Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Diabetes Mellitus Mediates the Relationship Between Atherogenic Index of Plasma and Gallstones: A Population-Based Cross-Sectional Study
Authors Du W, Wang Y, Song C, Tian Z, Liu Y , Shen W
Received 13 November 2023
Accepted for publication 18 January 2024
Published 23 January 2024 Volume 2024:17 Pages 317—332
DOI https://doi.org/10.2147/DMSO.S449562
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Wenyi Du,1,* Yixuan Wang,2,* Chen Song,1 Zhiqiang Tian,1 Yuan Liu,1 Wei Shen1
1Department of General Surgery, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi, People’s Republic of China; 2Medical Integration and Practice Center, Reproductive Hospital Affiliated to Shandong University, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuan Liu; Wei Shen, Department of General Surgery, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi, People’s Republic of China, Tel +86 17852061572 ; +86 13385110723, Email [email protected]; [email protected]
Purpose: Previous studies have shown a correlation between diabetes mellitus and gallstone formation. The atherogenic index of plasma (AIP) is associated with many metabolic diseases. However, insufficient evidence still exists to elucidate the association between AIP and gallstones. The primary objective of this study was to investigate the correlation between AIP and gallstones in US adults, and the secondary objective was to analyze whether diabetes plays a mediating role in the association.
Patients and Methods: Using data from the National Health and Nutrition Survey (NHANES) conducted between 2017 and March 2020, this study investigated the association between AIP and gallstone incidence in US adults. A variety of statistical methods were used to analyze the data in this study, including multivariate logistic regression, subgroup analyses, restricted cubic spline curves (RCS), and mediation effects analysis. In addition, two-stage linear regression was used to detect possible threshold and saturation effects.
Results: A total of 6952 subjects were enrolled in the trial, of which 748 patients were diagnosed with gallstones. A significant positive association between AIP and gallstones was observed by fully adjusted multivariate logistic regression analysis, with an odds ratio (OR) of 1.45 and a 95% confidence interval (CI) of (1.09, 1.93). In addition, a non-linear positive association and saturation effect between AIP and gallstones were found, with an inflection point of 0.2246. Mediation analysis showed that diabetes had a mediating effect of 16.9% in the association between AIP and gallstones.
Conclusion: This study suggests that elevated levels of AIP are linked to an augmented vulnerability to gallstone development, with diabetes serving as a mediating factor. These findings present a novel perspective on clinical approaches to prevent and manage gallstones.
Keywords: gallstones, atherogenic index of plasma, diabetes mellitus, National Health and Nutrition Examination Survey, cross-sectional studies
Introduction
Gallstones rank among the most prevalent gastrointestinal conditions globally.1 Gallstones affect approximately 6% of men and 9% of women in the United States.2 According to estimates, approximately $62 billion is allocated annually in the United States for the prevention and treatment of cholelithiasis.3 Around 10% of people with asymptomatic gallstones will experience symptoms or need treatment within five years.4 Between 50 and 70% of acute pancreatitis is caused by gallstones, which can lead to severe abdominal pain and life-threatening infections.5 Other serious complications that may result from gallstones include acute obstructive septic cholangitis, liver failure, and gallbladder cancer.6 Hence, it is crucial to recognize modifiable and manageable elements to decrease the occurrence of gallstones.
The atherogenic index of plasma (AIP) is a novel index of lipid metabolism that has emerged in recent years.7 AIP is a logarithmic transformation of the ratio of triglycerides (TG) to high-density lipoprotein (HDL) cholesterol (HDL-C), which was initially a reliable predictor of atherosclerosis and coronary artery disease.8,9 In addition to its traditional role in cardiovascular health, AIP has attracted scholarly attention for its potential impact on other endocrine diseases, including diabetes and metabolic syndrome.10 Therefore, AIP has become an important parameter in the field of lipidology.
The global population of individuals living with diabetes is estimated to be around 463 million, with projections indicating a projected increase of 51% by the year 2045.11 The association between diabetes mellitus and gallstones has been a topic of interest among researchers in recent years. Several epidemiological studies have reported that diabetes increases the risk of gallstones.12,13 The mechanisms mediating this association are not fully understood and may be related to factors such as insulin resistance, altered bile composition, and impaired gallbladder emptying.14 However, no study to date has reported an association between AIP and gallstones and whether the effect of AIP on gallstones is mediated through diabetes. Motivated by this knowledge gap, the present study aimed to conduct a large-scale cross-sectional investigation using data from the National Health and Nutrition Examination Survey (NHANES) conducted from 2017 to March 2020 to elucidate the association between AIP and gallstones and the mediating role of diabetes therein.
Materials and Methods
The Design of the Study and the Individuals Involved
Starting from 1998, the NHANES has conducted an extensive examination to assess the overall health and eating habits of the American people, including both grown-up individuals and those who are young. The National Center for Health Statistics (NCHS), which is a part of the Centers for Disease Control and Prevention (CDC), gathers survey information by conducting in-home interviews and health measurements at mobile testing centers. To ensure the precision and inclusiveness of its data, NHANES utilizes a complex cohort design that is stratified, multistage, and based on probability.15,16 Participants signed an informed consent form that was approved by the NCHS Research Ethics Review Board. The NHANES dataset and its documentation and protocols are freely available on the website.17
The study included individuals who were at least 20 years old and participated in the US National Health Survey between 2017 and March 2020. To guarantee the accuracy and dependability of the findings, our research employed precise criteria for exclusion. First, we excluded those who did not provide information on gallstones in the medical survey (n = 6350). 86 pregnant women, 21 participants without education and marriage information, and 1337 participants without TG and HDL-C test results were also excluded. Excluded participants with missing data on potential confounders (body mass index, asthma, heart disease, liver disease, cancer, smoking, and diet) from the sample group. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Wuxi People’s Hospital, with approval number KY23188. The original data presented in the study are included in the Supplementary Table 1.
Definition of Gallstones
Participants’ responses to the question “Has the doctor ever diagnosed you with gallstones?” were used to ascertain the presence or absence of gallstones. The questionnaire was administered, and a positive response of “yes” indicated the presence of gallstones, while a negative response of “no” indicated their absence.
Definition of AIP
Based on prior research, the computation of the atherogenic index of plasma (AIP) entails utilizing the equation 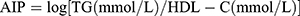 .18 To make future research easier, participants were divided into quartiles according to their AIP values. It is essential to highlight that, within the context of the study design, AIP was regarded as an exposure variable of particular interest.
.18 To make future research easier, participants were divided into quartiles according to their AIP values. It is essential to highlight that, within the context of the study design, AIP was regarded as an exposure variable of particular interest.
Diagnosis of Diabetes
Diabetes is characterized by fulfilling the requirements of having an HbA1c level that is 6.5% or higher, acknowledging the condition through self-reporting, and/or utilizing medication for managing diabetes.19
Ascertainment of Covariates
This study incorporated various variables, including age, sex, ethnicity, educational attainment, marital status, the ratio of family income to poverty (PIR), body mass index (BMI), smoking habits, alcohol consumption, existing medical conditions (diabetes, high blood pressure, asthma, heart disease, liver disease, cancer), and dietary factors such as carbohydrate, protein, fat, fiber, and water intake, to examine their potential impact on the relationship between AIP and gallstones.
In response to the query “Are you currently a smoker?”, the participants were categorized into three groups based on their smoking status: never smokers (individuals who have never smoked or have smoked fewer than 100 cigarettes in their lifetime), smokers (individuals who have smoked at least 100 cigarettes in their lifetime and currently smoke), and former smokers (individuals who have smoked at least 100 cigarettes in their lifetime but no longer smoke).20 Participants who consumed at least 12 alcoholic beverages per year or whose dietary intake contained >0g of alcohol were considered to be drinkers.21 Hypertension was operationally defined as meeting the criteria of having an average systolic blood pressure of 140 mmHg or higher and/or diastolic blood pressure of 90 mmHg or higher, self-reporting a diagnosis of hypertension, and/or utilizing antihypertensive medication.22 Between 2017 and 2020, each participant undertook one 24-hour review of dietary data, and our analyses will use the average consumption from both reviews or the first day’s dietary data if the second day’s dietary data were missing.
Statistical Analyses
Statistical analysis was conducted using R software (version 4.2.2) and Empower Stats (version 2.0), with a significance level of P < 0.05. The presence of multicollinearity was evaluated through the utilization of the Variance Inflation Factor (VIF) and tolerance. Severe multicollinearity was operationally defined as a VIF value equal to or exceeding 10.23 All the covariates included in the study had VIFs less than 5, and there was no significant multicollinearity observed. The statistical indicators were accompanied by reported odds ratios (OR) and ninety-five percent confidence intervals (CI). AIP was categorized into four equal parts, with the first quartile (Q1) acting as the benchmark group. Continuous variables were represented as mean and standard deviation, while categorical variables were presented as frequencies or percentages. AIP quartiles were tested using either the chi-square test or the Kruskal–Wallis H-test. To investigate the association between gallstones and AIP, four logistic regression models were constructed. The first model was unadjusted and the second model was adjusted for gender, age, and ethnicity. In addition to Model 2, Model 3 considered educational attainment, marital status, PIR, smoking, alcohol consumption, and medical comorbidities. To eliminate the potential influence of dietary factors on gallstones, Model 4 considered dietary intake of carbohydrates, protein, fat, dietary fiber, and water in addition to Model 3.24
Furthermore, subgroup analyses were conducted to investigate if AIP and gallstones exhibited potential variations in specific populations based on factors such as age, gender, ethnicity, educational background, smoking habits, alcohol consumption, and hypertension. To obtain a more accurate assessment of the non-linear correlation between AIP and gallstones, we also fitted restricted cubic spline (RCS) curves. Finally, mediation analysis allows us to calculate how many mediated effects are produced by diabetes. In addition to providing statistical evidence for mechanistic analyses, this strategy is ideal for revealing pathways. The direct effect indicates the association between AIP and gallstones; the indirect effect indicates the association is mediated by diabetes; and the mediation ratio indicates the percentage of mediated effects. Using these statistical methods, possible associations between AIP and gallstone risk can be examined more broadly.
Results
After applying appropriate exclusion criteria, a total of 6952 patients were included in this analysis, of whom 3406 were male and 3546 were female. Figure 1 illustrates the process of screening participants and provides detailed information.
 |
Figure 1 Flowchart of participants selection from NHANES 2017–2020. |
Gallstone-Based Baseline Characteristics of the Population
The baseline characteristics of patients with and without gallstones are summarized in Table 1. Gallstones were detected in 748 out of 6952 participants, giving an overall prevalence of gallstones in the population of 10.76%. Participants in the study were on average 51 years old. There were 49% males and 51% females in the gender distribution. Women, older adults, non-Hispanic whites, and non-drinkers had a higher risk of developing gallstones than other patients (all P < 0.05).
 |
Table 1 Baseline Characteristics of the Gallstones Group versus the Non-Gallstones |
Table 2 divides the participants into quartiles based on their AIP values. Q1 corresponded to values between −0.959 and −0.228, Q2 between −0.228 and −0.0237, Q3 between −0.0237 and 0.201, and Q4 between 0.201 and 1.64. The prevalence of gallstones increased significantly as participants’ AIP values increased (Q1: 7%, Q2: 10%, Q3: 13%, and Q4: 13%, p < 0.001).
 |
Table 2 Baseline Characteristics of the Study Population According to AIP in NHANES 2017–2020 |
Logistic Regression Analyses
The relationships between AIP and gallstones, as well as diabetes and gallstones, are illustrated in Table 3 through the findings obtained from multivariate logistic regression analyses. A strong and statistically significant positive correlation was found between AIP and gallstones in the original model, with an odds ratio of 1.84 (95% CI: 1.46–2.32; p-value < 0.0001). The robust and favorable association persisted as highly significant in Model 2, even after accounting for factors like gender, age, and ethnicity (OR = 2.35; 95% CI: 1.81–3.03; P < 0.0001). In Model 3, which is built upon Model 2, the correlation between AIP and gallstones remained statistically significant (OR = 1.45; 95% CI: 1.09–1.92; P = 0.0102) even after considering variables like educational background, marital status, BMI, PIR, smoking habits, alcohol intake, and related medical conditions (such as hypertension, asthma, coronary artery disease, liver disease, and malignancy). Despite accounting for all covariates in Model 4, the aforementioned significant correlation persisted as statistically significant (OR = 1.45; 95% CI: 1.09–1.93; P = 0.0108). This indicates that with every unit rise in AIP, the occurrence of gallstones escalated by 38%. The strong correlation between AIP and gallstones persisted even after dividing AIP into quartiles, as indicated by the statistical significance of the trend tests (P < 0.05). In Model 4, there was a 54% rise in gallstone occurrence when comparing the highest quartile of AIP to the lowest quartile. Furthermore, a notable correlation between diabetes and gallstones was detected. After accounting for all variables in Model 4, individuals with diabetes exhibited a 27% increased likelihood of developing gallstones in comparison to those without diabetes (OR = 1.27; 95% CI: 1.05–1.55; P = 0.0148).
 |
Table 3 Odds Ratios and 95% Confidence Intervals for Gallstones According to AIP and Diabetes |
The findings of the multiple logistic regression analysis examining the relationship between AIP and diabetes are presented in Table 4. In the unadjusted model, a statistically significant positive association between AIP and diabetes was observed (OR = 5.47; 95% CI: 4.51–6.63; p < 0.0001). Notably, this strong positive correlation remained significant even after controlling for gender, age, and race variables in Model 2 (OR = 7.33; 95% CI: 5.86–9.17; p < 0.0001). Model 3, which additionally adjusted for education, marital status, BMI, PIR, smoking, alcohol consumption, and associated comorbidities (hypertension, asthma, coronary heart disease, liver disease, and malignancy) based on Model 2, showed that the association between AIP and diabetes was still significant (OR = 4.70; 95% CI: 3.70–5.97; p < 0.0001). Furthermore, even after controlling for all covariates in Model 4, the aforementioned positive association continued to be statistically significant (OR = 5.08; 95% CI: 3.99–6.47; P < 0.0001).
 |
Table 4 Multiple Logistic Regression of the Association Between AIP and Diabetes Mellitus |
Subgroup Analyses and Interaction Tests
To assess the stability of the association between AIP and gallstones and to identify potential differences between different populations, we performed subgroup analyses, the results of which are shown in Figure 2. Notably, the association between AIP and high risk of gallstones was more pronounced in the subgroup of participants who were under 60 years of age, female, had a university degree or higher, smoked, drank alcohol, and were hypertensive (all P values < 0.05). In the interaction test, we found that the “P for interaction” was greater than 0.05, suggesting that the positive association between AIP and gallstones is generally stable and consistent in the general population.
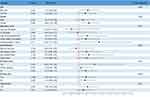 |
Figure 2 Subgroups analysis for the associations between AIP and gallstones. |
Gallstones Exhibit a Non-Linear Correlation with AIP
In Figure 3, the Restricted Cubic Spline Curve (RCS) illustrates the association between AIP and gallstones. Following adjustment for all covariates, a significant nonlinear relationship was observed between AIP and gallstones (nonlinear P < 0.001). To further explore this nonlinear relationship, a two-stage linear regression model was employed to calculate the threshold effect and inflection point. The inflection point for AIP was determined to be 0.2246. When the AIP value was below 0.2246, a robust positive correlation was observed between AIP and the occurrence of gallstones, with an odds ratio (OR) of 1.36 (95% CI: 1.24–1.50). However, this correlation weakened significantly when AIP measurements reached 0.2246.
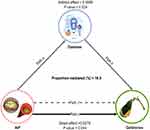
Mediation of Diabetes
Perform mediation analyses to assess whether diabetes mediates the association between AIP and gallstone occurrence. The model and pathway for the mediation analysis are shown in Figure 4. After adjusting for all potential confounders, the results showed a significant indirect effect of AIP on gallstone occurrence through diabetes (β [95% CI], 0.0056 [0.00032–0.01082]; P = 0.024), suggesting a partially mediated role of diabetes. The effect of AIP on gallstones remained statistically significant despite controlling for diabetes (β [95% CI], 0.02753 [0.00044–0.05846] p = 0.044), suggesting both direct and indirect effects. Approximately 16.9% of the effect of AIP on gallstones [95% CI, 0.5–83.7%] was mediated by diabetes. The results of the intermediation analysis are shown in Table 5.
 |
Table 5 Mediation Analysis for the Associations Between AIP and Gallstones |
Discussion
This study reports for the first time the mediating effect of diabetes in the association of AIP with gallstones. We analyzed a sample of 6952 adults and found a significant positive correlation between AIP and gallstones, suggesting that those with elevated AIP were more likely to develop gallstones. This correlation remains strong even when all confounding covariates are accounted for in the fully adjusted model (Model 4). According to subgroup analyses and interaction tests, the positive association between AIP and gallstones was more pronounced in participants who were under 60 years of age, female, with a bachelor’s degree or higher, and who smoked, consumed alcohol, and were hypertensive. Interaction tests between subgroups were not significantly different and were relatively stable. RCS curve fitting and threshold effect analyses showed that there was a nonlinear correlation between AIP and gallstones, with a turning point of 0.2246. Overall, AIP was positively correlated with the occurrence of gallstones. At the same time, diabetes played a partial mediating role in the relationship between AIP and gallstones, which highlights the importance of focusing on blood glucose and strengthening the management of diabetic patients while regulating lipid levels to prevent gallstones.
Recently, academics have increasingly ventured into the field of biomarkers associated with gallstones through extensive and comprehensive investigations using the NHANES database. In their study, Wang et al25 demonstrated a significant association between the metabolic index of insulin resistance score (METS-IR) and the prevalence of gallbladder stones. The results showed a strong and direct correlation, indicating that with every increment in the METS-IR index, there was a proportional rise of 3.3% in the occurrence of gallbladder stones. In contrast, a negative linear correlation was found between the METS-IR index and the age at which the first gallstone was formed. In another cross-sectional investigation conducted by Zhang et al,26 a significant discovery was made indicating a positive correlation between a higher visceral obesity index (VAI) and the prevalence of gallstones, potentially resulting in an earlier age at initial gallstone surgery. This association persisted when subgroup analyses were performed in different age groups, and races, with and without hypertension, and with and without diabetes. Notably, this positive association was more pronounced in women. Wang et al27 discovered a noteworthy correlation between the presence of heavy metals in the bloodstream and the occurrence of gallstones. Specifically, blood selenium emerged as an autonomous risk factor for gallstones, particularly among women, non-Hispanic whites, individuals below the age of 65, non-drinkers, those with obesity, and individuals with higher levels of education. Furthermore, blood cadmium (Cd) and mercury (Hg) exhibited a negative association within certain thresholds, indicating a potential decrease in the risk of gallstones. These combined results highlight the importance of incorporating multiple markers in the assessment of gallstones and shed light on the complex causes of the disease. However, to date, no academic studies have reported a potential relationship between AIP and gallstones.
The AIP, a lipid metabolism index, effectively integrates TG and HDL-C, providing insights into the ratio of TG to HDL-C as well as the size of lipoprotein particles. It serves as a superior indicator of dyslipidemia compared to elevated TG or reduced HDL-C levels, as it better reflects the pathogenicity and specificity of dyslipidemia.28,29 To our understanding, this study represents the initial cross-sectional analysis employing the NHANES database to examine the correlation between AIP and gallstones, as well as to explore the potential mediating influence of diabetes. Prior investigations have explored the relationship between AIP and various diseases through diverse epidemiological approaches, encompassing distinct target populations. Hernandez et al30 found that AIP ≥ 0.11 was significantly associated with severe suppurative tonsillitis in a Spanish case-control study (OR = 4.38; 95% CI = 1.09 to 17.50, p = 0.037). In a meticulous study of 112,200 Chinese patients, Liu et al31 found a strong association between AIP and NAFLD, and that AIP had a better discriminatory ability than other lipid markers in identifying NAFLD. A prospective study directed by Cho et al32 involving 323 Korean patients showed that according to the Atlanta classification, AIP was significantly and positively associated with pancreatitis severity. An extensive study by Sun et al,33 representing a large American cohort, revealed no significant association between periodontitis prevalence and AIP overall (P = 0.341), but a significant association was seen in non-smokers (P = 0.031). Shi et al34 reported a positive linear association between AIP and prediabetes or diabetes mellitus. Although an increase in AIP significantly increases the prevalence of diabetes, it remains stable only in the female population. A cross-sectional study by Yin et al35 found a J-shaped association between AIP and type 2 diabetes mellitus, and that higher AIP was significantly associated with a higher risk of insulin resistance and T2D. There is growing evidence that AIP is not only associated with cardiovascular disease but also with other metabolic and endocrine diseases.
Previous research has demonstrated a correlation between diabetes mellitus and gallstones, indicating that individuals with diabetes mellitus face an elevated likelihood of developing gallstones compared to the general population, particularly those with inadequately regulated blood glucose levels.36,37 In a cross-sectional investigation conducted in Ali, a cohort of 204 patients diagnosed with gallstones was examined, consisting of 74 individuals with diabetes, 79 without diabetes, 51 classified as pre-diabetic, 1 with well-managed diabetes, and 1 with poorly controlled diabetes (37 patients). The results indicate that diabetes is a contributing factor in the formation of gallstones.38 Conversely, controlled diabetes significantly reduces the risk of developing gallstones. A retrospective cohort study showed a significant reduction in the incidence of gallstones in diabetic patients on long-term metformin.39 Our study similarly found that diabetics had a 27% increased risk of gallstones compared to non-diabetics (in Model 4). However, the precise mechanism by which AIP enhances the susceptibility to gallstones, as well as the significance of diabetes about this correlation, remains uncertain. The connection between AIP and gallstones, as well as the heightened risk of gallstones due to diabetes, can be accounted for by several potential biological mechanisms. First, AIP is an indicator of the degree of atherosclerosis, which is strongly associated with metabolic syndrome and diabetes.10,34,40,41 One of the major contributing factors to the development of diabetes in patients with high AIP is dyslipidemia, which is usually manifested by elevated levels of low-density lipoprotein cholesterol (LDL-C) and triglycerides and reduced levels of high-density lipoprotein cholesterol (HDL-C). Abnormal lipid metabolism promotes the formation of atherosclerotic plaques, impairs glucose metabolism pathways, and leads to insulin resistance and the eventual development of diabetes.42,43 In our study, this fact was verified by using four integrated regression models. Interestingly, in Model 4, which adjusted for all potential confounders, the positive correlation between AIP and diabetes was more significant than in Model 3. For every one-unit rise in AIP, the risk of developing diabetes increased by 4.08-fold (OR = 5.08; 95% CI: 3.99–6.47; P < 0.0001). In addition, diabetes can exacerbate the reduced blood supply to the gallbladder through microvascular damage and neurological complications, which reduces the peristaltic capacity of the gallbladder. Prolonged siltation of bile in the gallbladder increases bile saturation, leading to cholecystitis and gallstones.44–47 It has been found that diabetic patients with sphincter Oddi dysfunction impede bile elimination, further increasing the risk of gallstones.48 Lastly, reduced sensitivity of gallbladder smooth muscle cells to cholecystokinin or a decrease in the number of cholecystokinin receptors in the gallbladder wall may be responsible. Insulin-related signaling pathways are aberrant in diabetic patients, mainly due to diminished punctate signaling of the insulin receptor in human hepatocytes and high levels of reactive oxygen species (ROS), which reduce the functional activity of clusters of insulin receptor molecules in otherwise insulin-sensitive cells.49
The study’s strength is derived from its utilization of a sample comprising US adults, acquired via a meticulously stratified, multi-stage probability sampling methodology, consequently enhancing the precision of the findings. In addition, we used four models to account for confounding variables, resulting in more reliable results. Finally, subgroup analyses and mediating effects analyses were performed to gain more insight into the association between AIP and gallstones in different populations and the mediating role that diabetes plays between the two.
Limitations
Of course, this study has its limitations. First, we extracted information only from the NHANES database, which is a cross-sectional survey and does not have prospective cohort follow-up data. In addition, the study’s eligibility criteria for participants were based on whether they had gallstones, and no information is available on the type of gallstones or when they first appeared. In conclusion, we did not perform additional analyses of the correlation between gallstones and the use of medication or the implementation of specific treatments in patients with gallstones. However, it was considered that many of the comorbidities were confounders. To summarize, our research provides a valuable understanding of the relationship between AIP and gallstones, thus improving comprehension in this specific field.
Conclusion
This study suggests that elevated levels of AIP are linked to an augmented vulnerability to gallstone development, with diabetes serving as a mediating factor. It implies that maintaining AIP within a lower range and prioritizing glycemia regulation is imperative for gallstone prevention. These findings present a novel perspective on clinical approaches to prevent and manage gallstones. However, additional research is necessary to elucidate the underlying mechanisms at play.
Abbreviations
BMI, Body mass index; CI, Confidence interval; OR, Odds Ratio; SD, Standard deviation; NHANES, National Health and Nutrition Examination Survey; PIR, The ratio of family income to poverty; AIP, Atherogenic index of plasma; GED, General Equivalency Diploma; TG, Triglyceride; HDL-C, High-density lipoprotein cholesterol; VIF, Variance Inflation Factor; NCHS, The National Center for Health Statistics; CDC, Centers for Disease Control and Prevention.
Data Sharing Statement
The original data presented in the study are included in Supplementary Table 1, and further inquiries can be directed to the corresponding authors ([email protected], [email protected]).
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Wuxi People’s Hospital, with approval number KY23188. All experimental protocols were approved by the NCHS Ethics Review Committee (https://www.cdc.gov/nchs/nhanes/irba98.htm). Informed consent for the study was obtained from all subjects and/or their legal guardians by signing an informed consent form.
Funding
This work was supported by the Top Talent Support Program for young and middle-aged people of the Wuxi Health Committee (Grant No. HB2020007).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Qin A, Wu J, Qiao Z, et al. Comparison on the efficacy of three duct closure methods after laparoscopic common bile duct exploration for choledocholithiasis. Med Sci Monit. 2019;25:9770–9775. doi:10.12659/MSM.918743
2. Markotic F, Grgic S, Poropat G, et al. Antibiotics for adults with acute cholecystitis or acute cholangitis or both. Cochrane Database Syst Rev. 2020;6:CD013646.
3. Grigor’eva IN, Romanova TI. Gallstone disease and microbiome. Microorganisms. 2020;8(6):835. doi:10.3390/microorganisms8060835
4. Madden MA, Daksha T, Smeeton NC, Alison C. Modified dietary fat intake for treatment of gallstone disease. Cochrane Database Syst Rev. 2017;6:CD012608.
5. Bálint ER, Fűr G, Kiss L, et al. Assessment of the course of acute pancreatitis in the light of aetiology: a systematic review and meta-analysis. Sci Rep. 2020;10(1):17936. doi:10.1038/s41598-020-74943-8
6. Liu Z, Kemp TJ, Gao YT, et al. Circulating levels of inflammatory proteins and survival in patients with gallbladder cancer. Sci Rep. 2018;8(1):5671. doi:10.1038/s41598-018-23848-8
7. Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA, Pérez-Maldonado IN. Atherogenic Index of plasma: novel predictive biomarker for cardiovascular illnesses. Arch Med Res. 2019;50(5):285–294. doi:10.1016/j.arcmed.2019.08.009
8. Huang H, Yu X, Li L, et al. Atherogenic index of plasma is related to coronary atherosclerotic disease in elderly individuals: a cross-sectional study. Lipids Health Dis. 2021;20(1):68. doi:10.1186/s12944-021-01496-8
9. Song P, Xu L, Xu J, et al. Atherogenic Index of plasma is associated with body fat level in type 2 diabetes mellitus patients. Curr Vasc Pharmacol. 2018;16(6):589–595. doi:10.2174/1570161116666180103125456
10. Samimi S, Rajabzadeh S, Rabizadeh S, et al. Atherogenic index of plasma is an independent predictor of metabolic-associated fatty liver disease in patients with type 2 diabetes. Eur J Med Res. 2022;27(1):112. doi:10.1186/s40001-022-00731-x
11. Aschner P, Gagliardino JJ, Ilkova H, et al. Persistent poor glycaemic control in individuals with type 2 diabetes in developing countries: 12 years of real-world evidence of the International Diabetes Management Practices Study (IDMPS). Diabetologia. 2020;63(4):711–721. doi:10.1007/s00125-019-05078-3
12. Lv J, Yu C, Guo Y, et al. Gallstone disease and the risk of type 2 diabetes. Sci Rep. 2017;7(1):15853. doi:10.1038/s41598-017-14801-2
13. Ratheesh R, Ulrich MT, Ghozy S, Al-Jaboori M, Nayak SS. The association between diabetes and gallstones: a nationwide population-based cohort study. Prz Gastroenterol. 2023;18(3):292–299. doi:10.5114/pg.2023.131395
14. Yuan S, Gill D, Giovannucci EL, Larsson SC. Obesity, type 2 diabetes, lifestyle factors, and risk of gallstone disease: a Mendelian randomization investigation. Clin Gastroenterol Hepatol. 2022;20(3):e529–e537. doi:10.1016/j.cgh.2020.12.034
15. Curtin LR, Mohadjer LK, Dohrmann SM, et al. National health and nutrition examination survey: sample design, 2007–2010. Vital Health Stat. 2013;160:1–23.
16. Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat. 2014;162:1–33.
17. Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–1621. doi:10.1001/jama.2021.18236
18. Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, et al. Atherogenic Index of Plasma (AIP): a marker of cardiovascular disease. Med J Islam Repub Iran. 2015;29:240. doi:10.1186/1476-511X-6-1
19. Aschner P, Beck Nielsen H, Bennet P, et al. Global guideline for type 2 diabetes. Diabet Res Clin Pract. 2014;104(1):1–52. doi:10.1016/j.diabres.2012.10.001
20. Wang J, Liu F, Kong R, Han X. Association between globulin and diabetic nephropathy in type2 diabetes mellitus patients: a cross-sectional study. Front Endocrinol. 2022;13:890273. doi:10.3389/fendo.2022.890273
21. Gong R, Luo G, Wang M, Ma L, Sun S, Wei X. Associations between TG/HDL ratio and insulin resistance in the US population: a cross-sectional study. Endocr Connect. 2021;10(11):1502–1512. doi:10.1530/EC-21-0414
22. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e143. doi:10.1161/CIR.0000000000000625
23. Liu M, Zhang Z, Zhou C, et al. Predicted fat mass and lean mass in relation to all-cause and cause-specific mortality. J Cachexia Sarcopenia Muscle. 2022;13(2):1064–1075. doi:10.1002/jcsm.12921
24. van Erpecum KJ. Biliary lipids, water and cholesterol gallstones. Biol Cell. 2005;97(11):815–822. doi:10.1042/BC20040088
25. Wang J, Yang J, Chen Y, Rui J, Xu M, Chen M. Association of METS-IR index with prevalence of gallbladder stones and the age at the first gallbladder stone surgery in US adults: a cross-sectional study. Front Endocrinol. 2022;13:1025854. doi:10.3389/fendo.2022.1025854
26. Zhang G, Ding Z, Yang J, et al. Higher visceral adiposity index was associated with an elevated prevalence of gallstones and an earlier age at first gallstone surgery in US adults: the results are based on a cross-sectional study. Front Endocrinol. 2023;14:1189553. doi:10.3389/fendo.2023.1189553
27. Wang J, Sun YX, Xiang S, et al. The association between blood heavy metals and gallstones: a cross-sectional study. Sci Total Environ. 2023;904:166735. doi:10.1016/j.scitotenv.2023.166735
28. Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50(7):1612–1617. doi:10.2337/diabetes.50.7.1612
29. Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34(7):583–588. doi:10.1016/S0009-9120(01)00263-6
30. Hernández JL, Baldeón C, López-Sundh AE, Ocejo-Vinyals JG, Blanco R, González-López MA. Atherogenic index of plasma is associated with the severity of Hidradenitis Suppurativa: a case-control study. Lipids Health Dis. 2020;19(1):200. doi:10.1186/s12944-020-01377-6
31. Liu J, Zhou L, An Y, Wang Y, Wang G. The atherogenic index of plasma: a novel factor more closely related to non-alcoholic fatty liver disease than other lipid parameters in adults. Front Nutr. 2022;9:954219. doi:10.3389/fnut.2022.954219
32. Cho SK, Kim JW, Huh JH, Lee KJ. Atherogenic Index of plasma is a potential biomarker for severe acute pancreatitis: a prospective observational study. J Clin Med. 2020;9(9):2982. doi:10.3390/jcm9092982
33. Sun J, Guo G. Association between atherogenic index of plasma and periodontitis among U.S. adults. BMC Oral Health. 2023;23(1):166. doi:10.1186/s12903-023-02853-y
34. Shi Y, Wen M. Sex-specific differences in the effect of the atherogenic index of plasma on prediabetes and diabetes in the NHANES 2011–2018 population. Cardiovasc Diabetol. 2023;22(1):19. doi:10.1186/s12933-023-01740-8
35. Yin B, Wu Z, Xia Y, Xiao S, Chen L, Li Y. Non-linear association of atherogenic index of plasma with insulin resistance and type 2 diabetes: a cross-sectional study. Cardiovasc Diabetol. 2023;22(1):157. doi:10.1186/s12933-023-01886-5
36. Attili AF. Diabetes and gallstones. Dig Liver Dis. 2011;43(9):672–673. doi:10.1016/j.dld.2011.06.018
37. Wang W, Li N. The association of gallstone disease and diabetes mellitus. A meta-analysis. Saudi Med J. 2014;35(9):1005–1012.
38. Ali S, Ahamad ST, Talpur AS, Parajuli S, Farooq J. Prevalence of non-insulin-dependent diabetes mellitus among patients with cholelithiasis: a single-centered, cross-sectional study. Cureus. 2018;10(4):e2444.
39. Liao KF, Chuang HY, Lai SW. Metformin use correlates with reduced risk of gallstones in diabetic patients: a 12-year follow-up study. Front Pharmacol. 2017;8:765. doi:10.3389/fphar.2017.00765
40. Li Z, Huang Q, Sun L, Bao T, Dai Z. Atherogenic Index in type 2 diabetes and its relationship with chronic microvascular complications. Int J Endocrinol. 2018;2018:1765835. doi:10.1155/2018/1765835
41. Yi Q, Ren Z, Bai G, et al. The longitudinal effect of the atherogenic index of plasma on type 2 diabetes in middle-aged and older Chinese. Acta Diabetol. 2022;59(2):269–279. doi:10.1007/s00592-021-01801-y
42. Škrha J. Diabetes, Lipids, and CV Risk. Curr Atheroscler Rep. 2021;23(3):8. doi:10.1007/s11883-021-00905-8
43. Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–899. doi:10.1007/s00125-015-3525-8
44. Arshad F, Laway BA, Rather TA, Kuchay MS, Khan SH. Impaired gallbladder motility in adults with newly detected type 2 diabetes and lack of reversibility after achieving euglycemia. Can J Diabetes. 2015;39(2):101–104. doi:10.1016/j.jcjd.2014.07.223
45. Bennion LJ, Grundy SM. Effects of diabetes mellitus on cholesterol metabolism in man. N Engl J Med. 1977;296(24):1365–1371. doi:10.1056/NEJM197706162962401
46. Bucceri AM, Brogna A, Ferrara R. Sonographic study of postprandial gallbladder emptying and common bile duct changes in patients with diabetes or cholelithiasis. Abdom Imaging. 1994;19(5):427–429. doi:10.1007/BF00206931
47. Ponz De Leon M, Ferenderes R, Carulli N. Composizione della bile in pazienti ad alto rischio di colelitiasi [Bile composition in patients with high risk of cholelithiasis]. Minerva Med. 1976;67(53):3483–3490. Italian.
48. Tan J, Kou J. Advances in research on the pathogenesis of type 2 diabetes complicated with gallstone. Int J Clin Med. 2019;10(03):161–173. doi:10.4236/ijcm.2019.103016
49. Dall’Agnese A, Platt JM, Zheng MM, et al. The dynamic clustering of insulin receptor underlies its signaling and is disrupted in insulin resistance. Nat Commun. 2022;13(1):7522. doi:10.1038/s41467-022-35176-7
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.