Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Diabetic Retinopathy and Cardiovascular Disease: A Literature Review
Authors Yu W, Yang B, Xu S, Gao Y, Huang Y, Wang Z
Received 1 September 2023
Accepted for publication 21 December 2023
Published 28 December 2023 Volume 2023:16 Pages 4247—4261
DOI https://doi.org/10.2147/DMSO.S438111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Wenhua Yu,1 Bo Yang,1 Siting Xu,1 Yun Gao,2 Yan Huang,3 Zhongqun Wang1
1Department of Cardiology, Affiliated Hospital of Jiangsu University, Zhenjiang, People’s Republic of China; 2Department of Pathology, Affiliated Hospital of Jiangsu University, Zhenjiang, People’s Republic of China; 3Department of Ophthalmology, Affiliated Hospital of Jiangsu University, Zhenjiang, People’s Republic of China
Correspondence: Zhongqun Wang; Yan Huang, Affiliated Hospital of Jiangsu University, Zhenjiang, People’s Republic of China, Tel +86-15262919468, Email [email protected]; [email protected]; [email protected]
Abstract: Diabetic complications can be divided into macrovascular complications such as cardiovascular disease and cerebrovascular disease and microvascular complications such as diabetic retinopathy, diabetic nephropathy and diabetic neuropathy. Among them, cardiovascular disease (CVD) is an important cause of death in diabetic patients. Diabetes retinopathy (DR) is one of the main reasons for the increasing disability rate of diabetes. In recent years, some studies have found that because DR and CVD have a common pathophysiological basis, the occurrence of DR and CVD are inseparable, and to a certain extent, DR can predict the occurrence of CVD. With the development of technology, the fundus parameters of DR can be quantitatively analyzed as an independent risk factor of CVD. In addition, the cytokines related to DR can also be used for early screening of DR. Although many advances have been made in the treatment of CVD, its situation of prevention and treatment is still not optimistic. This review hopes to discuss the feasibility of DR in predicting CVD from the common pathophysiological mechanism of DR and CVD, the new progress of diagnostic techniques for DR, and the biomarkers for early screening of DR.
Keywords: diabetic retinopathy, cardiovascular disease, pathophysiological mechanism, optical coherence tomography angiography, biomarkers
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Fang has been published for this article.
Introduction
Diabetic complications can be divided into macrovascular complications such as cardiovascular disease (CVD) and cerebrovascular disease and microvascular complications such as diabetic retinopathy (DR), diabetic nephropathy and diabetic neuropathy. Diabetes and its complications are posing a huge threat to human health. DR is an important cause of blindness among working-age people.1 About one fifth of diabetic patients in the world suffer from DR, and the number of patients with vision loss caused by DR will continue to rise.2 CVD is one of the leading causes of death, and the number of deaths increased by 14.5% between 2006 and 2016 globally.3
DR can be divided into non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) with or without macular edema (DME).4 PDR is the late stage of DR and its main pathophysiological changes include thickening of retinal capillary basement membrane, increased retinal vascular permeability, tissue ischemia and hypoxia, and neovascularization. Because the neovascularization are very fragile, it is prone to penetration, which can lead to vitreous hemorrhage and retinal detachment, ultimately resulting in vision loss.5
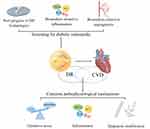
Cardiovascular disease is still the leading cause of death in diabetic patients, and in recent years, some studies have found that DR is closely related to the occurrence of CVD and can predict the occurrence of CVD.6,7 This may be due to the fact that DR and CVD share common risk factors such as hyperglycemia and hypertension, as well as pathophysiological mechanisms, including common oxidative stress, inflammation, and epigenetic modifications.8–13 However, there is currently no consensus on the feasibility of using DR to predict CVD. This review discusses the feasibility of DR in predicting the occurrence of CVD from the aspects of common risk factors and pathophysiological mechanisms of DR and CVD, new advances in diagnostic DR technology, and biomarkers for early screening of DR, hoping to provide new ideas for the prevention and detection of cardiovascular diseases (Figure 1).
Common Risk Factors for DR and CVD
DR and CVD are common vascular complications of diabetes, and the occurrence and development of vascular complications in diabetic patients are related to factors such as age of onset, course of disease, hyperglycemia, hypertension, abnormal lipid metabolism and obesity.14,15 Therefore, factors such as hyperglycemia, hypertension and obesity can be intervened to prevent the occurrence of DR and CVD. A prospective study showed that strengthening the control of blood glucose in patients with diabetes can significantly reduce the incidence of microvascular complications including diabetic retinopathy, but there is no significant reduction in the risk of cardiovascular disease.16 However, over time, the effect is cumulative, and continued follow-up for 10 years found that the relative risk of microvascular disease continued to decrease, and the risk of cardiovascular disease was significantly reduced.17 Diabetes with hypertension increases the risk of CVD and DR in patients with diabetes,15,18 and the study results show that strict control of blood pressure can significantly reduce the risk of CVD and DR in patients with diabetes.19 High BMI is one of the strongest risk factors for diabetes, and is associated with many metabolic abnormalities that lead to insulin resistance. Changing unhealthy diets and lifestyles is an effective way to address obesity and prevent complications of diabetes.14
Common Pathophysiological Mechanism of DR and CVD
Oxidative Stress
Studies have shown that the unified mechanism of diabetic microvascular and macrovascular complications may be an excess superoxide produced by the mitochondrial electron transport chain induced by hyperglycemia.8 Oxidative stress occurs when the production of reactive oxygen species (ROS) and the body’s endogenous antioxidant defense mechanisms are out of balance, damaging cells in target organs such as the heart and retina.20 DR and CVD are common microvascular and macrovascular complications of diabetes, oxidative stress has been proved to be one of the key factors leading to diabetic complications.
The abnormalities of the four metabolic pathways are related to the microvascular and cardiovascular complications of diabetes induced by oxidative stress, which are the activation of protein kinase C (PKC) pathway, the increase of polyol pathway flux, the activation of hexosamine pathway and the accumulation of advanced glycation end products (AGEs).21 Four abnormal metabolic pathways caused by hyperglycemia can lead to the increase of vascular endothelial growth factor (VEGF), Endothelin-1 (ET-1), transforming growth factor-β (TGF-β) and Insulin-like growth factor-1 (IGF-1), which in turn causes angiogenesis, vascular infiltration, blood-retinal barrier (BRB) damage, and ultimately result in DR.22 Metabolic abnormalities of diabetes can also promote each other with oxidative stress, and further cause inflammatory response mediated by Nuclear factor-kappa B (NF-κB), which eventually leads to the occurrence and development of DR.22 Metabolic abnormalities in diabetes lead to excessive production of ROS in vascular endothelial cells and myocardium.23 In addition, insulin resistance causes excessive ROS production by mitochondria in large vascular endothelial cells by increasing the flux of Free fatty acids (FFA) from adipocytes to arterial endothelial cells and oxidation.8 Excessive production of ROS may react with the NO produced by endothelial nitric oxide synthase (eNOS) to generate peroxynitrite, which in turn can uncouple eNOS and make it lose its anti-atherosclerotic activity.24 eNOS promotes the production of NO in blood vessels, and NO induces vascular smooth muscle relaxation through the activation of guanylate cyclase (GC) and the formation of cyclic guanosine monophosphate (cGMP) to dilate blood vessels.25 At the same time, NO can also increase the concentration of cGMP in platelets, resulting in the inhibition of intracellular Ca2+ level, and finally inhibit platelet aggregation.26 The functional manifestation of eNOS uncoupling is endothelial dysfunction, which is an early marker of most cardiovascular diseases.27 In addition, ROS can directly inactivate another key anti-atherosclerotic enzyme, prostacyclin synthase (PGIS).23 PGIS is the catalyst of prostacyclin synthesis. Prostacyclin can inhibit platelet aggregation and vasoconstriction, and PGIS gene has been proved to be related to cardiovascular disease28 (Figure 2).
Inflammation
Inflammation is a non-specific response of tissue to various injury stimuli. More and more evidence suggests that inflammation plays an important role in the occurrence and development of DR and CVD. Chronic low-grade inflammation exists in all stages of DR. Endothelial cells of diabetic retina increase the expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which leads to retinal leukocyte aggregation and the release of various cytokines, leading to low-grade inflammation.9 Inflammatory mediators destroy the tight junctions between endothelial cells, increase vascular permeability, and destroy BRB, leading to the occurrence of DME.29 Metabolic abnormalities caused by hyperglycemia lead to excessive production of ROS in mitochondria, increase oxidative stress, further activate NF-κB, and induce the up-regulation of inflammatory cytokines, and eventually lead to increased vascular permeability and further aggravate DR.8 The main pathological feature of PDR is neovascularization. Pro-inflammatory cytokines can bind with target endothelial cells to directly induce angiogenesis, or indirectly participate in angiogenesis by stimulating endothelial cells to produce proangiogenic mediators.30 On the contrary, angiogenic factors such as VEGF and Angiopoietin-1 (Ang-1) can also up-regulate the expression of inflammatory cytokines and cause pro-inflammatory response in endothelial cells.30
Inflammation is a common pathophysiological basis for atherosclerosis, which is the pathological basis of CVD. Unstable atherosclerotic plaque rupture, platelet aggregation and thrombosis will eventually lead to CVD.10,11 Leukocyte recruitment and pro-inflammatory cytokines are characteristics of the early stage of atherosclerosis, which is considered to be blood flow-mediated inflammatory changes of endothelial cells (ECs),31 and when ECs are activated by inflammation, the expression of various inflammatory factors increases, such as monocyte chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8), ICAM-1, VCAM-1 and so on, attracting lymphocytes and monocytes that bind to the endothelium and infiltrate the arterial wall,32 among which VCAM-1 seems to play a major role. When VCAM-1 adheres to the activated ECs, monocytes will penetrate between intact endothelial cells under the interaction of various chemokines, especially MCP-1 and its receptor CCR2. So far, monocytes have acquired the characteristics of tissue macrophages, combined with oxLDL, resulting in foam cells33 and foam cells secrete inflammatory cytokines, amplifying the local inflammatory response of the lesion site.34 Matrix metalloproteinases (MMPs) can degrade collagen fibers in the extracellular matrix of plaques. In advanced atherosclerosis, a large number of macrophages play a key role in plaque rupture, bleeding and thrombosis by secreting MMPs.35
In the presence of hyperglycemia, diabetic retinopathy may be driven by inflammation, resulting in uncontrolled synthesis of inflammatory mediators by endothelial cells, which leads to a surge of inflammatory mediators entering the systemic circulation, triggering the development of atherosclerosis, and eventually causing the occurrence of cardiovascular disease36 (Figure 3).
Epigenetic Modification
Epigenetic modification changes gene expression without affecting DNA sequence, and is an interaction between gene and environment. Epigenetic modification has been found to regulate oxidative stress, inflammation and angiogenesis in diabetes.37 DNA methylation, histone modification and non-coding RNA are important subtypes of epigenetic modification, which can affect gene transcription and regulate diabetic vascular complications.12,13,38
DNA Methylation
DNA methylation is the earliest discovered and the most important epigenetic modification. Under the catalysis of DNA methyltransferase (DNMTs), s-adenosine-methionine (SAM) is methylated at the 5’- position of cytosine residues, and this modification mainly occurs in rich regions of CpG island.39 DNA methylation is involved in the occurrence of diabetic vascular complications in many ways.40 It is well known that cardiovascular disease is the main cause of death in patients with diabetes. Studies have shown that hypomethylation occurs in atherosclerotic lesions, and this is associated with increased transcriptional activity.41,42 For example, during hyperglycemia, DNA hypomethylation of the p66Shc promoter in human aortic endothelial cells leads to overexpression of p66Shc, inducing oxidative stress and thereby accelerating endothelial dysfunction.43 In atherosclerosis, plaques develop preferentially in arterial regions with disturbed blood flow (d-flow). D-flow controls epigenomic DNA methylation patterns in a DNMT-dependent manner, which in turn alters endothelial gene expression and induces atherosclerosis.44 In addition, DNA methylation also occurs in several genes related to the pathogenesis of atherosclerosis, such as eNOS, hypoxia-inducible factor-ɑ (HIF-1ɑ) and MMPs.45 Other CVD risk factors, such as hyperhomocysteinemia, hypercholesterolemia and inflammation, are also associated with atherosclerosis-related DNA methylation.46 Other studies have shown that the DNA demethylase TET2 prevents atherosclerosis by inhibiting the upregulation of proinflammatory cytokines and chemokines and the activation of inflammasomes.47 This indicates that TET2 is a promising therapeutic target for the treatment of atherosclerosis. DR is another common vascular complication of diabetes, and DNA methylation plays a key role in different pathogenesis of DR, including oxidative stress, inflammation and neovascularization.48–50 ROS can activate DNMTs and promote DNA methylation by deprotonating cytosine molecules.51 On the other hand, DNA methylation promotes oxidative stress and ultimately contributes to the development of DR.52 Hyperglycemia can also inhibit DNMT1 recruitment to the matrix metalloproteinase-9 (MMP9) promoter region, resulting in an increase in MMP9, thus accelerating the inflammatory response of diabetic retinopathy.52 Maternal expression gene 3(MEG3) is a kind of lncRNA that can promote apoptosis and is widely expressed in eye tissues. MEG3 can effectively inhibit retinal neovascularization by down-regulating the expression of phosphatidylinositol 3-kinase (PI3K), serine / threonine kinase (AKT), VEGF and pro-inflammatory factor.53 Studies have shown that DNMT1 inhibits MEG3 expression by promoting MEG3 promoter methylation, thus accelerating endothelial-mesenchymal transformation (EndMT) in diabetic retinopathy,54 and promoting the proliferation, migration and angiogenesis of human retinal microvascular endothelial cells (hRMEC),55 In addition, DNA methylation also plays a role in other diabetic vascular complications, such as decreased methylation in the promoter region of the pro-inflammatory circulating protein ANGPTL2, which promotes the occurrence and development of albuminuria in patients with type 2 diabetes.56
Histone Modification
Histone methylation refers to the transfer of methyl groups from S-adenosine-L-methionine to lysine or arginine residues in histones by histone methyltransferases (HMTs). Lysine can be monomethylated, dimethylated or trimethylated, while arginine can be monomethylated, symmetrical or asymmetrically dimethylated.57 Histone methylation of specific lysine or arginine residues plays a key role in the occurrence of diabetic vascular complications.58 For example, aberrant histone methylation (H3K4me1, H3K9me2 and H3K9me3) at the promoters of NADPH oxidase (Nox4) and eNOS leads to continuous up-regulation of these two genes, which increases ROS production and further leads to endothelial dysfunction.59 Alkemade et al demonstrated that the level of H3K27me3 in VSMC decreased in apoE − / - rats.60 Another study confirmed that the overall H3K9me2 and H3K27me2 were significantly reduced in atherosclerotic lesions.61 Similarly, lower levels of H3K27Me3 were found in blood vessels in advanced atherosclerotic plaques without changes in the corresponding histone methyltransferase EZH2.62 In macrophages, JMJD3, a specific H3K27me3 demethylase, can regulate H3K27me3 levels in response to inflammation and transcriptional activity by binding to PcG. When exposed to lipopolysaccharide (LPS), the level of JMJD3 in macrophages increases and the inflammatory response is enhanced.63 This suggests that H3K27 demethylation is essential for atherosclerotic plaque formation. Protein arginine methyltransferase 4 (PRMT4) specifically catalyzes H3R17 methylation to regulate apoptosis of retinal pigment epithelial cells induced by oxidative stress.64 Hyperglycemia increases the level of H3K27me3 in human retinal endothelial cells and retinal microvessels of diabetic rats, activates Ezh2 in diabetes, and promotes the recruitment of enzymes responsible for regulating DNA methylation of MMP-9 promoter, which result in transcriptional activation and enhanced inflammatory response.65
Histone acetyltransferase (HATs) and histone deacetylase (HDACs) mediate histone acetylation and deacetylation, respectively. HATs use acetyl-CoA as a cofactor to catalyze the transfer of acetyl to the ε-amino group of the lysine side chains, thus reducing the binding of histone to DNA and activating gene expression. HDACs reverse lysine acetylation and repress gene expression.57 Studies have shown that HATs may participate in the pathophysiology of diabetic microvascular complications by regulating the expression of inflammatory pathway genes. For example, RelA/p65, a subunit of NF-κB, induces NF-κB activation through mutual regulation of acetylation and deacetylation, which promotes inflammation.66,67 The H3 acetylation of TNF-ɑ and COX-2 promoters in human blood monocytes increased in type 1 and type 2 diabetes subjects.68 Hyperglycemia leads to histone acetylation in the retina, which contributes to the upregulation of pro-inflammatory proteins induced by hyperglycemia, thereby promoting the development of diabetic retinopathy.69 OxLDL induces acetylation of IL-8 and chemokine MCP-1 promoter by recruiting p300 in endothelial cells, and oxLDL reduces the expression and binding affinity of HDAC1 and HDAC2, thus triggering inflammation and promoting atherosclerosis.70 In addition, hyperglycemia-induced superoxide overactivation is considered to be the main pathway of diabetic vascular complications,71 while ROS have been shown to increase HDAC activity and decrease HAT activity, as well as inhibit histone acetylation.72 It has been reported that lysine acetyltransferase 1 (KAT1) is significantly down-regulated in the retinal tissue of model mice, which promotes neovascularization and vascular leakage in mouse retinal tissue.73 Extracellular superoxide dismutase (SOD) is an antioxidant enzyme that protects vascular cells from oxidative stress. Exendin 4, a glucagon-like peptide-1 receptor agonist, induces the expression of SOD in human retinal microvascular endothelial cells through histone H3 acetylation.74
Non-Coding RNA
Non-coding RNA (ncRNAs) is mainly divided into micro-RNA (miRNAs), long non-coding RNA (lnc RNAs) and circular RNA (circ RNAs), which play a transcriptional regulatory role in diabetic vascular complications.75
MiRNA is a highly conserved small ncRNA of 20~40 nucleotides. The generation of miRNA first occurs in the nucleus, where the gene that encodes miRNA is transcribed into pri-miRNAs by RNA polymerase. Subsequently, the pri-miRNA is processed by RNase III in the nucleus to form pre-miRNA. In the cytoplasm, the pre-miRNA is transported to the cytoplasm by the exportin5 protein, and then it is cleaved by another RNase III to form mature miRNA. The mature miRNA binds to the RNA-induced silencing complex (RISC) to block protein translation or induce gene degradation by binding to the 3’-untranslated region of the target mRNA at the post-transcriptional level to regulate gene expression.76,77 The discovery of miRNA has shown that the non-histone coding region of the genome contains important information about life activities, which not only plays an important role in the normal development and physiology of organisms, but also participates in the pathological process of multiple diseases, including diabetes, cancer, cardiovascular disease and autoimmune diseases.78,79 MiRNAs has also been shown to contribute to vascular complications of diabetes. For example, miRNA-200b is down-regulated in retinal endothelial cells and cardiac microvascular endothelial cells exposed to high glucose, and its down-regulation leads to VEGF overexpression and EndMT, which is associated with the development of diabetic retinopathy and cardiovascular diseases.80,81 Circulating levels of miR-146a are decreased in diabetic patients.82 MiR-146a reduces endothelial inflammatory response induced by high glucose by inhibiting the expression of Nox4,83 decreases the expression of interleukin-1 receptor-associated kinase-1 (IRAK-1) and VCAM-1/ICAM-1,84 and reduces the transmission of TLR4 / NF-κB and TNF-α signal pathway.85
LncRNA is a class of ncRNA with a length of more than 200bp, which is thought to be involved in a variety of biological processes, such as epigenetic regulation, transcription, translation, splicing and cell differentiation.86 For example, LncRNA metastasis-associated lung adenocarcinoma transcript 1(MALAT1) has been extensively studied in diabetes. MALAT1 can promote ECs proliferation and retinal neovascularization.87 MALAT1 has also been found to promote cardiomyocyte proliferation by activating the phosphoinositide kinase (PI3K) / protein kinase B (PKB) signal pathway.88 Up-regulated expression of MALAT1 was found in ApoE−/−mice fed with high-fat diet, and overexpression of MALAT1 enhanced the effect of ox-LDL on EndMT in human umbilical vein endothelial cells (HUVEC), indicating that MALAT1 was involved in the development of atherosclerosis.89 In addition, MALAT1 can damage vascular endothelial cells by activating the NF-κB signal pathway and inducing diabetic inflammatory factors such as IL-6, TNF-α and IL-1β,90 Generally speaking, MALAT1 plays a role in both DR and CVD.
CircRNAs are closed circular RNAs, which are produced by reverse splicing of specific regions of heterogeneous nuclear RNA.91 CircRNAs mainly regulate the occurrence of diabetic vascular complications through the miRNA-mRNA axis. For example, circRNA (CircR)-284 is reported to promote atherosclerosis by targeting inhibition of micro-RNAs (miRs)-221, while CircR-284 increases the risk of plaque rupture.92 The down-regulation of circRNA DMNT3B increases the expression of miR-20b-5p and promotes the proliferation, migration and angiogenesis of human retinal microvascular endothelial cells (HRMECs).93 Under the condition of HG, circ_001209 is overexpressed in HRVECs, which indirectly regulates the expression of COL12A1 by down-regulating miR-15b-5p, resulting in vascular endothelial cell dysfunction.94 However, the epigenetic modification of circRNA-miRNA-mRNA axis in DR and CVD is still limited and needs further study.
In summary, epigenetic modification may play an important role in the pathophysiology of diabetes and its related vascular complications, including atherosclerotic cardiovascular disease and retinopathy, and provide a unique opportunity to develop new treatments for diabetic complications.
The above is a summary of the common pathophysiological mechanisms of DR and CVD. At present, it is known that there is a certain correlation between DR And CVD, but the specific mechanism and common regulatory pathways need to be further studied.
The Predictive Effect of DR on CVD
Diabetic complications can interact with each other. For example, chronic kidney disease can be detected from retinal images, which provides the feasibility of using retinal photography to screen for chronic kidney disease in the community population.95 Carotid atherosclerosis parameters can predict the outcome of microvascular and cardiovascular complications in patients with type 2 diabetes.96 Many studies have suggested that diabetic retinopathy can predict the occurrence of cardiovascular disease, and patients with diabetic retinopathy have an increased risk of cardiovascular disease. Studies have found that there is a close relationship between microvascular and macrovascular complications of diabetes. A clinical study on type 2 diabetic retinopathy found that DR is associated with an increased incidence of CVD after adjusting for risk factors such as age, gender, blood pressure, smoking status, and total cholesterol/high-density lipoprotein ratio, and DR is an independent predictor of CVD.97 A 10-year follow-up study found that functional and structural retinal microvascular changes can predict cardiovascular events in patients with type 1 diabetes.98 Therefore, non-invasive imaging of retinal microvessels can be performed to detect changes in microvessels and to predict the occurrence of cardiovascular events.99 In addition, whether serological biomarkers, which measure the severity of DR, can be used for early warning of cardiovascular events is also an interesting topic. Figure 4).
New Progress of Screening DR Technologies
The traditional imaging examinations of DR mainly include fundus color photography and fundus fluorescein angiography (FFA). FFA is extremely important for evaluating the clinical fundus characteristics of DR, and has important guiding significance for the staging, treatment guidance and prognosis of DR, which is considered as the gold standard for identifying the existence of neovascularization. However, traditional angiography techniques have the limitations of affecting vascular imaging due to contrast medium leakage, inability to image the retinal capillary network in layers, and inability to observe neovascularization directly,100 and injection of contrast medium may bring adverse reactions such as nausea and vomiting. The emergence of optical coherence tomography angiography (OCTA) breaks the deadlock, and its high-resolution and non-invasive characteristics enable in-depth analysis of a series of pathophysiological changes of DR.101 Compared with FFA, OCTA is more accurate in evaluating capillary non-perfusion, but its ability to detect microaneurysms is lower than FFA.102 Meanwhile, OCTA can better detect the formation of early retinal neovascularization.103
The grading of the severity of DR is very important for the diagnosis, treatment and prognosis evaluation of the disease, and the warning of CVD through DR is also inseparable from the quantitative analysis of the severity of DR. In recent years, researchers have been trying to determine vascular changes in DR and whether they can inform the development of CVD. A prospective study of DR predicting CVD risk found that the addition of retinal microvascular parameters greatly improved CVD risk prediction.104 With the development of ophthalmological instruments, OCTA can quantitatively analyze the retinal capillary microvasculature of diabetic patients in a non-invasive manner.105 The common parameters for quantitative analysis of retinal microvessels are perfusion density and vessel density.106–108 OCTA has the potential to show retinal capillary perfusion and its control.106 Studies have shown that the perfusion index of retinal vessels may be a useful biomarker for judging the severity of DR.107 Another study shows that the vascular density measured by OCTA is related to the severity of DR, which may guide the stage of DR.108 In addition, vessel diameter index and fractal dimension are also visible quantitative parameters. Studies have found that there is a negative correlation between the severity of DR and the vessel density and fractal dimension, and a positive correlation with vessel diameter index.109
Early microvascular damage can also be detected by OCTA in patients with subclinical diabetic retinopathy.110 Compared with healthy controls, patients with subclinical DR have microvascular changes in the superficial and deep capillary plexus, as shown by increased foveal vessel density, parafoveal and foveal ischemic zone (FAZ) area.111 Therefore, OCTA is an effective tool for early screening of DR.
In addition to OCTA, the severity of DR can also be evaluated by using quantitative ultra-widefield angiography indicators such as leakage index, ischemic index, and microaneurysm count.112
In recent years, with the development of information technology and the scientific progress of big data, the research of artificial intelligence (AI) has made unprecedented progress. Methods based on machine learning (ML), especially deep learning (DL), can not only identify, locate and quantify the pathological features of DR, but also diagnose or classify DR stages.113 In addition to identifying DR, AI recognition of retinal fundus images is also possible to predict cardiovascular risk factors that were previously thought to be absent or unquantifiable in retinal images, such as age, sex, smoking status, and systolic blood pressure.114 Although AI has certain limitations in clinical diagnosis and management and cannot completely replace ophthalmologists, recently, fully automated systems based on AI have been further developed and preliminarily approved for DR screening,115 and it is believed that AI will play an important role in DR Screening in the near future.
Serum Biomarker
Inflammation and angiogenesis play an important role in the occurrence and development of DR. Various pathological changes in diabetic patients can up-regulate the expression of VEGF, which can lead to angiogenesis and activate various inflammatory mediators,116 At the same time, inflammatory mediators can also induce the expression of VEGF.117 Changes in the concentrations of various pro-inflammatory and angiogenic mediators were found in the serum of patients with DR, which are related to the severity of DR and can be used as biomarkers of the severity of DR.
Biomarkers Related to Inflammation
As mentioned above, chronic low-grade inflammation exists at every stage of DR, and many studies have confirmed that inflammatory mediators can be used as biomarkers to measure the severity of DR and predict the progress of DR. C-reactive protein (CRP) is produced in liver and adipose tissue and is largely regulated by IL-6.118 It has been found that the concentration of serum CRP is significantly increased in DR patients and positively correlated with the severity of DR.119 TNF-ɑ can promote leukocyte siltation, increase the production of ROS, and promote the destruction of BRB.120 It has been reported that the level of serum TNF-ɑ is highly correlated with PDR and can be used as an independent inflammatory marker of PDR.121 Shimizu et al also found that serum IL-6 concentration was significantly correlated with the severity of DME and may also be a predictor of PDR.122 In addition, the levels of serum inflammatory factors such as IL-1β and IL-8 are also reported to be related to DR stages.123 However, other studies have shown that there is little association between inflammatory markers and diabetic retinopathy after controlling for established risk factors including duration of diabetes, A1C, systolic blood pressure, waist-to-hip ratio, and use of diabetes medications.124 Due to the uncertainty of the results, the clinical value of serum inflammatory mediators as DR biomarkers should be reconsidered. Furthermore, regional concentrations of inflammatory mediators in the retina may be more meaningful than the serum levels of inflammation that reflect the systemic effects of diabetes. IL-1, IL-6, IL-8, TNF-A, MCP-1, ICAM-1 and VCAM1 were found to be elevated in the vitreous of diabetic patients with DR, and were related to the pathogenesis of DR.125–128 It is worth noting that although inflammatory levels in diabetic retinopathy are generally considered to be chronically low, the levels of IL-8 and MCP-1 in vitreous fluids are comparable to those found in the pleural effusion of patients with pneumonia or tuberculosis.129 It has also been reported that the levels of inflammatory cytokines IL-1β, IL-6, IL-8 and TNF-ɑ in aqueous humor reflect the severity of DR and are related to prognosis.130 Moreover, a prospective study observed that TNF-ɑ levels in tears were highly correlated with the severity of DR.131 It has also been reported that inflammatory biomarkers such as IL-6 and TNF-α in saliva are also associated with the severity of DR, suggesting that these salivary biomarkers are potential biomarkers for predicting the progression of DR.132
Biomarkers Related to Angiogenesis
VEGF is considered to be the main angiogenic growth factor related to the development of DR.133 The VEGF family consists of seven members, namely VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PlGF (placental growth factor), of which VEGF-A is the prototype of the VEGF family and is therefore sometimes referred to simply as VEGF. Hyperglycemia induces ischemia and hypoxia, oxidative stress, and overactivation of PKC, which eventually leads to various VEGF-mediated pathological processes, such as angiogenesis, increased endothelial permeability, reduced pro-apoptotic protein inhibition and disruption of vascular homeostasis.116 VEGF increases the levels of VCAM-1, ICAM-1 and MCP-1 through NF-κB -mediated pathway, and increases leukocyte adhesion, which further aggravates endothelial dysfunction.134,135 Ahuja et al measured the level of serum VEGF in healthy control group, non-DR group, NPDR group and PDR group, and found that it showed a significant increasing trend, which is a reliable biomolecule biomarker to judge the severity of DR.136 A meta-analysis conducted by Zhou et al yielded the same results.137 Similarly, VEGF levels in the vitreous are significantly associated with DR.138 Through a cross-sectional study, Ang et al found that the level of VEGF in tears of patients with DR was correlated with the severity of DR.139 Fibroblast growth factor 21 (FGF-21) also has proangiogenic effects, and Lin et al found that serum FGF21 levels were independently associated with the severity of DR.140
In summary, biomarkers related to the pathogenesis of DR can predict the severity of DR, but their serum levels may come from systemic effects, so levels of cytokines in vitreous and aqueous humor may be more meaningful. Compared with aqueous humor, vitreous, tears and saliva are more easily obtained and noninvasive, so the levels of cytokines in tears and saliva may be more promising tools for screening and predicting DR. However, these results need to be confirmed in larger studies.
Conclusion
The prediction of cardiovascular risk in people with diabetes can carry out targeted preventive treatment for asymptomatic patients who are at high risk of developing diabetes. The severity of DR can be used to predict the occurrence of CVD, which may be because diabetic retinopathy and cardiovascular disease are in the same pathophysiological environment, in which oxidative stress, inflammation and epigenetic modification seem to play a key role, but its specific mechanism needs to be further studied. Early screening of DR by fundus imaging to prevent CVD may be feasible, but the new technology is not mature and has not been widely implemented. In addition, the related serum biomarkers are not highly specific and can not accurately reflect the pathological degree of DR. Therefore, more specific markers of vitreous, aqueous humor and tears are needed to prove their significance.
Funding
This work was supported by the Key Research and Development Project of Jiangsu Province (BE2022780).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179–183. doi:10.1080/09286580701396720
2. Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–1591. doi:10.1016/j.ophtha.2021.04.027
3. Collaborators GBDCo D. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi:10.1016/S0140-6736(17)32152-9
4. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi:10.1016/S0161-6420(03)00475-5
5. Alghadyan AA. Diabetic retinopathy - an update. Saudi J Ophthalmol. 2011;25(2):99–111. doi:10.1016/j.sjopt.2011.01.009
6. Xie J, Ikram MK, Cotch MF, et al. Association of diabetic macular edema and proliferative diabetic retinopathy with cardiovascular disease: a systematic review and meta-analysis. JAMA Ophthalmol. 2017;135(6):586–593. doi:10.1001/jamaophthalmol.2017.0988
7. Rajala U, Pajunpaa H, Koskela P, Keinanen-Kiukaanniemi S. High cardiovascular disease mortality in subjects with visual impairment caused by diabetic retinopathy. Diabetes Care. 2000;23(7):957–961. doi:10.2337/diacare.23.7.957
8. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi:10.2337/diabetes.54.6.1615
9. Khalfaoui T, Lizard G, Ouertani-Meddeb A. Adhesion molecules (ICAM-1 and VCAM-1) and diabetic retinopathy in type 2 diabetes. J Mol Histol. 2008;39(2):243–249. doi:10.1007/s10735-007-9159-5
10. Kanter JE, Kramer F, Barnhart S, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A. 2012;109(12):E715–E724. doi:10.1073/pnas.1111600109
11. Li JJ, Chen JL. Inflammation may be a bridge connecting hypertension and atherosclerosis. Med Hypotheses. 2005;64(5):925–929. doi:10.1016/j.mehy.2004.10.016
12. Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21(4):214–222. doi:10.1016/j.tem.2009.12.007
13. Devaskar SU, Raychaudhuri S. Epigenetics--a science of heritable biological adaptation. Pediatr Res. 2007;61(5 Pt 2):1R–4R. doi:10.1203/pdr.0b013e31805cdbd8
14. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
15. Liu Y, Yang J, Tao L, et al. Risk factors of diabetic retinopathy and sight-threatening diabetic retinopathy: a cross-sectional study of 13 473 patients with type 2 diabetes mellitus in mainland China. BMJ open. 2017;7(9):e016280. doi:10.1136/bmjopen-2017-016280
16. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. doi:10.1016/S0140-6736(98)07019-6
17. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. New Engl J Med. 2008;359(15):1577–1589. doi:10.1056/NEJMoa0806470
18. Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57. doi:10.1186/s12933-018-0703-2
19. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. doi:10.1136/bmj.317.7160.703
20. Betteridge DJ. What is oxidative stress? Metabolism. 2000;49(2 Suppl 1):3–8. doi:10.1016/S0026-0495(00)80077-3
21. Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61(1):29–38. doi:10.1007/s00125-017-4435-8
22. Kang Q, Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. doi:10.1016/j.redox.2020.101799
23. Giacco F, Brownlee M, Schmidt AM. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi:10.1161/CIRCRESAHA.110.223545
24. Munzel T, Daiber A, Ullrich V, Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25(8):1551–1557. doi:10.1161/01.ATV.0000168896.64927.bb
25. Rapoport RM, Draznin MB, Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983;306(5939):174–176. doi:10.1038/306174a0
26. Bassenge E. Antiplatelet effects of endothelium-derived relaxing factor and nitric oxide donors. Eur Heart J. 1991;12(Suppl E):12–15. doi:10.1093/eurheartj/12.suppl_E.12
27. Steven S, Frenis K, Oelze M, et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. 2019;2019:7092151. doi:10.1155/2019/7092151
28. Nakayama T. Prostacyclin synthase gene: genetic polymorphisms and prevention of some cardiovascular diseases. Curr Med Chem Cardiovasc Hematol Agents. 2005;3(2):157–164. doi:10.2174/1568016053544327
29. Semeraro F, Morescalchi F, Cancarini A, Russo A, Rezzola S, Costagliola C. Diabetic retinopathy, a vascular and inflammatory disease: therapeutic implications. Diabetes Metab. 2019;45(6):517–527. doi:10.1016/j.diabet.2019.04.002
30. Aplin AC, Gelati M, Fogel E, Carnevale E, Nicosia RF. Angiopoietin-1 and vascular endothelial growth factor induce expression of inflammatory cytokines before angiogenesis. Physiol Genomics. 2006;27(1):20–28. doi:10.1152/physiolgenomics.00048.2006
31. Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209(1):13–22. doi:10.1083/jcb.201412052
32. Chistiakov DA, Melnichenko AA, Grechko AV, Myasoedova VA, Orekhov AN. Potential of anti-inflammatory agents for treatment of atherosclerosis. Exp Mol Pathol. 2018;104(2):114–124. doi:10.1016/j.yexmp.2018.01.008
33. Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. doi:10.1155/2013/152786
34. Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi:10.1038/nature01323
35. Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104(3):365–372. doi:10.1161/01.CIR.104.3.365
36. Cheung N, Wong TY. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res. 2008;27(2):161–176. doi:10.1016/j.preteyeres.2007.12.001
37. Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015;48:40–61. doi:10.1016/j.preteyeres.2015.05.001
38. Zhang Y, Sun Z, Jia J, et al. Overview of histone modification. Adv Exp Med Biol. 2021;1283:1–16.
39. Miranda TB, Jones PA. DNA methylation: the nuts and bolts of repression. J Cell Physiol. 2007;213(2):384–390. doi:10.1002/jcp.21224
40. Zheng J, Cheng J, Zhang Q, Xiao X. Novel insights into DNA methylation and its critical implications in diabetic vascular complications. Biosci Rep. 2017;37(2). doi:10.1042/BSR20160611
41. Hiltunen MO, Turunen MP, Hakkinen TP, et al. DNA hypomethylation and methyltransferase expression in atherosclerotic lesions. Vasc Med. 2002;7(1):5–11. doi:10.1191/1358863x02vm418oa
42. Hiltunen MO, Yla-Herttuala S. DNA methylation, smooth muscle cells, and atherogenesis. Arterioscler Thromb Vasc Biol. 2003;23(10):1750–1753. doi:10.1161/01.ATV.0000092871.30563.41
43. Keating ST, Plutzky J, El-Osta A. Epigenetic changes in diabetes and cardiovascular risk. Circ Res. 2016;118(11):1706–1722. doi:10.1161/CIRCRESAHA.116.306819
44. Dunn J, Qiu H, Kim S, et al. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest. 2014;124(7):3187–3199. doi:10.1172/JCI74792
45. Matouk CC, Marsden PA. Epigenetic regulation of vascular endothelial gene expression. Circ Res. 2008;102(8):873–887. doi:10.1161/CIRCRESAHA.107.171025
46. Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr. 2005;135(1):5–8. doi:10.1093/jn/135.1.5
47. Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842–847. doi:10.1126/science.aag1381
48. Duraisamy AJ, Mishra M, Kowluru A, Kowluru RA. Epigenetics and regulation of oxidative stress in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59(12):4831–4840. doi:10.1167/iovs.18-24548
49. Chen H, Zhang X, Liao N, et al. Identification of NLRP3 inflammation-related gene promoter hypomethylation in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2020;61(13):12. doi:10.1167/iovs.61.13.12
50. Berdasco M, Gomez A, Rubio MJ, et al. DNA methylomes reveal biological networks involved in human eye development, functions and associated disorders. Sci Rep. 2017;7(1):11762. doi:10.1038/s41598-017-12084-1
51. Afanas’ev I. New nucleophilic mechanisms of ros-dependent epigenetic modifications: comparison of aging and cancer. Aging Dis. 2014;5(1):52–62. doi:10.14336/AD.2014.050052
52. Kowluru RA, Shan Y. Role of oxidative stress in epigenetic modification of MMP-9 promoter in the development of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):955–962. doi:10.1007/s00417-017-3594-0
53. Di Y, Wang Y, Wang YX, Wang X, Ma Y, Nie QZ. Maternally expressed gene 3 regulates retinal neovascularization in retinopathy of prematurity. Neural Regen Res. 2022;17(6):1364–1368. doi:10.4103/1673-5374.327358
54. He Y, Dan Y, Gao X, Huang L, Lv H, Chen J. DNMT1-mediated lncRNA MEG3 methylation accelerates endothelial-mesenchymal transition in diabetic retinopathy through the PI3K/Akt/mTOR signaling pathway. Am J Physiol Endocrinol Metab. 2021;320(3):E598–E608. doi:10.1152/ajpendo.00089.2020
55. Chen J, Liao L, Xu H, Zhang Z, Zhang J. Long non-coding RNA MEG3 inhibits neovascularization in diabetic retinopathy by regulating microRNA miR-6720-5p and cytochrome B5 reductase 2. Bioengineered. 2021;12(2):11872–11884. doi:10.1080/21655979.2021.2000721
56. Ishii T, Furuya F, Takahashi K, et al. Angiopoietin-like protein 2 promotes the progression of diabetic kidney disease. J Clin Endocrinol Metab. 2019;104(1):172–180. doi:10.1210/jc.2017-02705
57. Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–395. doi:10.1038/cr.2011.22
58. Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–455. doi:10.1007/s00125-014-3462-y
59. Liao Y, Gou L, Chen L, et al. NADPH oxidase 4 and endothelial nitric oxide synthase contribute to endothelial dysfunction mediated by histone methylations in metabolic memory. Free Radic Biol Med. 2018;115:383–394. doi:10.1016/j.freeradbiomed.2017.12.017
60. Alkemade FE, van Vliet P, Henneman P, et al. Prenatal exposure to apoE deficiency and postnatal hypercholesterolemia are associated with altered cell-specific lysine methyltransferase and histone methylation patterns in the vasculature. Am J Pathol. 2010;176(2):542–548. doi:10.2353/ajpath.2010.090031
61. Greissel A, Culmes M, Napieralski R, et al. Alternation of histone and DNA methylation in human atherosclerotic carotid plaques. Thromb Haemost. 2015;114(2):390–402. doi:10.1160/TH14-10-0852
62. Wierda RJ, Rietveld IM, van Eggermond MC, et al. Global histone H3 lysine 27 triple methylation levels are reduced in vessels with advanced atherosclerotic plaques. Life Sci. 2015;129:3–9. doi:10.1016/j.lfs.2014.10.010
63. De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130(6):1083–1094. doi:10.1016/j.cell.2007.08.019
64. Kim DI, Park MJ, Choi JH, et al. PRMT1 and PRMT4 regulate oxidative stress-induced retinal pigment epithelial cell damage in SIRT1-dependent and SIRT1-independent manners. Oxid Med Cell Longev. 2015;2015:617919. doi:10.1155/2015/617919
65. Duraisamy AJ, Mishra M, Kowluru RA. Crosstalk between histone and DNA methylation in regulation of retinal matrix metalloproteinase-9 in diabetes. Invest Ophthalmol Vis Sci. 2017;58(14):6440–6448. doi:10.1167/iovs.17-22706
66. Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21(23):6539–6548. doi:10.1093/emboj/cdf660
67. Kiernan R, Bres V, Ng RW, et al. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem. 2003;278(4):2758–2766. doi:10.1074/jbc.M209572200
68. Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 2004;279(17):18091–18097. doi:10.1074/jbc.M311786200
69. Kadiyala CS, Zheng L, Du Y, et al. Acetylation of retinal histones in diabetes increases inflammatory proteins: effects of minocycline and manipulation of histone acetyltransferase (HAT) and histone deacetylase (HDAC). J Biol Chem. 2012;287(31):25869–25880. doi:10.1074/jbc.M112.375204
70. Lee HT, Oh S, Ro DH, Yoo H, Kwon YW. The key role of DNA methylation and histone acetylation in epigenetics of atherosclerosis. J Lipid Atheroscler. 2020;9(3):419–434. doi:10.12997/jla.2020.9.3.419
71. Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19(3):257–267. doi:10.2337/diacare.19.3.257
72. Berthiaume M, Boufaied N, Moisan A, Gaudreau L. High levels of oxidative stress globally inhibit gene transcription and histone acetylation. DNA Cell Biol. 2006;25(2):124–134. doi:10.1089/dna.2006.25.124
73. Qi Y, Yao R, Zhang W, Cui Q. KAT1 triggers YTHDF2-mediated ITGB1 mRNA instability to alleviate the progression of diabetic retinopathy. Pharmacol Res. 2021;170:105713. doi:10.1016/j.phrs.2021.105713
74. Yasuda H, Ohashi A, Nishida S, et al. Exendin-4 induces extracellular-superoxide dismutase through histone H3 acetylation in human retinal endothelial cells. J Clin Biochem Nutr. 2016;59(3):174–181. doi:10.3164/jcbn.16-26
75. Beltrami C, Angelini TG, Emanueli C. Noncoding RNAs in diabetes vascular complications. J Mol Cell Cardiol. 2015;89(Pt A):42–50. doi:10.1016/j.yjmcc.2014.12.014
76. Lucas T, Bonauer A, Dimmeler S. RNA therapeutics in cardiovascular disease. Circ Res. 2018;123(2):205–220. doi:10.1161/CIRCRESAHA.117.311311
77. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/S0092-8674(04)00045-5
78. Suh N, Blelloch R. Small RNAs in early mammalian development: from gametes to gastrulation. Development. 2011;138(9):1653–1661. doi:10.1242/dev.056234
79. Fu X, Dong B, Tian Y, et al. MicroRNA-26a regulates insulin sensitivity and metabolism of glucose and lipids. J Clin Invest. 2015;125(6):2497–2509. doi:10.1172/JCI75438
80. McArthur K, Feng B, Wu Y, Chen S, Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60(4):1314–1323. doi:10.2337/db10-1557
81. Feng B, Cao Y, Chen S, Chu X, Chu Y, Chakrabarti S. miR-200b mediates endothelial-to-mesenchymal transition in diabetic cardiomyopathy. Diabetes. 2016;65(3):768–779. doi:10.2337/db15-1033
82. Baldeon RL, Weigelt K, de Wit H, et al. Decreased serum level of miR-146a as sign of chronic inflammation in type 2 diabetic patients. PLoS One. 2014;9(12):e115209. doi:10.1371/journal.pone.0115209
83. Wang HJ, Huang YL, Shih YY, Wu HY, Peng CT, Lo WY. MicroRNA-146a decreases high glucose/thrombin-induced endothelial inflammation by inhibiting NAPDH oxidase 4 expression. Mediators Inflamm. 2014;2014:379537. doi:10.1155/2014/379537
84. Lo WY, Peng CT, Wang HJ. MicroRNA-146a-5p mediates high glucose-induced endothelial inflammation via targeting interleukin-1 receptor-associated kinase 1 expression. Front Physiol. 2017;8:551. doi:10.3389/fphys.2017.00551
85. Ye EA, Steinle JJ. miR-146a attenuates inflammatory pathways mediated by TLR4/NF-kappaB and TNFalpha to protect primary human retinal microvascular endothelial cells grown in high glucose. Mediators Inflamm. 2016;2016:3958453. doi:10.1155/2016/3958453
86. Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354–361. doi:10.1016/j.tcb.2011.04.001
87. Michalik KM, You X, Manavski Y, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114(9):1389–1397. doi:10.1161/CIRCRESAHA.114.303265
88. Zhao J, Li L, Peng L. MAPK1 up-regulates the expression of MALAT1 to promote the proliferation of cardiomyocytes through PI3K/AKT signaling pathway. Int J Clin Exp Pathol. 2015;8(12):15947–15953.
89. Li H, Zhao Q, Chang L, et al. LncRNA MALAT1 modulates ox-LDL induced EndMT through the Wnt/beta-catenin signaling pathway. Lipids Health Dis. 2019;18(1):62. doi:10.1186/s12944-019-1006-7
90. Gong YP, Zhang YW, Su XQ, Gao HB. Inhibition of long noncoding RNA MALAT1 suppresses high glucose-induced apoptosis and inflammation in human umbilical vein endothelial cells by suppressing the NF-kappaB signaling pathway. Biochem Cell Biol. 2020;98(6):669–675. doi:10.1139/bcb-2019-0403
91. Bayoumi AS, Aonuma T, Teoh JP, Tang YL, Kim IM. Circular noncoding RNAs as potential therapies and circulating biomarkers for cardiovascular diseases. Acta Pharmacol Sin. 2018;39(7):1100–1109. doi:10.1038/aps.2017.196
92. Bazan HA, Hatfield SA, Brug A, Brooks AJ, Lightell DJ Jr, Woods TC. Carotid plaque rupture is accompanied by an increase in the ratio of serum circR-284 to miR-221 levels. Circ Cardiovasc Genet. 2017;10(4). doi:10.1161/CIRCGENETICS.117.001720
93. Zhu K, Hu X, Chen H, et al. Downregulation of circRNA DMNT3B contributes to diabetic retinal vascular dysfunction through targeting miR-20b-5p and BAMBI. EBioMedicine. 2019;49:341–353. doi:10.1016/j.ebiom.2019.10.004
94. Wang F, Zhang M. Circ_001209 aggravates diabetic retinal vascular dysfunction through regulating miR-15b-5p/COL12A1. J Transl Med. 2021;19(1):294. doi:10.1186/s12967-021-02949-5
95. Sabanayagam C, Xu D, Ting DSW, et al. A deep learning algorithm to detect chronic kidney disease from retinal photographs in community-based populations. Lancet Digit Health. 2020;2(6):e295–e302. doi:10.1016/S2589-7500(20)30063-7
96. Cardoso CRL, Salles GC, Leite NC, Salles GF. Prognostic impact of carotid intima-media thickness and carotid plaques on the development of micro- and macrovascular complications in individuals with type 2 diabetes: the Rio de Janeiro type 2 diabetes cohort study. Cardiovasc Diabetol. 2019;18(1):2. doi:10.1186/s12933-019-0809-1
97. Gimeno-Orna JA, Faure-Nogueras E, Castro-Alonso FJ, Boned-Juliani B. Ability of retinopathy to predict cardiovascular disease in patients with type 2 diabetes mellitus. Am J Cardiol. 2009;103(10):1364–1367. doi:10.1016/j.amjcard.2009.01.345
98. Garofolo M, Gualdani E, Giannarelli R, et al. Microvascular complications burden (nephropathy, retinopathy and peripheral polyneuropathy) affects risk of major vascular events and all-cause mortality in type 1 diabetes: a 10-year follow-up study. Cardiovasc Diabetol. 2019;18(1):159. doi:10.1186/s12933-019-0961-7
99. Farrah TE, Dhillon B, Keane PA, Webb DJ, Dhaun N. The eye, the kidney, and cardiovascular disease: old concepts, better tools, and new horizons. Kidney Int. 2020;98(2):323–342. doi:10.1016/j.kint.2020.01.039
100. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi:10.1016/j.preteyeres.2017.11.003
101. Kashani AH, Chen CL, Gahm JK, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66–100. doi:10.1016/j.preteyeres.2017.07.002
102. Couturier A, Mane V, Bonnin S, et al. Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina. 2015;35(11):2384–2391. doi:10.1097/IAE.0000000000000859
103. Hwang TS, Jia Y, Gao SS, et al. Optical coherence tomography angiography features of diabetic retinopathy. Retina. 2015;35(11):2371–2376. doi:10.1097/IAE.0000000000000716
104. Ho H, Cheung CY, Sabanayagam C, et al. Retinopathy signs improved prediction and reclassification of cardiovascular disease risk in diabetes: a prospective cohort study. Sci Rep. 2017;7:41492. doi:10.1038/srep41492
105. Ting DSW, Tan GSW, Agrawal R, et al. Optical coherence tomographic angiography in type 2 diabetes and diabetic retinopathy. JAMA Ophthalmol. 2017;135(4):306–312. doi:10.1001/jamaophthalmol.2016.5877
106. Yu DY, Cringle SJ, Yu PK, et al. Retinal capillary perfusion: spatial and temporal heterogeneity. Prog Retin Eye Res. 2019;70:23–54. doi:10.1016/j.preteyeres.2019.01.001
107. Lin AD, Lee AY, Zhang Q, et al. Association between OCT-based microangiography perfusion indices and diabetic retinopathy severity. Br J Ophthalmol. 2017;101(7):960–964. doi:10.1136/bjophthalmol-2016-309514
108. Durbin MK, An L, Shemonski ND, et al. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017;135(4):370–376. doi:10.1001/jamaophthalmol.2017.0080
109. Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT362–OCT370. doi:10.1167/iovs.15-18904
110. Zhang B, Chou Y, Zhao X, Yang J, Chen Y. Early detection of microvascular impairments with optical coherence tomography angiography in diabetic patients without clinical retinopathy: a meta-analysis. Am J Ophthalmol. 2021;222:226–237. doi:10.1016/j.ajo.2020.09.032
111. Park YG, Kim M, Roh YJ. Evaluation of foveal and parafoveal microvascular changes using optical coherence tomography angiography in type 2 diabetes patients without clinical diabetic retinopathy in South Korea. J Diabetes Res. 2020;2020:6210865. doi:10.1155/2020/6210865
112. Ehlers JP, Jiang AC, Boss JD, et al. Quantitative ultra-widefield angiography and diabetic retinopathy severity: an assessment of panretinal leakage index, ischemic index and microaneurysm count. Ophthalmology. 2019;126(11):1527–1532. doi:10.1016/j.ophtha.2019.05.034
113. Schmidt-Erfurth U, Sadeghipour A, Gerendas BS, Waldstein SM, Bogunovic H. Artificial intelligence in retina. Prog Retin Eye Res. 2018;67:1–29. doi:10.1016/j.preteyeres.2018.07.004
114. Poplin R, Varadarajan AV, Blumer K, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat Biomed Eng. 2018;2(3):158–164. doi:10.1038/s41551-018-0195-0
115. Abramoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39. doi:10.1038/s41746-018-0040-6
116. Behl T, Kotwani A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res. 2015;99:137–148. doi:10.1016/j.phrs.2015.05.013
117. Ben-Av P, Crofford LJ, Wilder RL, Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995;372(1):83–87. doi:10.1016/0014-5793(95)00956-A
118. Genest J. C-reactive protein: risk factor, biomarker and/or therapeutic target? Can J Cardiol. 2010;26(Suppl A):41A–44A. doi:10.1016/S0828-282X(10)71061-8
119. Jia ZT, Liu CY, Li H. Changes of the concentration of serum ischemia modified albumin and high sensitivity C-reactive protein in type 2 diabetic patients with retinopathy. Zhonghua Yan Ke Za Zhi. 2009;45(9):805–808.
120. Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59(11):2872–2882. doi:10.2337/db09-1606
121. Gustavsson C, Agardh E, Bengtsson B, Agardh CD. TNF-alpha is an independent serum marker for proliferative retinopathy in type 1 diabetic patients. J Diabetes Complications. 2008;22(5):309–316. doi:10.1016/j.jdiacomp.2007.03.001
122. Shimizu E, Funatsu H, Yamashita H, Yamashita T, Hori S. Plasma level of interleukin-6 is an indicator for predicting diabetic macular edema. Jpn J Ophthalmol. 2002;46(1):78–83. doi:10.1016/S0021-5155(01)00452-X
123. Doganay S, Evereklioglu C, Er H, et al. Comparison of serum NO, TNF-alpha, IL-1beta, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye. 2002;16(2):163–170. doi:10.1038/sj/eye/6700095
124. Nguyen TT, Alibrahim E, Islam FM, et al. Inflammatory, hemostatic, and other novel biomarkers for diabetic retinopathy: the multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32(9):1704–1709. doi:10.2337/dc09-0102
125. Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye. 2006;20(12):1366–1369. doi:10.1038/sj.eye.6702138
126. Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales’ disease. Retina. 2008;28(6):817–824. doi:10.1097/IAE.0b013e31816576d5
127. Patel JI, Saleh GM, Hykin PG, Gregor ZJ, Cree IA. Concentration of haemodynamic and inflammatory related cytokines in diabetic retinopathy. Eye. 2008;22(2):223–228. doi:10.1038/sj.eye.6702584
128. Loporchio DF, Tam EK, Cho J, et al. Cytokine levels in human vitreous in proliferative diabetic retinopathy. Cells. 2021;10(5):1069. doi:10.3390/cells10051069
129. Hernandez C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simo R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22(6):719–722. doi:10.1111/j.1464-5491.2005.01538.x
130. Feng S, Yu H, Yu Y, et al. Levels of inflammatory cytokines IL-1beta, IL-6, IL-8, IL-17A, and TNF-alpha in aqueous humour of patients with diabetic retinopathy. J Diabetes Res. 2018;2018:8546423. doi:10.1155/2018/8546423
131. Costagliola C, Romano V, De Tollis M, et al. TNF-alpha levels in tears: a novel biomarker to assess the degree of diabetic retinopathy. Mediators Inflamm. 2013;2013:629529. doi:10.1155/2013/629529
132. Khairul-Anwar I, Wan-Nazatul-Shima S, Siti-Lailatul-Akmar Z, Siti-Azrin AH, Zunaina E. Evaluation of TNF-alpha and IL-6 in saliva among diabetic retinopathy patients in East Coast Malaysia. Trop Med Int Health. 2022;27(3):310–316. doi:10.1111/tmi.13724
133. Wirostko B, Wong TY, Simo R. Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res. 2008;27(6):608–621. doi:10.1016/j.preteyeres.2008.09.002
134. Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48(5):1131–1137. doi:10.2337/diabetes.48.5.1131
135. Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276(10):7614–7620. doi:10.1074/jbc.M009705200
136. Ahuja S, Saxena S, Akduman L, Meyer CH, Kruzliak P, Khanna VK. Serum vascular endothelial growth factor is a biomolecular biomarker of severity of diabetic retinopathy. Int J Retina Vitreous. 2019;5:29. doi:10.1186/s40942-019-0179-6
137. Zhou Z, Ju H, Sun M, Chen H. Serum vascular endothelial growth factor levels correlate with severity of retinopathy in diabetic patients: a systematic review and meta-analysis. Dis Markers. 2019;2019:9401628. doi:10.1155/2019/9401628
138. Mitamura Y, Tashimo A, Nakamura Y, et al. Vitreous levels of placenta growth factor and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Diabetes Care. 2002;25(12):2352. doi:10.2337/diacare.25.12.2352
139. Ang WJ, Zunaina E, Norfadzillah AJ, et al. Evaluation of vascular endothelial growth factor levels in tears and serum among diabetic patients. PLoS One. 2019;14(8):e0221481. doi:10.1371/journal.pone.0221481
140. Lin Y, Xiao YC, Zhu H, et al. Serum fibroblast growth factor 21 levels are correlated with the severity of diabetic retinopathy. J Diabetes Res. 2014;2014:929756. doi:10.1155/2014/929756
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.