Nevertheless, a few challenges have been observed in explaining the complete mechanism that contributes to the effectiveness of probiotics in depression, including the heterogeneity of depression and the gut microbiota’s complexity.48 However, despite these challenges and the unclear mechanism, increasing evidence supports that probiotics are an excellent alternative for depression treatment, compared to standard antidepressants.
The Combination of Vitamins and Nutrients
Table 7 shows six studies reporting a combination of vitamin and nutrient supplementation in depression. We observed that four studies conducted by Nguyen,50 Berens,51 Oliver-Baxter,52 and Harris53 revealed beneficial effects of these classes on depression. Nguyen et al50 reported that the intervention of multiple micronutrient and iron-folic acid groups had a significant impact on lowering depression scores during the first and second trimesters of pregnancy compared to folic acid alone (P < 0.05) among women in the highest tertile for CES-D scores. Multiple micronutrients, such as vitamin B12, vitamin D, folates, zinc, and selenium, have been recommended for preventing maternal depression.
 |
Table 7 Effects of Combining Vitamins and Nutrient Supplementation as Depression Treatments |
Oliver-Baxter52 also reported that the consumption of supplements containing 12 vitamins, minerals, and herbs within eight weeks to have significant results in decreasing depression scores and all pro-inflammatory cytokines levels related to depression, such as IL-1β, IL-5, IL-6, TNF-α, and TNF-β. Significant changes in IL-1, TNF-α, and TNF-β caused supplement effects on TNF-related cytokines that modulated fundamental cellular processes, such as inflammation. Withania somnifera, one of the active ingredients, has shown effects on proinflammatory cytokines that interfere with hypothalamic-pituitary-gonadal axis down-regulation of local inflammatory.52 When the hypothalamic-pituitary-adrenal axis is repeatedly activated, interrelated systems produce by-products to compensate for failure in other systems, such as decreased lymphocytes and increased pro-inflammatory activities that resulted in inflammation.54 Previous studies have shown that depression is highly associated with increased inflammatory marker levels, such as IL-1 and IL-6.52
Several ingredients in multivitamin supplements can potentially improve symptoms of depression, such as folate, vitamin C, vitamin D, and herbal ingredients (Ginseng and Ginkgo biloba), by oxidative-stress reduction and mood enhancement. Zinc, vitamin D, and α-tocopherol have antioxidant and anti-inflammatory activities linked to the mechanism of increased telomere length of leukocyte DNA.55
The findings of another study indicated that a small quantity of lipid-based nutrient supplement (SQ-LNS) containing vitamin, mineral, and essential fatty acids did not affect maternal postpartum depression (PDD),56 which was proposed to be a result of the dosage of essential fatty acids (alpha-linolenic acid [ALA] and linoleic acid) in SQ-LNS formulation was insufficient to treat depression.56 Results from this study are in concurrence with a meta-analysis study where EPA/DHA supplements with substantially greater dosages (>60%) of high dose EPA (0.2–2.2 gram/day) were found to offer more significant results in treating depression than supplements with a lower dosage (<60%).57
Other Supplementations
Table 8 shows other supplementations that significantly reduced depression scores, such as carnitine, coenzyme Q10, nanocurcumin, curcumin, magnesium oxide, melatonin, myo-inositol, sumac (Rhus coriaria L.), vitamin A, vitamin B, folic acid, zinc, and zinc sulfate. However, other supplementations, such as vitamin E, C, and ferrous sulfate, did not offer a significant reduction in the depression score in this study due to many factors, such as the short trial duration, limited sample size in each group, low effective doses, and inadequate measurement at the start and end of the trial.58,59
 |
Table 8 Effects of Other Supplements in Treating Depression |
Furthermore, we observed that carnitine’s effect in lowering depression parameter scores was linked to improved mitochondrial performance, antioxidant effects, increased cholinergic neurotransmission, enhanced protein, and gene expression, which affected brain metabolism, thereby aiding the improvement of neuropsychological function.60
Coenzyme Q10, nanocurcumin, and curcumin also had neuroprotective properties, including antioxidant and anti-inflammatory activities. Previously, several studies reported that depression was associated with higher TNF-α levels in individuals with depression than in healthy controls. Therefore, these supplements effectively protect brain cells and neurons from significant neurotoxic damage and lower pro-inflammatory cytokine levels (TNF-α and IL-1β), thereby reducing the overproduction of circulating TNF-α and IL-1β caused by lipopolysaccharides.61 These regulatory effects would help develop depression treatments in the future. Additionally, the studies showed that curcumin and nanocurcumin protected the brain from oxidative stress and regulated serotonin and dopamine release.62,63 The low oral bioavailability of curcumin in humans is the cause for low levels of curcumin in the plasma and insufficient pharmacological effects.62 Thus, many formulations, such as nanoparticles, liposomes, micelles, and phospholipid complexes, have been developed to address this issue and improve curcumin bioavailability. Nanocurcumin is more potential than curcumin in suppressing nuclear factor B and has a higher cellular absorption rate, offering better efficacy at the pharmacological level as compared to curcumin.62,64 Previous studies have reported that different curcumin formulae administered result in effects of different magnitudes at the behavioral, electrophysiological, and molecular levels in depression. Therefore, future clinical trials on the effects of different curcumin formulations on depression are essential, to evaluate the potential difference in efficacy on depression.65–67
Rajizadeh et al68 investigated the consumption of 500 mg magnesium oxide daily for eight weeks in people with depression and hypomagnesemia. They observed that magnesium oxide improved depression by reducing BDI scores. Although the advised dose for treating depression ranged from 125 mg to 450 mg based on the previous study, the duration of therapeutic supplementation ranged from 7 to 20 months. Based on these findings, magnesium was proposed to be essential for improving synaptic functions, particularly in the hippocampal neurons of the brain. In addition to its role in blocking N-methyl-D- aspartate (NMDA) channels, magnesium also has an excitatory influence on the production of the brain-derived neurotrophic factor (BDNF) involved in brain function and neuron maintenance.69 Additionally, magnesium influences the production of cyclic adenosine monophosphate response element-binding proteins that control genes related to brain function, particularly those involved in dopamine synthesis. Thus, magnesium can help alleviate depression.68
Melatonin also has a similar mechanism in reducing depression. As observed, melatonin exerts its neuroprotective properties by controlling glutamate’s NMDA-mediated excitotoxic effects, such as through decreased BDNF signaling and necrosis in cells.70 Furthermore, melatonin regulates dopamine signaling, which is a precursor of noradrenaline.
MDD has been linked to a decline in monoaminergic neurotransmitters, such as noradrenaline, dopamine, and serotonin.71 Jamalian et al72 observed that myo-inositol (2 g) with 200 ug folate twice a day compared to the metformin group significantly improved BDI and DASS scores in women with polycystic ovary syndrome (PCOS) after 12 weeks of supplementation. Furthermore, myo-inositol lowered the oxidation of thiol groups that prevented the progressive rise of oxidative stress in women with PCOS.
Likewise, Hariri et al73 investigated the effects of sumac (Rhus coriaria L.) supplementation with a total dose of three grams daily in obese women. Although BDI scores reduced significantly in both groups, no significant difference compared with the placebo was observed. Sumac contains gallotannins that have anti-inflammatory activities through their ability to scavenge ROS and suppress inflammatory mediators (COX-2) linked with depression.74,75
Some vitamin supplementations also help in depression treatment, such as vitamins A and B. Bitarafan et al,76 reported that consuming a vitamin A (retinyl palmitate) dose of 2500 IU daily within six months continued by 10.000 IU per day for another six months, significantly improved depression scales in patients with multiple sclerosis. These patients had immune dysfunction and psychosocial stressors that can cause depression.76 Therefore, vitamin A influences anti-inflammatory effects by suppressing the growth of inflammatory T-helper cells and lowering the gene expression of inflammatory cytokines.77 This modulation can help reduce depression symptoms in patients with MS can be reduced. In contrast, vitamin B, such as B12, folic acid, and B6, also improves depression symptoms. It has been reported that combining vitamin B12 and folic acid lowered homocysteine levels, thereby lowering harmful effects and enhancing the efficacy of antidepressant treatments. In the brain, vitamin B12-dependent methylation influences neurological symptoms and disorders. Thus, vitamin B deficiency can increase homocysteine levels, resulting in increased oxidative stress that can cause depression.78
Furthermore, Solati et al79 reported that 30 mg of zinc daily in obese women for 12 weeks improved depression scores by increasing BDNF levels. Thus, zinc had a similar mechanism to antidepressants and other supplementations. In turn, the rise of BDNF levels in the hippocampus triggered therapeutic effects in depressed patients. Likewise, Ranjbar et al80 investigated the consumption of 25 mg zinc sulfate daily in addition to antidepressants (SSRI citalopram or fluoxetine). As observed, depression scores were significantly different compared to the control group. Zinc also mediated antidepressant effects through other pathways, such as zinc antagonism of the NMDA receptor.80 Zinc supplementation results in its storage in the synaptic vesicles of glutamatergic neurons and following neuronal activity that can be transferred into the synaptic cleft, inhibiting glutamate receptors like NMDA is observed.81
Alternatively, the study by Mazloom reported that although the effect of vitamins C and E did not have significant results on depression levels compared with the placebo in patients with diabetes type-2, vitamin C caused a substantial change in anxiety levels.58 This change was proposed to be caused by the short duration of treatment and inadequate doses of vitamin C and E. However, Amr et al82 reported that vitamin C supplementation of 500 mg twice a day with fluoxetine (SSRI antidepressant) of 10 mg per day, administered orally in pediatric patients with MDDs, led to a significant reduction in depressive symptoms compared to the group given fluoxetine alone. This finding shows that vitamin C can be an adjunctive therapy for antidepressant drugs. Furthermore, vitamin C offers neuroprotective properties as an additional protection from oxidative free radicals that can cause brain damage and result in depression.83 Similarly, a previous study observed that vitamin C modulated dopamine and glutamate-mediated neurotransmission as a neuromodulator in the brain. Moreover, the complicated connection of vitamin C with the dopaminergic pathway can be another potential mechanism of action, accounting for its efficacy in treating depression.84
Implications and Future Directions
Given the positive results of the dietary supplements in reducing depressive symptoms, we suggest that people with depression consult with a specialist before selecting the type of supplementation. The lower and upper permissible levels of consumption of these supplementations also need to be considered (Table 5). Further studies focusing on substances that showed no positive results, with more carefully selected groups, doses, and combinations are needed to provide a more comprehensive and up-to-date overview of existing supplementations to reduce depressive symptoms.
Conclusion
Based on this study, we observed that the most common supplementation used in clinical studies for depression treatment was the PUFA group and its combination (n = 17, 26.98%), followed by vitamin D (n = 15, 23.81%), then probiotics (n = 8, 12.7%). The total daily dosage of PUFA showed its beneficial effect with 0.7–2 g EPA and 0.4–0.8 g DHA daily for three weeks to 4 months. Furthermore, its positive effect was 2000 IU/day–50,000 IU/week vitamin D for eight weeks to 24 months of supplementation. We also observed that microbes from the genus Lactobacillus and Bifidobacterium with various dosage ranges above 108 CFU in yogurt, capsule, and sachets in the probiotic group showed beneficial adjunctive therapy effects in treating depression. The depression scale was therefore valuable to assess the effect of an intervention on depression. Nevertheless, we propose that PUFA, vitamin D, and probiotics be considered beneficial dietary supplementations in depression treatment and management.
Funding
This work is supported by Universitas Padjadjaran, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rao AL, Hong ES. Understanding depression and suicide in college athletes: emerging concepts and future directions. Br J Sports Med. 2016;50(3):136–137. doi:10.1136/bjsports-2015-095658
2. Metrics GH. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990 – 2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;1990–2017. DOI:10.1016/S0140-6736(18)32279-7
3. Koo SK. Depression status in Korea. Osong Public Heal Res Perspect. 2018;9(4):141–142. doi:10.24171/j.phrp.2018.9.4.01
4. World Health Organization. Depression; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/depression.
5. Nikam YL, Choudhary SE, Gochade M. Depression in teens: a overview. Int J Recent Adv Multidisciplin Topics. 2021;2(8):46–47.
6. Yamasaki K, Hasegawa T, Takeda M. Serum level of soluble interleukin 6 receptor is a useful biomarker for identification of treatment-resistant major depressive disorder. Neuropsychopharmacol Rep. 2020;40(2):130–137. doi:10.1002/npr2.12100
7. Klengel T, Binder EB. Gene-environment interactions in major depressive disorder. Can J Psychiatry. 2013;58(2):76–83. doi:10.1177/070674371305800203
8. Muneer A. Mixed states in bipolar disorder: etiology, pathogenesis and treatment. Chonnam Med J. 2017;53(1):1. doi:10.4068/cmj.2017.53.1.1
9. Patel R, Rose G. Persistent Depressive Disorder. StatPearls Publishing LLC; 2021.
10. Hidaka BH. Depression as a disease of modernity: explanations for increasing prevalence. J Affect Disord. 2012;140(3):205–214. doi:10.1016/j.jad.2011.12.036
11. Khushboo BS. Antidepressants: mechanism of action, toxicity and possible amelioration. J Appl Biotechnol Bioeng.2017;3(5). doi:10.15406/jabb.2017.03.00082
12. Gartlehner G, Wagner G, Matyas N, et al. Pharmacological and non-pharmacological treatments for major depressive disorder: review of systematic reviews. BMJ Open. 2017;7(6):1–13. doi:10.1136/bmjopen-2016-014912
13. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366. doi:10.1016/S0140-6736(17)32802-7
14. Forneris CA, Nussbaumer-Streit B, Morgan LC, et al. Psychological therapies for preventing seasonal affective disorder. Cochrane Database Syst Rev. 2019;2019(5). doi:10.1002/14651858.CD011270.pub3
15. Haller H, Anheyer D, Cramer H, Dobos G. Complementary therapies for clinical depression: an overview of systematic reviews. BMJ Open. 2019;9(8):1–15. doi:10.1136/bmjopen-2018-028527
16. FDA. What you need to know about dietary supplements; 2017. Available from: https://www.fda.gov/food/buy-store-serve-safe-food/what-you-need-know-about-dietary-supplements.
17. Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr. 2018;1–7. doi:10.1016/j.clnu.2018.04.010
18. Hoffmann K, Emons B, Brunnhuber S, Karaca S, Juckel G. The role of dietary supplements in depression and anxiety-a narrative review. Pharmacopsychiatry. 2019;52(6):261–279. doi:10.1055/a-0942-1875
19. Syed EU, Wasay M, Awan S. Vitamin B12 supplementation in treating major depressive disorder: a randomized controlled trial. Open Neurol J. 2013;7(1):44–48. doi:10.2174/1874205x01307010044
20. Wang J, Um P, Dickerman BA, Liu J. Zinc, magnesium, selenium and depression: a review of the evidence, potential mechanisms and implications. Nutrients. 2018;10(5):1–19. doi:10.3390/nu10050584
21. Ng QX, Koh SSH, Chan HW, Ho CYX. Clinical use of curcumin in depression: a meta-analysis. J Am Med Dir Assoc. 2017;18(6):503–508. doi:10.1016/j.jamda.2016.12.071
22. McNamara RK, Jandacek R, Rider T, et al. Effects of fish oil supplementation on prefrontal metabolite concentrations in adolescents with major depressive disorder: a preliminary 1H MRS study. Nutr Neurosci. 2016;19(4):145–155. doi:10.1179/1476830514Y.0000000135
23. Nishi D, Su KP, Usuda K, et al. Differences between Japan and Taiwan in the treatment of pregnant women with depressive symptoms by omega-3 fatty acids: an open-label pilot study. Nutr Neurosci. 2019;22(1):63–71. doi:10.1080/1028415X.2017.1354540
24. Hsu MC, Tung CY, Chen HE. Omega-3 polyunsaturated fatty acid supplementation in prevention and treatment of maternal depression: putative mechanism and recommendation. J Affect Disord. 2018;238:47–61. doi:10.1016/j.jad.2018.05.018
25. Wani AL, Bhat SA, Ara A. Omega-3 fatty acids and the treatment of depression: a review of scientific evidence. Integr Med Res. 2015;4:1–10. doi:10.1016/j.imr.2015.07.003
26. Park Y, Park YS, Kim SH, Oh DH, Park YC. Supplementation of n-3 polyunsaturated fatty acids for major depressive disorder: a randomized, double-blind, 12-week, placebo-controlled trial in Korea. Ann Nutr Metab. 2015;66(2–3):141–148. doi:10.1159/000377640
27. Ravi S, Khalili H, Abbasian L, Arbabi M, Ghaeli P. Effect of omega-3 fatty acids on depressive symptoms in HIV-positive individuals: a randomized, placebo-controlled clinical trial. Ann Pharmacother. 2016;50(10):797–807. doi:10.1177/1060028016656017
28. Lotrich FE, Sears B, McNamara RK. Elevated ratio of arachidonic acid to long-chain omega-3 fatty acids predicts depression development following interferon-alpha treatment: relationship with interleukin-6. Brain Behav Immun. 2013;31:48–53. doi:10.1016/j.bbi.2012.08.007
29. Ginty AT, Conklin SM. Short-term supplementation of acute long-chain omega-3 polyunsaturated fatty acids may alter depression status and decrease symptomology among young adults with depression: a preliminary randomized and placebo controlled trial. Psychiatry Res. 2015;229(1–2):485–489. doi:10.1016/j.psychres.2015.05.072
30. Jamilian M, Shojaei A, Samimi M, et al. The effects of omega-3 and vitamin E co-supplementation on parameters of mental health and gene expression related to insulin and inflammation in subjects with polycystic ovary syndrome. J Affect Disord. 2018;229:41–47. doi:10.1016/j.jad.2017.12.049
31. Raygan F, Ostadmohammadi V, Bahmani F, Asemi Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Prog Neuro Psychopharmacol Biol Psychiatry. 2018;84:50–55. doi:10.1016/j.pnpbp.2018.02.007
32. Yosaee S, Soltani S, Esteghamati A, et al. Effects of zinc, vitamin D and their co-supplementation on mood, serum cortisol, and brain-derived neurotrophic factor in patients with obesity and mild to moderate depressive symptoms: a 12-week, 2 × 2 factorial design, double-blind, randomized, placebo-. Nutrition. 2019;71:110601. doi:10.1016/j.nut.2019.110601
33. Hansen JP, Pareek M, Hvolby A, et al. Vitamin D3 supplementation and treatment outcomes in patients with depression (D3 ‑ vit ‑ dep). BMC Res Notes. 2019;12:1–6. doi:10.1186/s13104-019-4218-z
34. Bivona G, Agnello L, Ciaccio M. The immunological implication of the new vitamin D metabolism. Cent Eur J Immunol. 2018;43(3):331–334. doi:10.5114/ceji.2018.80053
35. Spedding S. Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014;6:1501–1518. doi:10.3390/nu6041501
36. Jhee JH, Kim H, Park S, et al. Vitamin D deficiency is significantly associated with depression in patients with chronic kidney disease. Cytokine. 2017;103:1–13. doi:10.1371/journal.pone.0171009
37. Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69:80–92. doi:10.1124/pr.116.013227
38. Beilfuss J, Berg V, Sneve M, Jorde R, Kamycheva E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine. 2012;60(3):870–874. doi:10.1016/j.cyto.2012.07.032
39. Grossmann RE, Zughaier SM, Liu S, Lyles RH, Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr. 2012;66(9):1072–1074. doi:10.1038/ejcn.2012.82
40. Wei R, Christakos S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients. 2015;7(10):8251–8260. doi:10.3390/nu7105392
41. Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015;29(6):2207–2222. doi:10.1096/fj.14-268342
42. Catena-Dell’Osso M, Bellantuono C, Consoli G, Baroni S, Rotella F, Marazziti D. Inflammatory and neurodegenerative pathways in depression: a new avenue for antidepressant development? Curr Med Chem. 2011;18(2):245–255. doi:10.2174/092986711794088353
43. Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11(1):1–16. doi:10.1186/1741-7015-11-200
44. Andreazza AC, Wang JF, Salmasi F, Shao L, Young LT. Specific subcellular changes in oxidative stress in prefrontal cortex from patients with bipolar disorder. J Neurochem. 2013;127(4):552–561. doi:10.1111/jnc.12316
45. Galior K, Grebe S, Singh R. Development of vitamin d toxicity from overcorrection of vitamin D deficiency: a review of case reports. Nutrients. 2018;10(8):953. doi:10.3390/nu10080953
46. Shahrokhi M, Nagalli S. Probiotics. In: StatPearls. StatPearls Publishing; 2021.
47. Chao L, Liu C, Sutthawongwadee S, et al. Effects of probiotics on depressive or anxiety variables in healthy participants under stress conditions or with a depressive or anxiety diagnosis: a meta-analysis of randomized controlled trials. Front Neurol. 2020;11. doi:10.3389/fneur.2020.00421
48. Yong SJ, Tong T, Chew J, Lim WL. Antidepressive mechanisms of probiotics and their therapeutic potential. Front Neurosci. 2020;13. doi:10.3389/fnins.2019.01361
49. Louzada ER, Ribeiro SML. Synbiotic supplementation, systemic inflammation, and symptoms of brain disorders in elders: a secondary study from a randomized clinical trial. Nutr Neurosci. 2018;23(2):93–100. doi:10.1080/1028415X.2018.1477349
50. Nguyen PH, DiGirolamo AM, Gonzalez-Casanova I, et al. Impact of preconceptional micronutrient supplementation on maternal mental health during pregnancy and postpartum: results from a randomized controlled trial in Vietnam. BMC Womens Health. 2017;17(1):1–9. doi:10.1186/s12905-017-0401-3
51. Von Berens Å, Fielding RA, Gustafsson T, et al. Effect of exercise and nutritional supplementation on health-related quality of life and mood in older adults: the VIVE2 randomized controlled trial. BMC Geriatr. 2018;18(1):1–8. doi:10.1186/s12877-018-0976-z
52. Oliver-Baxter JM, Whitford HS, Turnbull DA, Bond MJ. Effects of vitamin supplementation on inflammatory markers and psychological wellbeing among distressed women: a randomized controlled trial. J Integr Med. 2018;16(5):322–328. doi:10.1016/j.joim.2018.06.001
53. Harris E, Kirk J, Rowsell R, et al. The effect of multivitamin supplementation on mood and stress in healthy older men. Hum Psychopharmacol Clin Exp. 2011;26(8):560–567. doi:10.1002/hup.1245
54. Jaremka L, Lindgren M, Kiecolt-Glaser J. Synergistic relationships among stress, depression, and troubled relationships: insights from psychoneuroimmunology. Depress Anxiety. 2013;30(4):1–15. doi:10.1002/da.22078
55. Harris E, Kirk J, Rowsell R, et al. A double-blind trial of bupropion versus desipramine for bipolar depression. Hum Psychopharmacol Clin Exp. 2011;26(8):560–567. doi:10.1002/hup.1245
56. Okronipa H, Adu-Afarwuah S, Lartey A, et al. Maternal supplementation with small-quantity lipid-based nutrient supplements during pregnancy and lactation does not reduce depressive symptoms at 6 months postpartum in Ghanaian women: a randomized controlled trial. Arch Womens Ment Health. 2018;21(1):55–63. doi:10.1007/s00737-017-0752-7
57. Sublette ME, Ellis SP, Geant AL, Mann JJ. NIH public access. J Clin Psychiatr. 2011;72(12):1577–1584. doi:10.4088/JCP.10m06634.Meta-analysis
58. Mazloom Z, Ekramzadeh M, Hejazi N. Efficacy of supplementary vitamins C and E on anxiety, depression and stress in type 2 diabetic patients: a randomized, single-blind, placebo-controlled trial. Pakistan J Biol Sci. 2013;16(22):1597–1600. doi:10.3923/pjbs.2013.1597.1600
59. Vaucher P, Druais PL, Waldvogel S, Favrat B. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247–1254. doi:10.1503/cmaj.110950
60. Jamilian H, Jamilian M, Samimi M, et al. Oral carnitine supplementation influences mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Gynecol Endocrinol. 2017;33(6):442–447. doi:10.1080/09513590.2017.1290071
61. Sanoobar M, Dehghan P, Khalili M, Azimi A, Seifar F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: a double blind randomized clinical trial. Nutr Neurosci. 2015;19(3):138–143. doi:10.1179/1476830515Y.0000000002
62. Asadi S, Gholami MS, Siassi F, Qorbani M, Sotoudeh G. Beneficial effects of nano-curcumin supplement on depression and anxiety in diabetic patients with peripheral neuropathy: a randomized, double-blind, placebo-controlled clinical trial. Phyther Res. 2020;34(4):896–903. doi:10.1002/ptr.6571
63. Yu JJ, Pei LB, Zhang Y, Wen ZY, Yang JL. Chronic supplementation of curcumin enhances the efficacy of antidepressants in major depressive disorder. J Clin Psychopharmacol. 2015;35(4):406–410. doi:10.1097/JCP.0000000000000352
64. Rahimi HR, Nedaeinia R, Sepehri Shamloo A, Nikdoust S, Kazemi Oskuee R. Novel delivery system for natural products: nano-curcumin formulations. Avicenna J Phytomed. 2016;6(4):383–398.
65. Mohammed HS, Khadrawy YA, El-Sherbini TM, Amer HM. Electrocortical and biochemical evaluation of antidepressant efficacy of formulated nanocurcumin. Appl Biochem Biotechnol. 2019;187(3):1096–1112. doi:10.1007/s12010-018-2866-4
66. Ramaholimihaso T, Bouazzaoui F, Kaladjian A. Curcumin in depression: potential mechanisms of action and current evidence—a narrative review. Front Psychiatry. 2020;11:1–16. doi:10.3389/fpsyt.2020.572533
67. Fidelis EM, Savall ASP, da Luz Abreu E, et al. Curcumin-loaded nanocapsules reverses the depressant-like behavior and oxidative stress induced by β-amyloid in mice. Neuroscience. 2019;423:122–130. doi:10.1016/j.neuroscience.2019.09.032
68. Rajizadeh A, Mozaffari-Khosravi H, Yassini-Ardakani M, Dehghani A. Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: a randomized, double-blind, placebo-controlled trial. Nutrition. 2017;35:56–60. doi:10.1016/j.nut.2016.10.014
69. Szewczyk B, Poleszak E, Sowa-Kuæma M, et al. Shear wave elastography of abdominal and retroperitoneal masses and inflammatory processes: a feasibility study. Ultrasound Med Biol. 2008;37(8):S33–S34. doi:10.1016/j.ultrasmedbio.2011.05.163
70. Bavithra S, Sugantha Priya E, Selvakumar K, Krishnamoorthy G, Arunakaran J. Effect of melatonin on glutamate: BDNF signaling in the cerebral cortex of Polychlorinated Biphenyls (PCBs)—exposed adult male rats. Neurochem Res. 2015;40(9):1858–1869. doi:10.1007/s11064-015-1677-z
71. Tonon AC, Pilz LK, Markus RP, Hidalgo MP, Elisabetsky E. Melatonin and depression: a translational perspective from animal models to clinical studies. Front Psychiatr.2021;12:1–13. doi:10.3389/fpsyt.2021.638981
72. Jamilian H, Jamilian M, Foroozanfard F, Afshar Ebrahimi F, Bahmani F, Asemi Z. Comparison of myo-inositol and metformin on mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Psychosom Obstet Gynecol. 2018;39(4):307–314. doi:10.1080/0167482X.2017.1383381
73. Hariri N, Darafshi Ghahroudi S, Jahangiri S, Borumandnia N, Narmaki E, Saidpour A. The beneficial effects of sumac (Rhus coriaria L.) supplementation along with restricted calorie diet on anthropometric indices, oxidative stress, and inflammation in overweight or obese women with depression: a randomized clinical trial. Phyther Res. 2020;34(11):3041–3051. doi:10.1002/ptr.6737
74. Shabbir A. Rhus coriaria Linn, a plant of medicinal, nutritional and industrial importance: a review. J Anim Plant Sci. 2012;22(2):505–512.
75. Rahideh ST, Shidfar F, Khandozi N, Rajab A, Hosseini SP, Mirtaher SM. The effect of sumac (Rhus coriaria L.) powder on insulin resistance, malondialdehyde, high sensitive C-reactive protein and paraoxonase 1 activity in type 2 diabetic patients. J Res Med Sci. 2014;19(10):933–938.
76. Bitarafan S, Saboor-yaraghi A, Sahraian M, Soltani D, Nafissi S. Effect of vitamin A supplementation on fatigue and depression in multiple sclerosis patients. Iran J Allergy Asthma Immunol. 2016;15:13–19.
77. Jafarirad S, Siassi F, Harirchian MH, et al. The effect of vitamin A supplementation on stimulated T-cell proliferation with myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. J Neurosci Rural Pract. 2012;3(3):294–298. doi:10.4103/0976-3147.102609
78. Sangle P, Sandhu O, Aftab Z, Anthony AT, Khan S. Vitamin B12 supplementation: preventing onset and improving prognosis of depression. Cureus. 2020;12(10):1–7. doi:10.7759/cureus.11169
79. Solati Z, Jazayeri S, Tehrani-Doost M, Mahmoodianfard S, Gohari MR. Zinc monotherapy increases serum brain derived neurotrophic factor (BDNF) levels and decreases depressive symptoms in overweight or obese subjects: a double-blind, randomized, placebo-controlled trial. Nutr Neurosci. 2015;18(4):162–168. doi:10.1179/1476830513Y.0000000105
80. Ranjbar E, Shams J, Sabetkasaei M, et al. Effects of zinc supplementation on efficacy of antidepressant therapy, inflammatory cytokines, and brain-derived neurotrophic factor in patients with major depression. Nutr Neurosci. 2014;17(2):65–71. doi:10.1179/1476830513y.0000000066
81. Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158(1):126–136. doi:10.1016/j.neuroscience.2008.01.061
82. Amr M, El-Mogy A, Shams T, Vieira K, Lakhan S. Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder. Clin Nutr. 2013;33–50. doi:10.1201/b16308-5
83. Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23(5):209–216. doi:10.1016/S0166-2236(99)01543-X
84. Papakostas GI. Dopaminergic-based pharmacotherapies for depression. Eur Neuropsychopharmacol. 2006;16(6):391–402. doi:10.1016/j.euroneuro.2005.12.002
85. Gharekhani A, Khatami MR, Dashti-Khavidaki S, et al. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: a randomized, placebo-controlled clinical trial. Eur J Clin Pharmacol. 2014;70(6):655–665. doi:10.1007/s00228-014-1666-1
86. Keshavarz SA, Mostafavi SA, Akhondzadeh S, et al. Omega-3 supplementation effects on body weight and depression among dieter women with co-morbidity of depression and obesity compared with the placebo: a randomized clinical trial. Clin Nutr ESPEN. 2018;25:37–43. doi:10.1016/j.clnesp.2018.03.001
87. Rizzo AM, Corsetto PA, Montorfano G, et al. Comparison between the AA/EPA ratio in depressed and non depressed elderly females: omega-3 fatty acid supplementation correlates with improved symptoms but does not change immunological parameters. Nutr J. 2012;11(1):1–11. doi:10.1186/1475-2891-11-82
88. Jiang W, Whellan DJ, Adams KF, et al. Long-chain omega-3 fatty acid supplements in depressed heart failure patients: results of the OCEAN trial. JACC Hear Fail. 2018;6(10):833–843. doi:10.1016/j.jchf.2018.03.011
89. Vaz S, Farias DR, Rodrigues A, Adegboye A, Nardi AE, Kac G. Omega-3 supplementation from pregnancy to postpartum to prevent depressive symptoms: a randomized placebo- controlled trial. BMC Pregnancy Childbirth.2017;1–13. DOI:10.1186/s12884-017-1365-x
90. Antypa N, Smelt AHM, Strengholt A, Van Der Does AJW. Effects of omega-3 fatty acid supplementation on mood and emotional information processing in recovered depressed individuals. J Psychopharmacol. 2012;26(5):738–743. doi:10.1177/0269881111424928
91. Maltais M, de Souto Barreto P, Pothier K, et al. Lifestyle multidomain intervention, omega-3 supplementation, or both for reducing the risk of developing clinically relevant depressive symptoms in older adults with memory complaints? Secondary analysis from the MAPT trial. Exp Gerontol. 2019;120:28–34. doi:10.1016/j.exger.2019.02.010
92. Giltay E, Geleijnse J, Kromhout D. Effects of n 2 3 fatty acids on depressive symptoms and dispositional. Am J Clin Nutr. 2011;94(6):1442–1450. doi:10.3945/ajcn.111.018259.INTRODUCTION
93. Kuszewski JC, Howe PRC, Wong RHX. An exploratory analysis of changes in mental wellbeing following curcumin and fish oil supplementation in middle-aged and older adults. Nutrients. 2020;12(10):1–13. doi:10.3390/nu12102902
94. Meyer BJ, Grenyer BFS, Crowe T, Owen AJ, Grigonis-Deane EM, Howe PRC. Improvement of major depression is associated with increased erythrocyte DHA. Lipids. 2013;48(9):863–868. doi:10.1007/s11745-013-3801-7
95. Andreeva VA, Galan P, Torrès M, Julia C, Hercberg S, Kesse-Guyot E. Supplementation with B vitamins or N-3 fatty acids and depressive symptoms in cardiovascular disease survivors: ancillary findings from the SUpplementation with FOLate, vitamins B-6 and B-12 and/or OMega-3 fatty acids (SU.FOL.OM3) randomized trial. Am J Clin Nutr. 2012;96(1):208–214. doi:10.3945/ajcn.112.035253
96. Sepehrmanesh Z, Kolahdooz F, Abedi F, et al. Vitamin D supplementation affects the beck depression inventory, insulin resistance, and biomarkers of oxidative stress in patients with major depressive disorder: a randomized. J Nutr.2016;(5):1–6. doi:10.3945/jn.115.218883
97. Vaziri F, Nasiri S, Tavana Z, Dabbaghmanesh MH, Sharif F. A randomized controlled trial of vitamin D supplementation on perinatal depression: in Iranian pregnant mothers. BMC Pregnancy Childbirth. 2016;16:1–12. doi:10.1186/s12884-016-1024-7
98. Kjærgaard M, Waterloo K, Wang CEA, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case – control study and randomised clinical trial. Br J Psychiatry. 2012;25:360–368. doi:10.1192/bjp.bp.111.104349
99. Zheng S, Tu L, Cicuttini F, et al. Effect of vitamin D supplementation on depressive symptoms in patients with knee osteoarthritis. J Am Med Dir Assoc. 2018;20:1634–1640.e1. doi:10.1016/j.jamda.2018.09.006
100. Sharifi A, Vahedi H, Nedjat S. Vitamin D decreases beck depression inventory score in patients with mild to moderate ulcerative colitis: a double-blind randomized placebo- controlled trial vitamin D decreases beck depression inventory score in patients with mild to moderate ulcerative. J Diet Suppl. 2018;0211. DOI:10.1080/19390211.2018.1472168
101. Omidian M, Mahmoudi M, Abshirini M. Diabetes & metabolic syndrome: clinical research & reviews effects of vitamin D supplementation on depressive symptoms in type 2 diabetes mellitus patients: randomized placebo-controlled double- blind clinical trial. Diabetes Metab Syndr Clin Res Rev. 2019;13(4):2375–2380. doi:10.1016/j.dsx.2019.06.011
102. Alavi NM, Khademalhoseini S, Vakili Z, Assarian F. Effect of vitamin D supplementation on depression in elderly patients: a randomized clinical trial. Clin Nutr. 2018;38:2065–2070. doi:10.1016/j.clnu.2018.09.011
103. Hansen JP, Pareek M, Hvolby A, et al. Vitamin D3 supplementation and treatment outcomes in patients with depression (D3-vit-dep). BMC Res Notes. 2019;12(1):1–6. doi:10.1186/s13104-019-4218-z
104. Okereke OI, Iii CFR, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. Jama. 2020;02114(5):471–480. DOI:10.1001/jama.2020.10224
105. De Koning EJ, Lips P, Heijboer AC, Den Heijer M, Bet PM, Van Schoor NM. Vitamin D supplementation for the prevention of depression and poor physical function in older persons: the D-Vitaal study, a randomized clinical trial. Am J Clin Nutr. 2019;110:1119–1130. doi:10.1093/ajcn/nqz141
106. Rolf L, Muris A, Bol Y, Smolders J, Smolders J, Hupperts R. Vitamin D3 supplementation and the IL-2/IL-2R pathway in multiple sclerosis: attenuation of progressive disturbances? J Neuroimmunol. 2017;314:50–57. doi:10.1016/j.jns.2017.04.017
107. Mousa A, Naderpoor N, De Courten MPJ, De Courten B. Vitamin D and symptoms of depression in overweight or obese adults: a cross-sectional study and randomized placebo-controlled trial. J Steroid Biochem Mol Biol. 2017. doi:10.1016/j.jsbmb.2017.08.002
108. Wang Y, Liu Y, Lian Y, Li N, Liu H, Li G. Efficacy of high-dose supplementation with oral vitamin D3 on depressive symptoms in dialysis patients with vitamin D3 insufficiency. Nan Fang Yi Ke da Xue Xue Bao. 2016;36(3):1–7. doi:10.1097/JCP.0000000000000486
109. Moludi J, Alizadeh M, Mohammadzad MHS, Davari M. The effect of probiotic supplementation on depressive symptoms and quality of life in patients after myocardial infarction: results of a preliminary double-blind clinical trial. Psychosom Med. 2019;81(9):770–777. doi:10.1097/PSY.0000000000000749
110. Inoue T, Kobayashi Y, Mori N, et al. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef Microbes. 2018;9(6):843–853. doi:10.3920/BM2017.0193
111. Mohammadi AA, Jazayeri S, Khosravi-Darani K, et al. The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr Neurosci. 2016;19(9):387–395. doi:10.1179/1476830515Y.0000000023
112. Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36(5):1245–1249. doi:10.1016/j.clnu.2016.08.015
113. Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32(3):315–320. doi:10.1016/j.nut.2015.09.003
114. Reininghaus EZ, Platzer M, Kohlhammer-Dohr A, et al. Provit: supplementary probiotic treatment and vitamin b7 in depression—a randomized controlled trial. Nutrients. 2020;12(11):1–17. doi:10.3390/nu12113422
115. Bot M, Brouwer IA, Roca M, et al. Effect of multinutrient supplementation and food-related behavioral activation therapy on prevention of major depressive disorder among overweight or obese adults with subsyndromal depressive symptoms: the MooDFOOD randomized clinical trial. JAMA. 2019;321(9):858–868. doi:10.1001/jama.2019.0556
116. Liu QS, Deng R, Fan Y, et al. Low dose of caffeine enhances the efficacy of antidepressants in major depressive disorder and the underlying neural substrates. Mol Nutr Food Res. 2017;61(8):1–43. doi:10.1002/mnfr.201600910
117. Shabani A, Foroozanfard F, Kavossian E, et al. Effects of melatonin administration on mental health parameters, metabolic and genetic profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Affect Disord. 2019;250(November2018):51–56. doi:10.1016/j.jad.2019.02.066
118. de Koning EJ, van der Zwaluw NL, van Wijngaarden JP, et al. Effects of two-year vitamin B12 and folic acid supplementation on depressive symptoms and quality of life in older adults with elevated homocysteine concentrations: additional results from the B-proof study, an RCT. Nutrients. 2016;8(11):748. doi:10.3390/nu8110748
119. Bochyńska A, Lipczyńska-ŁOjkowska W, Gugała-Iwaniuk M, et al. The effect of vitamin B supplementation on homocysteine metabolism and clinical state of patients with chronic epilepsy treated with carbamazepine and valproic acid. Seizure. 2012;21(4):276–281. doi:10.1016/j.seizure.2012.01.013
120. Loria-Kohen V, Gómez-Candela C, Palma-Milla S, Amador-Sastre B, Hernanz A, Bermejo LM. Estudio piloto sobre el efecto de la suplementación con ácido fólico en la mejor de los niveles de homocisteína, función cognitiva y estado depresivo en trastornos de la conducta alimentaria [A pilot study of folic acid supplementation for improving homocysteine levels, cognitive, and depressive status in eating disorders]. Nutr Hosp. 2013;28(3):807–815. Spanish. doi:10.3305/nh.2013.28.3.6335
121. National Institutes of Health. Omega-3 fatty acids; 2021. Available from: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/#h8.
122. Forester B, Zuo C, Ravichandran C, et al. Coenzyme Q10 effects on creatine kinase activity and mood in geriatric bipolar depression. J Geriatr Psychiatry Neurol. 2012;25(1):1–16. doi:10.1177/0891988712436688
123. National Institutes of Health. Nutrient recommendations: Dietary Reference Intakes (DRI); 2022. Available from: https://ods.od.nih.gov/HealthInformation/Dietary_Reference_Intakes.aspx.
124. Centers for Disease Control and Prevention (CDC). Folic acid safety, interactions, and effects on other outcomes; 2017. Available from: https://www.cdc.gov/ncbddd/folicacid/faqs/faqs-safety.html.
125. Institute of Medicine Panel on Micronutrients. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK222309/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Vitamin D Deficiency Participates in Depression of Patients with Diabetic Peripheral Neuropathy by Regulating the Expression of Pro-Inflammatory Cytokines
Zhou J, Li D, Wang Y
Neuropsychiatric Disease and Treatment 2024, 20:389-397
Published Date: 27 February 2024
