Back to Journals » Drug Design, Development and Therapy » Volume 16
Dietary Supplement of Anoectochilus roxburghii (Wall.) Lindl. Polysaccharides Ameliorates Cognitive Dysfunction Induced by High Fat Diet via “Gut-Brain” Axis
Authors Fu L, Zhu W, Tian D, Tang Y, Ye Y, Wei Q, Zhang C, Qiu W, Qin D, Yang X, Huang Y
Received 14 January 2022
Accepted for publication 12 June 2022
Published 20 June 2022 Volume 2022:16 Pages 1931—1945
DOI https://doi.org/10.2147/DDDT.S356934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Liya Fu,1,2,* Wanlong Zhu,1,2,* Dongmei Tian,1,2 Yong Tang,2,3 Yun Ye,1 Qiming Wei,1 Chengbin Zhang,1 Wenqiao Qiu,2 Dalian Qin,2 Xuping Yang,1,2 Yilan Huang1,2
1Department of Pharmacy, The Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China; 2School of Pharmacy, Southwest Medical University, Luzhou, People’s Republic of China; 3State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Macau, 999078, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xuping Yang; Yilan Huang, Department of Pharmacy, The Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Anoectochilus roxburghii (Wall.) Lindl. polysaccharides (ARPs) have been reported to exhibit multiple pharmacological activities including anti-inflammatory and anti-hyperglycemia. This study aims to investigate the effect of ARPs on cognitive dysfunction induced by high fat diet (HFD).
Methods: Six-week-old male mice were treated with ARPs by dietary supplementation for 14 weeks. The effect of ARPs on cognitive function was determined by assessing the changes in spatial learning and memory ability, neurotrophic factors in hippocampus, inflammatory parameters, intestinal barrier integrity, and gut microbiota.
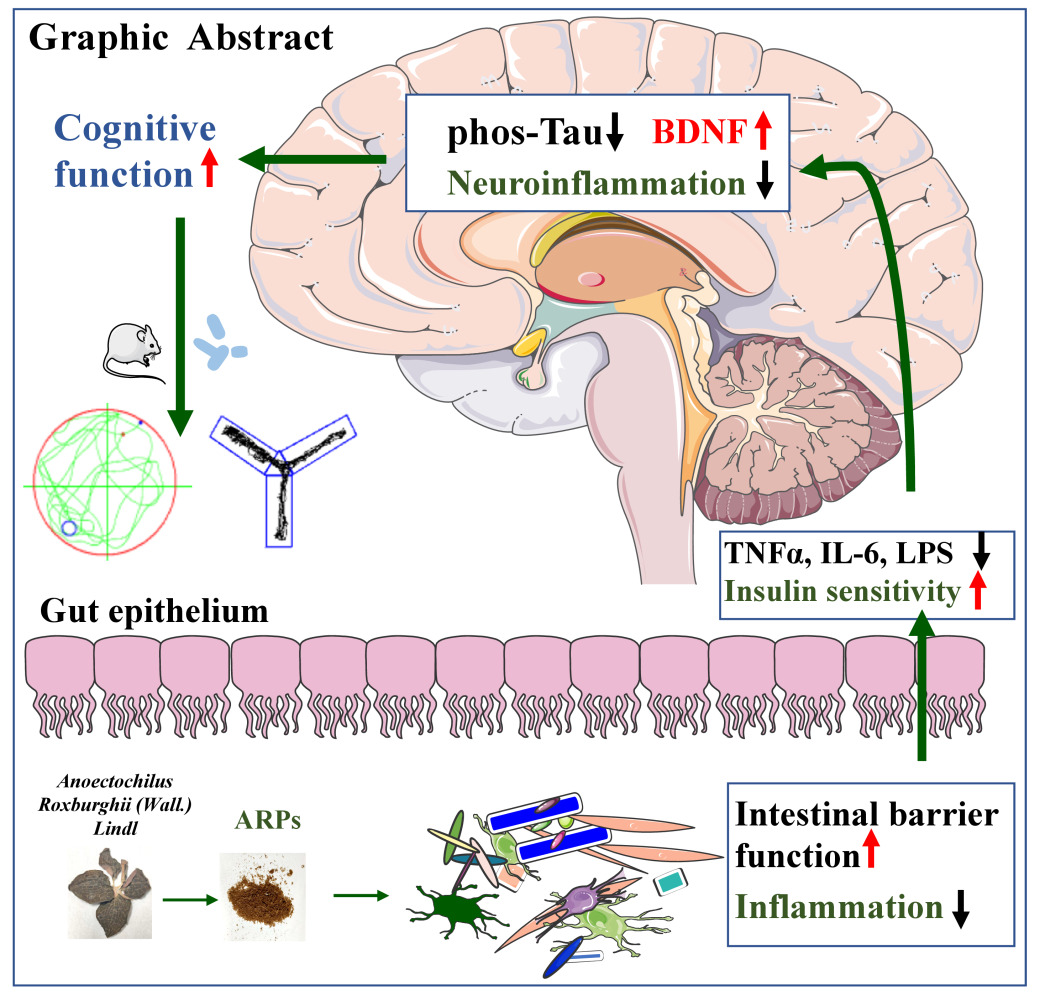
Results: ARPs supplementation can effectively ameliorate cognitive dysfunction, decrease the phosphorylation levels of Tau protein in hippocampus. Meanwhile, the increased body weight, plasma glucose, total cholesterol, inflammatory factors induced by HFD were abolished by ARPs treatment. Furthermore, ARPs treatment restored the intestinal epithelial barrier as evidenced by upregulation of intestinal tight junction proteins. Additionally, ARPs supplementation significantly decreased the relative abundance of several bacteria genus such as Parabacteroides, which may play regulatory roles in cognitive function.
Conclusion: These results suggest that ARPs might be a promising strategy for the treatment of cognitive dysfunction induced by HFD. Mechanistically, alleviation of cognitive dysfunction by ARPs might be associated with the “gut-brain” axis.
Keywords: Anoectochilus roxburghii (Wall.) Lindl. polysaccharides, diet-induced-obesity, cognitive dysfunction, gut microbiota, “gut-brain” axis
Graphical Abstract:
Introduction
Cognitive dysfunction is a syndrome manifested primarily by impairment of learning and memory functions.1 Numerous researches suggest obesity or over-weight are associated with deficits in learning, memory, executive function as well as potential brain atrophy.2–4 Inflammation is considered to be involved in the pathophysiology of cognitive impairment and dementia, which is associated with the neuropathological features of Alzheimer’s disease.5 Multiple studies including obese subjects have shown that systemic inflammation is positively associated with symptoms of cognitive decline.6–8 Neuroinflammation promotes central insulin resistance, which ultimately leads to hyperphosphorylation of Tau protein, inducing hippocampal neuronal apoptosis.9 Thus, ameliorating obesity and the related inflammation may have a beneficial impact on cognitive function.
It is well documented that changes in intestinal microbiota can modulate the peripheral and central nervous systems, influencing brain function via a pathway called “gut-brain” axis.4,10,11 A review by Chang et al reported that the conversion of L-glutamate to D-glutamate by Bacteroides may affect cognitive function in Alzheimer’s disease, which supports the importance of intestinal bacteria metabolism on cognitive function and memory.12 Meanwhile, metabolic diseases such as type 2 diabetes or fatty liver disease are closely related to the gut microflora dysbiosis.13 Long-term high-fat diet not only promotes changes in the composition of intestinal microflora and metabolites, but also leads to extensive accumulation of lipids in intestinal cells, thus inducing oxidative stress and damaged intestinal barrier.10 In obese individuals, large quantities of lipopolysaccharides (LPS) in intestinal enter the blood circulation and stimulate the secretion of pro-inflammatory factors, which trigger or intensify neuroinflammation and aggravate cognitive dysfunction.5,14
It has been shown that some herbal prescriptions and monomers such as ginkgo biloba preparations and Luteolin can protect against cognitive deficits in obese mice.15–17 Anoectochilus roxburghii (Wall.) Lindl. is a traditional Chinese medicine. Anoectochilus roxburghii (Wall.) Lindl. polysaccharides (ARPs), acts as one of the main components, has multiple pharmacological activities including anti-oxidant, anti-inflammation, anti-hyperglycemia and anti-hyperlipidemia.18,19 Liu et al found that ARPs improve obesity-associated inflammation by regulating the p38 mitogen-activated protein kinase (p38/MAPK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways.20 Moreover, Wang et al showed that Anoectochilus roxburghii (Wall.) Lindl. extract significantly delayed aging in a D-galactose-induced dementia model.21 Another study showed that ARPs lowered the pH value in the cecum and increased its content of probiotic bifidobacteria.22 Therefore, we speculate that ARPs may ameliorate inflammation and cognitive dysfunction induced by HFD. In this study, we constructed a diet-induced-obese mouse model and investigated the effects of ARPs on cognitive function and the related mechanisms.
Materials and Methods
ARPs Extraction
Anoectochilus roxburghii (Wall.) Lindl. (60 mesh) was purchased from Sichuan Tianzhi Co., Ltd. The extraction was performed as described in Zeng’s study.19 In brief, Anoectochilus roxburghii (Wall.) Lindl. powder was extracted with distilled water (1:50, w/v) at 70 °C for 1.5 hrs. After suction, the aqueous extracts were concentrated to 1/10 of the initial volume with a rotary vacuum evaporator (RE-5210A, Shanghai Yarong Co., China) at 54°C. Then, 95% ethanol was added to the concentrated supernatant and this solution was kept at 4°C for 24 hrs, ARPs were collected by centrifugation (12,000 rpm for 8 min, 20°C). The content of polysaccharide was measured by phenol-sulfuric acid method (sulfuric: phenol: polysaccharide = 5:1:2). Sulfuric acids (95%) were added and placed the samples in water bath at 100°C for 10 min, absorbance was measured at 490 nm. Finally, the precipitates were washed with distilled water and freeze dried for further experiment. The ARPs components was identified by using High-performance liquid chromatography (HPLC, Agilent, Palo Alto, CA, USA), and separation was accomplished using a Shiseido C18 column (4.6 mm × 250 mm, 5 µm pore size). Molecular weight of each component was determined by gel permeation chromatography (GPC) on the chromatograph Viscotek GPCmax VE2001 (Malvern Instruments, UK).
Animal and Experimental Design
Six-week-old male C57BL/6J mice purchased from HFK Biological Technology Co. Ltd were randomly divided into predefined groups (n = 6 per group). All mice were housed in environmentally controlled animal rooms (23 ± 2°C, humidity 50%, range 30–70%) under a 12/12‐hr day/night cycle and had access to food and water ad libitum. For treatments, ARPs were mixed with powdered high-fat diet (HFD, D12492, 60 kcal% fat). HFD supplemented with ARPs 1 mg/g and 3 mg/g (w/w) were used as low-dosage and high-dosage group separately, while HFD supplemented with Metformin (Meilunbio®, Dalian, China, 2 mg/g, w/w) was used as positive control group. Body weight, water intake and food intake were measured every week. Glucose tolerance test and insulin tolerance test were measured at 10 weeks, learning and memory ability were evaluated at 12 weeks. Mice at 14 weeks were euthanized with pentobarbital (100 mg/kg, intraperitoneal injection), the hippocampus and colon were dissected rapidly. All experiments involving animals were approved by the Animal Ethics Committee of the Affiliated Hospital of Southwest Medical University.
Morris Water Maze (MWM) Test
The water maze apparatus consisted of a round black pool (120 cm in diameter, 60 cm in height) containing Titanium oxide-mixed water (23–25°C) and a platform (10 cm in diameter) submerged 1.0 cm under the water. The pool was divided into four quadrants equally named I, II, III, and IV, respectively. A black curtain was arranged around the pool to isolate interference from irrelevant visual cues, and four cues of assorted colours and shapes were fixed on the curtain opposite each quadrant as the only way for the mice to discriminate their position. During the training trial, mice were allowed to swim in the water while searching for the hidden platform for 90s, and they were allowed to stay on it for 10s upon finding the platform. Mice that failed to locate the platform in 90s were guided to it, and then returned to their cage. The mice were trained twice per day at intervals of 60 min over five consecutive days. Twenty-four hrs after the last training trial, the mice were tested for memory retention in a probe trial in the absence of the platform. The mice were allowed to swim in the pool for 90s, and the time spent in the target quadrant where the platform had been fixed during the acquisition test was recorded. The time spent in the target quadrant, and the number of platform crossing times were monitored using a video camera (Sony, Tokyo, Japan) mounted over the maze and automatically recorded via a video tracking system.
Y Maze Test
Y maze test was performed as described in Walrave’s study.23 Briefly, the Y-maze apparatus consisted of three arms (30-cm long and 6-cm wide with 15-cm high walls) separated by an angle of 120°. The maze walls were constructed from dark black, polyvinyl plastic. The test consisted of two trials separated by an intertrial interval (2 hrs). Using a ceiling-mounted charge-coupled device camera, all trials were analyzed for the number of entries the mice made into each arm. (1) Spontaneous alternation behavior test: mice were placed in the Y-maze individually and allowed to freely explore the three arms for 6 min. The spontaneous alternation percentage (%) was used as a measure of spatial working memory. Spontaneous alternation percentage (%) = [number of alternations/total arm entries - 2] × 100%. (2) Spatial novelty recognition test (used as a measure for spatial short-term memory): mice were placed in the start arm of the maze facing the terminal and allowed to explore two arms 10 min (the start arm and familiar arm) with the novel arm being blocked. Next, mice were placed back in the maze in the same starting arm, with free access to all three arms for 5 min. Resting time and distance in novel arm zone were recorded for each mouse.
Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
For GTT analysis, mice were injected intraperitoneally with glucose (1 g/kg) after an overnight fasting. Blood glucose levels were measured at 0, 15, 30, 60, 90 and 120 min. For ITT analysis, after 4 hrs of fasting, mice were injected intraperitoneally with insulin (0.5 U/kg), blood glucose levels were measured at 0, 15, 30, 60, 90 and 120 min.
Enzyme-Linked Immunosorbent Assay (ELISA)
According to the manufacturer’s instructions, commercial ELISA kit was used to determine the concentration level of serum LPS (Cloud-Clone Corp, Katy, TX, USA) and TNF- α (R&D systems, Minneapolis, USA).
Hematoxylin-Eosin (H&E) Staining
Hippocampus and colon samples were dissected and fixed in 4% paraformaldehyde for 24 hrs, then the tissue was dehydrated and buried in paraffin. Tissue wax was cut into slices with a thickness of about 5 –30 μm (colon section, 5 μm; brain serial sagittal section, 30 μm). Each section was dewaxed, rehydrated and stained with hematoxylin and eosin. Tissue morphology was observed using a microscope (Leica, Milano, Italy) equipped with DFC350FX digital camera (Leica).
Immunohistochemistry
Sections (colon section, 5 μm; brain serial sagittal section, 30 μm) were treated for 20 min with 0.6% H2O2 in PBS, permeabilised in PBS-Triton X-100 0.25% (PBS-Tx) for 30 min and blocked for 1 hr with 10% fetal bovine serum (FBS) in PBST×0.1%. TNFα (1:500 dilution, Abcam, ab34674) and ZO-1 (1:500 dilution, Abcam, ab59720) were incubated overnight at 4°C, followed by secondary antibodies (1:500 dilution) for 1hr at room temperature. Positive signals were visualized using 3,3-diaminobenzidine (DAB) (Sigma-Aldrich, Saint Louis, MO, USA). Images were acquired with DM2500 microscope (Leica, Milano, Italy) equipped with DFC350FX digital camera (Leica).
Immunofluorescence
Sections (brain serial sagittal section, 30 μm) were permeabilized in 1×PBS containing 0.25% Triton X-100 (PBS-TX) for 10 min, blocked with PBS-TX containing 10% FBS/1% bovine serum albumin/PBSTx 0.1% for 30 min at room temperature. After incubation with primary antibodies (anti-BDNF, ab108319, Abcam, 1:500) overnight, the sections were washed with PBS and incubated with the appropriate fluorescent secondary antibodies (1:500, Alexa Fluor; Invitrogen) at room temperature for 1 hr. Confocal imaging was performed with a Leica TCS SP5 laser scanning confocal microscope (Leica, Milano, Italy). Image J software was used to quantify the intensity of fluorescent signals of each image (Image J function “Analyze > measure.”).
16S rDNA Sequencing
Following the manufacturer’s instructions, QIAamp DNA fecal Mini Kit 206 was used to extract total DNA from mouse fecal samples. Amplification of V3-V4 region of bacterial 16s rRNA gene by PCR, and extracted from 2% agarose gel, then purified by VAHTS DNA Clean reads. OTU clustering (software: UPARSE), taxonomic assignment (software: RDP classifier), α-diversity (Shannon), and β-diversity (Bray Curtis) analyses (software: QIIME) were conducted. Analysis was performed by using R software and parametric test and non-parametric test were conducted, respectively. The Tukey’s test and the Wilcox test of the agricolae package were used for statistical analysis.
Quantitative Real-Time Polymerase Chain Reaction Analyses (RT-PCR)
Total mRNA was isolated from the hippocampus or colon tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). 1 μg RNA was then reverse transcribed with the QuantiTect® Reverse Transcription Kit (Takara Ltd., Otsu, Japan) according to the manufacturer’s protocol. Quantitative Realtime PCR was performed using SYBR Green Realtime PCR Master Mix (Bio-Rad, Hercules, CA, USA) to detect mRNA expression levels of target genes. The PCR reaction cycle program was as follows: 3 min of initial denaturation at 95°C, then 40 cycles at 95°C for 30 sec, 50°C for 30 sec, 68°C for 1 min 30 sec. All primer sequences are listed in Supplementary Table 1.
Western-Blot Analysis
Western blotting analyses of tissues were performed as described.24 Briefly, 1 mL 1×loading buffer was used to extract the total proteins of hippocampus, and the concentration was measured by using a Bradford Protein Assay kit. Equal amounts of proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% polyacrylamide gel and transferred to PVDF membranes (Millipore, Billerica, MA). The membrane was incubated with primary antibody (anti-BDNF, Abcam, 1:2000; anti-Tau, HuaAn, 1:5000; anti-phos-Tau, HuaAn, 1:1000; anti-GAPDH, HuaAn, 1:10,000) overnight at 4°C. Then, the membrane was incubated with the corresponding secondary antibodies for 1 hr at room temperature. Band visualization was performed using an ultra-sensitive chemiluminescence (ECL) detection kit.
Statistical Analysis
Statistical analysis and graphing were performed using GraphPad Prism 8.4.2. Quantile-Quantile plots were used to assess data distribution for all variables. A detailed breakdown of the results is shown in Supplementary Materials. Statistical significance was calculated using two-tailed Student’s t-test for comparisons between two groups and one-way ANOVA followed by post-hoc test adjustments using Bonferroni correction for comparisons among multiple groups. Post-hoc tests were run only if F achieved p< 0.05 and there was no significant variance inhomogeneity. Data are presented as mean ± SEM, p<0.05 was considered statistically significant.
Results
ARPs Ameliorated Memory Impairments Induced by HFD
The monosaccharide composition in ARPs was analyzed by HPLC. Five major monosaccharides including arabinose, galactaric acid, mannose, glucose and galactose were identified in polysaccharides, which further confirmed that ARPs were heteropolysaccharides (Supplementary Figure 1 and Supplementary Table 2).
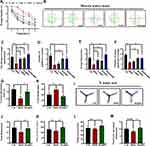
Spatial learning and reference memory ability were often investigated using the MWM test.25 As shown in Figure 1A, compared with HFD, mice in ARPs group rapidly learned and memorized the location of the platform in the target quadrant, exhibited a significant decrease in the escape latency during the acquisition phase (Figure 1A). Next, a probe trial was conducted by removing the platform. Mice in the ARPs-treated group spent a significantly more time swimming in the target quadrant than mice under HFD, and exhibited greater numbers of platform crossings, and these effects presented as a dose-dependent manner (Figure 1B–F). Moreover, we evaluated the effect of ARPs on short-term spatial memory using the Y-maze test. HFD resulted in markedly decreased ratio of spontaneous alternation behavior compared with that of the CD mice, and these behaviors were partly recovered by administration of ARPs (Figure 1G–J). Meanwhile, in spatial novelty recognition test, the resting time and distance in novel arms were only slightly reduced in mice fed with HFD compared with that of CD mice, with no statistical significance. However, ARPs treatment led to a significant increase in these parameters compared with HFD mice (Figure 1K–M). The above results suggest that dietary supplementation of ARPs effectively recovers the impairment of learning and memory in obese mice induced by HFD.
ARPs Attenuated Glucose and Lipid Metabolic Disorders Induced by HFD
In order to investigated whether ARPs can regulate glucose and lipid homeostasis, we next investigated the metabolic consequences of these mice. As shown in Figure 2A, mice treated with ARPs protected mice from diet-induced obesity (Figure 2A). Compared with mice in HFD group, the ARPs-treated mice exhibited markedly decreased serum glucose and total cholesterol (TC) (Figure 2B–D). To observe changes in insulin sensitivity, we performed GTT and ITT analysis, mice on HFD showed higher insulin resistance and glucose intolerance compared with that in CD, while ARPs administration reversed these impairments (Figure 2E and F). All these data suggest that ARPs effectively alleviate obesity, hyperglycemia and hypercholesterolemia caused by HFD.
ARPs Modulated Regulators Involved in Cognitive Function in Hippocampus
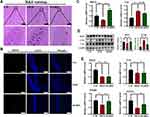
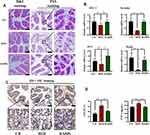
Based on the observation that BDNF is essential in maintaining neural cell survival and promoting neurite outgrowth, synaptogenesis, memory and learning,26 while the microtubule-associated protein Tau is a key molecule in the pathogenesis of Alzheimer’s disease.27 We next performed experiments to determine whether ARPs modulate regulators involved in cognitive function. As shown in H&E staining, the neurons in hippocampus from CD mice displayed regular appearance with large and round nuclei but densely stained, and distorted cells with pyknotic appearance were observed in HFD group, while ARPs treatment effectively restored these pathological changes (Figure 3A). Furthermore, we examined the expression of BDNF by immunofluorescence and observed an increase in hippocampus from ARPs-treated mice in comparison with mice on HFD (Figure 3B and Supplementary Figure 2). Consistently, RT-PCR and Western-blot analysis of BDNF in hippocampus also reached the similar result (Figure 3C and D). Meanwhile, ARPs administration significantly decreased the phosphorylation of Tau protein (the main component of amyloid plaques) (Figure 3D). Moreover, the mRNA expression of postsynaptic density protein-95 (PSD95, an important postsynaptic scaffolding protein) and fibroblast growth factor-21 (FGF21) in hippocampus were upregulated by ARPs compared with mice on HFD (Figure 3E). Taken together, these results suggest that ARPs promote the survival of neuronal cells and improve cognitive impairment.
ARPs Attenuated HFD-Induced Neuroinflammation in Hippocampus
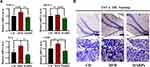
Inflammation is considered to be the most important pathophysiological mechanism of cognitive disorders, and Tumor necrosis factor-α (TNF-α) is reported to be closely associated with these impairments.28 In this study, compared with CD group, mRNA expression of TNF-α, interleukin-6 (IL-6) and mouse EGF-like module-containing mucin-like hormone receptor-like 1 (F4/80) in hippocampus were increased in mice on HFD, while ARPs effectively abolished these effects (Figure 4A). Meanwhile, immunohistochemistry analysis showed that mice on HFD showed abnormal increased TNF- α levels in hippocampus, and this abnormal expression was improved by ARPs (Figure 4B). These data demonstrate that dietary supplementation of ARPs ameliorates hippocampal inflammation caused by HFD.
ARPs Attenuated HFD-Induced Inflammation in Colon
It is well documented that gut microorganisms and metabolites can stimulate inflammatory cytokines production and secretion, which triggers or exacerbates central inflammation and cognitive dysfunction.5 In this study, RT-PCR analysis revealed that increased mRNA expression of TNF- α, MCP-1, IL-6 and F4/80 in colon were increased by HFD, and dietary supplementation of ARPs effectively reversed this trend (Figure 5A). Meanwhile, immune-histochemistry results showed that supplementation of ARPs attenuated the abnormal increase of TNF-α in colon from mice on HFD (Figure 5B). Our findings demonstrate that ARPs can effectively improve HFD-induced inflammation in colon.
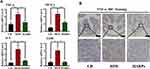
ARPs Ameliorated Impaired Intestinal Barrier Induced by HFD
The integrity of intestinal epithelium is considered to be the first defense line of the gastrointestinal tract. Related studies have shown that long-term HFD can destroy the integrity of intestinal barrier and cause intestinal leakage.29 In this study, H&E staining results demonstrated that inflammatory cell infiltrate in the lamina propria of the colon from HFD mice, PAS staining further showed that the number of mucus-secreting goblet cells, intestinal wall thickness and villus height were decreased by HFD, ARPs significantly improved these detrimental effects (Figure 6A). By performing RT-PCR analysis, we detected the gene expression of the main components of intestinal mucus layer Mucoprotein-2 (Muc‐2), and no significant differences were observed between groups. However, the expression of key tight junction proteins such as Occludin and Zonula Occludens-1 (ZO-1) in colon were greatly increased by ARPs compared with mice on HFD (Figure 6B). Additionally, the expression of tight junction protein zonula occludens-1 (ZO-1) in colon from HFD mice was down-regulated (Figure 6C). Moreover, ARPs treatment also reversed the decreased mRNA levels of recombinant regenerating islet-derived protein 3 gamma (Reg3g, an antimicrobial molecule highly expressed in the intestine) in HFD (Figure 6B). It has been reported that HFD induces intestinal microbial disorders and enhance LPS production, which ultimately causing or aggravating systemic inflammation.30 Our ELISA analysis revealed that increased serum LPS and TNF-α levels induced by HFD were partly abolished by ARPs treatment (Figure 6D). The above results suggest that dietary supplementation of ARPs can improve the integrity of intestinal barrier induced by HFD.
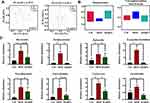
ARPs Reshaped the Gut Microbiome and Ameliorated HFD-Induced Intestinal Microbial Disorders
It has been suggested that gut microbiota structure can influence plasticity and cognitive function of brain.31 In this study, 16S rDNA sequencing analysis was used to detect whether ARPs could affect the intestinal microflora. Firstly, principal component analysis (PCA) showed different clustering pattern in the structure of intestinal microbiota among the three groups (Figure 7A), and α-diversity and β-diversity of the microbiota from mice treated by ARPs were similar to mice on CD (Figure 7B). In order to further study the specific changes of bacterial communities, we compared the relative abundance of dominant phyla and genera in the three groups, especially those that responded to ARPs. The results showed that compared with mice on CD, HFD led to a significant increase in the abundance of several bacterial genus including Parabacteroides, Erysipelatoclostridium, Faecalibaculum, Enterorhabdus and Lactococcus, while ARPs supplementation restored these abnormities (Figure 7C).
Discussion
Long-term high-fat diet has been shown to lead to central nervous system inflammation and damage of brain tissue, and eventually develop to neurodegenerative brain diseases such as Alzheimer’s disease.32 ARPs, one of the main components of Anoectochilus roxburghii (Wall.) Lindl., has been reported to improve glucolipid metabolism and obesity-related complications. Our previous study has found that dietary supplementation of ARPs protects mice against HFD-induced weight gain and hyperlipidemia.24 Zeng et al discovered that ARPs administration for 14 days possessed hepatoprotective effect against CCl4-induced hepatotoxicity in mice and the action might in part be through reducing oxidative stress, inflammation and apoptosis.33 This study demonstrated that ARPs supplementation can effectively ameliorate cognitive dysfunction of HFD-induced obese mice. Mechanistically, these beneficial effects were associated with the alleviation of disorders of glucolipid metabolism, neuroinflammation and impaired intestinal function.
Cognitive ability and memory are influenced by neurotrophic factors including BDNF, which is primarily expressed in the central nervous system. Its biological effects include protection against neuronal injury and maintenance of neuronal survival and physiological function in the mature central and peripheral nervous systems.26 Wang et al reported that BDNF levels were significantly reduced in hippocampus of HFD-induced obese mice, which was associated with the induction of cognitive impairment.34 Additionally, accumulation of hyperphosphorylated tau proteins positively correlates with cognitive dysfunction in Alzheimer’s disease. Reduction of Tau protein prevented neuronal loss and reversed pathological deposition.35 In this study, dietary supplementation with ARPs increased the expression of BDNF and decreased Tau protein phosphorylations in the hippocampus of obese mice, which may partly account for the improved cognitive function.
As previous study pointed, ARPs is a heteropolysaccharide with an average molecular weight of about 22,235~66,004 Da, mainly composed of arabinose, mannose, glucose and galactose, most of which have high molecular weight and polarity.36 However, the blood-brain barrier (BBB) restricts the passage of molecules larger than 500Da,37 Thus, we speculated that ARPs is hard to pass through the BBB and act directly on the central nervous system. It is well documented that HFD can promote inflammation and insulin resistance in the whole body including the brain, thus damaging hippocampal neurons and synapses and causing cognitive dysfunction.38 Moreover, HFD induces gut microbiota disorders and increases the LPS production, which further aggravated the inflammation and the consequent cognitive dysfunction.39 Our research has revealed the positive role of ARPs in improving the impaired intestinal barrier and microbiota disorders, accompanied by decreased LPS and TNF-α levels. Therefore, we conclude that the alleviation of ARPs on cognitive impairment induced by obesity might be associated with the “gut-brain” axis.
Previous studies have shown that the gut microbiota can influence brain plasticity and cognitive function.6,40 Huang et al found that Bacteroides paracasei can modulate age-related cognitive decline by altering communication in the gut-brain axis.41 In this study, 16S rDNA sequencing analysis confirmed that dietary supplementation with ARPs reduced the relative abundance of certain taxa. Lactobacillus and Parabacteroides have been considered as potential beneficial bacteria in some researches.42,43 However, these species display some degree of pathogenicity under particular conditions. A recent study found that cognitive impairment can affect the relative abundance of Parabacteroides, which has been regarded as a risk indicator of mild cognitive impairment.44 Considering that the relationship between gut microbiota and cognitive function is complicated, more studies should be carried out to understand the underlying mechanism of ARPs on cognitive dysfunction induced by HFD.
These studies have several potential limitations. First, our study suggested that ARPs show benefits on cognitive dysfunction in obese mice induced by HFD, whether these benefits of ARPs exist in other animal models such as diabetic mice or APP/PS1 mice remains to be determined. Second, our study only investigated the changes in bacterial abundance after ARPs treatment, and the flora-metabolites are also proved to play an important regulatory role in neuroinflammation and cognitive function.12 Therefore, the changes of microflora products and their regulatory role need to be further studied. Finally, ARPs has been previously shown to ameliorate oxidative stress,24,33 which is a critical factor involved in the intestinal barrier disruption,45 whether ARPs can directly act in the cells of intestinal wall needs to be confirmed via in vitro experiments.
Conclusion
In summary, ARPs ameliorated memory and cognitive impairment in obese mice by improving neuroinflammation induced by HFD. Meanwhile, ARPs supplementation restored intestinal barrier integrity and balanced gut microbial dysbiosis. The results confirmed the potential effect of ARPs on ameliorating cognitive impairment and provided a theoretical basis for the development of functional herbal extracts.
Abbreviations
ARPs, Anoectochilus roxburghii (Wall.) Lindl. polysaccharides; HFD, high-fat diet; CD, chow diet; HARPs, HFD supplemented with Anoectochilus roxburghii (Wall.) Lindl. polysaccharides; MWM, Morris water maze; GTT, glucose tolerance test; ITT, insulin tolerance test; ELISA, enzyme-linked immunosorbent assay; H&E, hematoxylin-eosin; RT-PCR, quantitative real-time polymerase chain reaction analyses; TG, triglyceride; TC, total cholesterol; PAS, periodic acid-Schiff; PCA, principal component analysis; LPS, lipopolysaccharide; MUC-2, mucoprotein-2; Reg3g, recombinant regenerating islet-derived protein 3 gamma; ZO-1, Zonula Occludens-1; TNF-α, tumor necrosis factor-α; MCP-1, monocyte chemoattractant protein-1; IL-6, interleukin-6; BDNF, brain-derived neurotrophic factor; NQO1, quinone oxidoreductase-1; NT3, neurotrophin-3; PSD95, postsynaptic density protein-95; F4/80, mouse EGF-like module-containing mucin-like hormone receptor-like 1; FGF21, fibroblast growth factor-21; BBB, blood-brain barrier.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. We have uploaded the 16S rDNA raw data on the NCBI database, and the accession number is PRJNA754503. The link is as follows: https://submit.ncbi.nlm.nih.gov/subs/.
Ethics Approval
All the experimental protocols were approved by the Animal Experimental Ethical Committee, the Affiliated Hospital of Southwest Medical University (Ref No.20210223-078). All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Acknowledgment
All authors acknowledge the support received from Dr. Yuanzhi Liu (the Laboratory of Traditional Chinese Medicine and Laboratory of Pharmacological, Southwest Medical University).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by research funding from the Science and Technology Planning Project of Sichuan Province (No.2019YFS0180 to H.Y.), Doctoral Research Initiation Fund of Affiliated Hospital of Southwest Medical University (No.21032 to Y.X.), the Collaborative Project of Luzhou Government and Southwest Medical University (2018LZXNYD-PT02 to Y.Y.), and The Foundation for Young Scholars of Southwest Medical University (2019-ZQN-125, 2020-ZQN-151).
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101(40):14515–14520. doi:10.1073/pnas.0406344101
2. Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer’s disease. J Alzheimers Dis. 2009;16(4):693–704. doi:10.3233/jad-2009-1022
3. West RK, Livny A, Ravona-Springer R, et al. Higher BMI is associated with smaller regional brain volume in older adults with type 2 diabetes. Diabetologia. 2020;63(11):2446–2451. doi:10.1007/s00125-020-05264-8
4. Chunchai T, Thunapong W, Yasom S, et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J Neuroinflammation. 2018;15(1):11. doi:10.1186/s12974-018-1055-2
5. Solas M, Milagro FI, Ramírez MJ, et al. Inflammation and gut-brain axis link obesity to cognitive dysfunction: plausible pharmacological interventions. Curr Opin Pharmacol. 2017;37:87–92. doi:10.1016/j.coph.2017.10.005
6. Agustí A, García-Pardo MP, López-Almela I, et al. Interplay between the gut-brain axis, obesity and cognitive function. Front Neurosci. 2018;12:155. doi:10.3389/fnins.2018.00155
7. Morys F, Dadar M, Dagher A. Association between midlife obesity and its metabolic consequences, cerebrovascular disease, and cognitive decline. J Clin Endocrinol Metab. 2021;106(10):e4260–e4274. doi:10.1210/clinem/dgab135
8. Pathak GA, Silzer TK, Sun J, et al. Genome-wide methylation of mild cognitive impairment in Mexican Americans highlights genes involved in synaptic transport, alzheimer’s disease-precursor phenotypes, and metabolic morbidities. J Alzheimers Dis. 2019;72(3):733–749. doi:10.3233/jad-190634
9. Xia Y, Prokop S, Giasson BI. “Don’t Phos Over Tau”: recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer’s disease and other tauopathies. Mol Neurodegener. 2021;16(1):37. doi:10.1186/s13024-021-00460-5
10. Zhang P, Yu Y, Qin Y, et al. Alterations to the microbiota-colon-brain axis in high-fat-diet-induced obese mice compared to diet-resistant mice. J Nutr Biochem. 2019;65:54–65. doi:10.1016/j.jnutbio.2018.08.016
11. Kesika P, Suganthy N, Sivamaruthi BS, et al. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021;264:118627. doi:10.1016/j.lfs.2020.118627
12. Chang CH, Lin CH, Lane HY. d-glutamate and gut microbiota in Alzheimer’s disease. Int J Mol Sci. 2020;21(8):2676. doi:10.3390/ijms21082676
13. Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129(10):4050–4057. doi:10.1172/jci129194
14. Qin L, Wu X, Block ML, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. doi:10.1002/glia.20467
15. Le Bars PL. Magnitude of effect and special approach to Ginkgo biloba extract EGb 761 in cognitive disorders. Pharmacopsychiatry. 2003;36:S44–S49. doi:10.1055/s-2003-40458
16. Xie Z, Lu H, Yang S, et al. Salidroside attenuates cognitive dysfunction in senescence-accelerated mouse prone 8 (SAMP8) mice and modulates inflammation of the gut-brain axis. Front Pharmacol. 2020;11:568423. doi:10.3389/fphar.2020.568423
17. Liu Y, Fu X, Lan N, et al. Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav Brain Res. 2014;267:178–188. doi:10.1016/j.bbr.2014.02.040
18. Tang T, Duan X, Ke Y, et al. Antidiabetic activities of polysaccharides from Anoectochilus roxburghii and Anoectochilus formosanus in STZ-induced diabetic mice. Int J Biol Macromol. 2018;112:882–888. doi:10.1016/j.ijbiomac.2018.02.042
19. Zeng B, Su M, Chen Q, et al. Antioxidant and hepatoprotective activities of polysaccharides from Anoectochilus roxburghii. Carbohydr Polym. 2016;153:391–398. doi:10.1016/j.carbpol.2016.07.067
20. Liu ZL, Zhang JG, Liu Q, et al. The vascular protective effects of Anoectochilus roxburghii polysaccharose under high glucose conditions. J Ethnopharmacol. 2017;202:192–199. doi:10.1016/j.jep.2017.03.012
21. Wang L, Chen Q, Zhuang S, et al. Effect of Anoectochilus roxburghii flavonoids extract on H(2)O(2) - Induced oxidative stress in LO2 cells and D-gal induced aging mice model. J Ethnopharmacol. 2020;254:112670. doi:10.1016/j.jep.2020.112670
22. Liu Q, Li Y, Sheng SM. Anti-aging effects and mechanisms of Anoectochilus roxburghii (Wall.) Lindl polysaccharose. J Huaqiao Univ. 2020;41(01):77–83.
23. Walrave L, Vinken M, Albertini G, et al. Inhibition of Connexin43 hemichannels impairs spatial short-term memory without affecting spatial working memory. Front Cell Neurosci. 2016;10:288. doi:10.3389/fncel.2016.00288
24. Tian D, Zhong X, Fu L, et al. Therapeutic effect and mechanism of polysaccharides from Anoectochilus Roxburghii (Wall.) Lindl. in diet-induced obesity. Phytomedicine. 2022;99:154031. doi:10.1016/j.phymed.2022.154031
25. Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi:10.1038/nprot.2006.116
26. Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–250. doi:10.1007/978-3-642-45106-5_9
27. Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82(4):756–771. doi:10.1016/j.neuron.2014.05.004
28. Cacquevel M, Lebeurrier N, Chéenne S, et al. Cytokines in neuroinflammation and Alzheimer’s disease. Curr Drug Targets. 2004;5(6):529–534. doi:10.2174/1389450043345308
29. Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147(6):1363–1377.e1317. doi:10.1053/j.gastro.2014.08.033
30. Kim KA, Gu W, Lee IA, et al. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7(10):e47713. doi:10.1371/journal.pone.0047713
31. Bruce-Keller AJ, Salbaum JM, Luo M, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77(7):607–615. doi:10.1016/j.biopsych.2014.07.012
32. Freeman LR, Zhang L, Nair A, et al. Obesity increases cerebrocortical reactive oxygen species and impairs brain function. Free Radic Biol Med. 2013;56:226–233. doi:10.1016/j.freeradbiomed.2012.08.577
33. Zeng B, Su M, Chen Q, et al. Protective effect of a polysaccharide from Anoectochilus roxburghii against carbon tetrachloride-induced acute liver injury in mice. J Ethnopharmacol. 2017;200:124–135. doi:10.1016/j.jep.2017.02.018
34. Wang S, Huang XF, Zhang P, et al. Dietary teasaponin ameliorates alteration of gut microbiota and cognitive decline in diet-induced obese mice. Sci Rep. 2017;7(1):12203. doi:10.1038/s41598-017-12156-2
35. DeVos SL, Miller RL, Schoch KM, et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. 2017;9(374):eaag0481. doi:10.1126/scitranslmed.aag0481
36. Zhang Z, Guo L, Yan A, et al. Fractionation, structure and conformation characterization of polysaccharides from Anoectochilus roxburghii. Carbohydr Polym. 2020;231:115688. doi:10.1016/j.carbpol.2019.115688
37. Bleier BS, Kohman RE, Feldman RE, et al. Permeabilization of the blood-brain barrier via mucosal engrafting: implications for drug delivery to the brain. PLoS One. 2013;8(4):e61694. doi:10.1371/journal.pone.0061694
38. Saiyasit N, Chunchai T, Prus D, et al. Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet-induced obese condition. Nutrition. 2020;69:110576. doi:10.1016/j.nut.2019.110576
39. Bilski J, Mazur-Bialy A, Wojcik D, et al. Role of obesity, mesenteric adipose tissue, and adipokines in inflammatory bowel diseases. Biomolecules. 2019;9(12):780. doi:10.3390/biom9120780
40. Xu Y, Zhou H, Zhu Q. The impact of microbiota-gut-brain axis on diabetic cognition impairment. Front Aging Neurosci. 2017;9:106. doi:10.3389/fnagi.2017.00106
41. Huang SY, Chen LH, Wang MF, et al. Lactobacillus paracasei PS23 delays progression of age-related cognitive decline in senescence accelerated mouse prone 8 (SAMP8) mice. Nutrients. 2018;10(7):894. doi:10.3390/nu10070894
42. Wang L, Chen C, Cui S, et al. Adhesive bifidobacterium induced changes in cecal microbiome alleviated constipation in mice. Front Microbiol. 2019;10:1721. doi:10.3389/fmicb.2019.01721
43. Gao G, Ma T, Zhang T, et al. Adjunctive probiotic lactobacillus rhamnosus probio-M9 administration enhances the effect of anti-PD-1 antitumor therapy via restoring antibiotic-disrupted gut microbiota. Front Immunol. 2021;12:772532. doi:10.3389/fimmu.2021.772532
44. Khine WWT, Voong ML, Ng TKS, et al. Mental awareness improved mild cognitive impairment and modulated gut microbiome. Aging. 2020;12(23):24371–24393. doi:10.18632/aging.202277
45. Grosheva I, Zheng D, Levy M, et al. High-throughput screen identifies host and microbiota regulators of intestinal barrier function. Gastroenterology. 2020;159(5):1807–1823. doi:10.1053/j.gastro.2020.07.003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.