Back to Journals » Drug Design, Development and Therapy » Volume 17
Development of a Fast Onset Proton Pump Inhibitor: Comparison of Fixed-Dose Combination of Rabeprazole and Sodium Bicarbonate (YPI-011) to the Conventional Enteric-Coated Rabeprazole
Authors Bae S , Kwon J, Lee MH, Yu KS , Lee S
Received 5 October 2022
Accepted for publication 13 January 2023
Published 15 February 2023 Volume 2023:17 Pages 497—506
DOI https://doi.org/10.2147/DDDT.S391716
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Sungyeun Bae,1 Jihoon Kwon,2 Mi-Hye Lee,3 Kyung-Sang Yu,1 SeungHwan Lee1
1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Republic of Korea; 2Department of Statistics, APACE, Seoul, Republic of Korea; 3Yungjin Pharmaceutical Co., Ltd, Seoul, Republic of Korea
Correspondence: SeungHwan Lee, Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea, Tel +82-2-2072-1920, Fax +82-2-742-9252, Email [email protected]
Purpose: Proton pump inhibitors (PPIs) are the first-line therapy for gastroesophageal reflux disorder (GERD). Unlike conventional PPIs, non-enteric coated PPIs with antacid salt enable a faster acid suppression through the rapid absorption of the PPI. YPI-011 is a newly developed fixed-dose combination of a rabeprazole with sodium bicarbonate (NaHCO3). This study compared the pharmacokinetics (PKs) and pharmacodynamics (PDs) of YPI-011 to the conventional enteric-coated rabeprazole (Pariet®).
Materials and Methods: A randomized, open-label, two-treatment, two-sequence crossover study was conducted with two different doses (10 and 20 mg) and 44 subjects in each group. They randomly received either a test or reference treatment for 7 days in the first period and the other treatment in the second period. Blood samples for the PK analysis were taken after the single- and multiple-dose. Intragastric pH monitoring for the PD analysis was implemented for baseline and after the single- and multiple-dose.
Results: Gastric acid suppression evaluated by the percentage decrease from baseline in the integrated gastric acidity for a 24-hour interval after the multiple-dose was similar between the treatments in both dose groups. The systemic exposure of rabeprazole at steady state after the multiple-dose was also similar between the treatments in both dose groups. The time to reach the maximum rabeprazole concentration was faster in the test treatment. The PK-PD relationship of PPI is well known, and the faster absorption of rabeprazole resulted in a more rapid mode of action in acid suppression.
Conclusion: The fixed dose combination of rabeprazole with NaHCO3 showed a faster absorption and consequently, a more rapid gastric acid suppression with a similar systemic exposure of rabeprazole at steady state compared to the conventional enteric-coated rabeprazole.
Keywords: comparative pharmacokinetics/pharmacodynamics, intragastric pH monitoring, gastroesophageal reflux disease, immediate release
Introduction
Patients with gastroesophageal reflux disease (GERD) suffer from heartburn, chest pain and extra-esophageal symptoms like cough and sore throat.1 GERD should not be neglected because it can cause more serious complications such as esophagitis or Barrett’s esophagus when not treated.2 Not only does GERD deteriorate quality of life but it also poses a significant burden on society.3 This is even more serious considering that the prevalence of GERD is increasing, especially in the developed countries.2,3
Proton pump inhibitors (PPIs) are recommended as the first-line therapy for GERD, and clinicians are recommended to start with a 4- to 8-week trial of PPI therapy for the treatment of GERD.4 Because PPIs are easily degraded in acidic conditions, they are usually developed as enteric-coated forms. While an enteric coating ensures the stability of PPIs, it inevitably delays their absorption. To solve this problem, Zegerid® (Santarus Inc, USA), the combination of non-enteric coated omeprazole with sodium bicarbonate (NaHCO3), was developed and approved by the FDA in 2004.5 Compared to the conventional enteric-coated omeprazole, it was characterized as an “immediate-release omeprazole” with a faster absorption and consequently, a more rapid onset of anti-secretory effects.5 For this type of fixed dose combination (FDC) to get an investigational new drug (IND) submission, the Korean Ministry of Food and Drug Safety (MFDS) requires the following features: i) The investigational product (IP) should meet the predefined equivalent criteria in systemic exposure and pharmacodynamic (PD) marker at steady state compared to the conventional PPI and ii) There should be additional therapeutic excellence compared to the conventional PPI such as a fast onset of action. Numerous medications have been approved after meeting these criteria such as the combination of esomeprazole with NaHCO3, calcium carbonate (CaCO3) and magnesium hydroxide (Mg(OH)2).
YPI-011 is a FDC of rabeprazole with NaHCO3 developed by Yungjin Pharmaceutical Corp, Korea. It was developed in two different doses: 10 and 20 mg of the non-enteric coated rabeprazole with 500 mg of NaHCO3. The NaHCO3 in YPI-011 was designed to be immediately released in the stomach, neutralizing the acidic condition and protecting against the degradation of rabeprazole. It was expected that the faster absorption of rabeprazole in YPI-011 should contribute to a quicker neutralization of the gastric pH compared to the conventional enteric-coated rabeprazole. So far, the pharmacokinetic (PK) and PD characteristics when combining rabeprazole with antacid salts have not been reported. Therefore, this study compared the PK and PD characteristics of YPI-011 (test treatment, T) to the conventional enteric-coated rabeprazole (Pariet®, reference treatment, R).
Methods
This study was approved by the Korean MFDS and the Institutional Review Board (IRB) of Seoul National University Hospital at Seoul National University Hospital Clinical Trials Center in accordance with the Declaration of Helsinki and Korean Good Clinical Practice (KGCP) (ClinicalTrials.gov identifier: NCT04703868). All subjects provided informed consent before any study-related procedures. Due to the confidentiality issue, individual data will not be shared.
Study Population
Healthy Korean subjects aged between 19 and 55 years with a body weight of 50 to 90 kg and a body mass index (BMI) of 18.0 to 28.0 kg/m2 were able to participate in this study. Previous medical history, physical examination, vital signs, 12-lead electrocardiography (ECG) and clinical laboratory tests were comprehensively evaluated. Subjects with a history of gastrointestinal surgery that can affect either the PK or PD of rabeprazole were excluded. Moreover, subjects with any anatomical abnormalities that can interfere with the insertion or maintenance of an intragastric catheter were unable to participate in the study. Positive results on H. pylori were also one of the exclusion criteria because it could affect the intragastric pH.6 Subjects had to quit smoking and drinking alcohol during the study period.
Study Design
This study was a randomized, open-label, two-treatment study with two different doses of rabeprazole (10 and 20 mg). In the 10-mg dose group, YPI-011, 10 mg of rabeprazole with 500 mg of NaHCO3, was compared to the conventional enteric-coated rabeprazole 10 mg. Likewise, in the 20-mg dose group, YPI-011, 20 mg of rabeprazole with 500 mg of NaHCO3, was compared to the conventional enteric-coated rabeprazole 20 mg. Subjects were randomly assigned to one of the two sequences (T-R or R-T) and administered either a test or reference treatment for 7 consecutive days in each period with 150 mL of water. There was a 14-day washout period between the two periods based on the PK characteristics of rabeprazole and the turnover rate of gastric H+, K(+)-adenosine triphosphatase (ATPase).7
Pharmacokinetic Evaluation
Blood samples for the PK evaluation of rabeprazole were collected at 0 (pre-dose), 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10 and 24 hours post dose after the single-dose (Day 1) and at steady state after the multiple-dose (Day 7) in each period. At each sampling time point, 7 mL of blood were collected in a sodium heparin tube and centrifuged at 4°C and 3000 rpm for 10 minutes within 30 minutes after sampling the blood. The supernatant was stored at −70°C until analysis.
The plasma concentration of rabeprazole was measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS) with rabeprazole-d4 sodium salt as the internal standard. The mass spectrometer was operated in the positive ion mode, and the mass transition ion pair was selected with a mass-to-charge ratio (m/z) at 360.3 → 242.2 for rabeprazole and 364.1 → 242.2 for the internal standard. The lower limit of quantification (LLOQ) was 1 ng/mL.
The PK parameters were estimated by non-compartmental methods using the Phoenix WinNonlin® software version 8.3 (Pharsight Co, Mountain View, CA, USA). Area under the concentration–time curve (AUC) within a dosing interval (AUCtau) calculated by the linear-up/log-down trapezoidal method, maximum rabeprazole plasma concentration (Cmax), time to reach Cmax (Tmax), time point immediately prior to the first quantifiable concentration (tlag), half-life (t1/2), apparent total clearance (CL/F) and apparent volume of distribution (Vz/F) after the single- and multiple-dose were estimated.
Pharmacodynamic Evaluation
The 24-hour intragastric pH monitoring was performed 1 day before the first dose (Day −1) for a baseline evaluation and after the single-dose (Day 1) and multiple-dose at steady state (Day 7) in each period. The procedure was performed using an impedance-pH recorder (Digitrapper™ pH-Z recorder, Medtronic Co. Ltd., Ireland). The pH catheter was calibrated with a standard solution of pH 4 and 7 beforehand. After the calibration, the pH catheter was connected to the pH recorder and inserted to the subjects. Investigators checked whether gastric pH measured by the distal sensor was below 3 and esophageal pH measured by the proximal sensor of the catheter was within 6 to 8 to ensure that the catheter was positioned properly. Subjects were asked to drink the same amount of water (150 mL) in the baseline period (Day −1) to compensate for the impact of the water on the intragastric pH. While monitoring the 24-hour intragastric pH, the subjects were required to start their standardized meals exactly 4.5 and 10.5 hours after administration of the treatment (for baseline and after the expected time for the administration of treatment on Day 1) and were recommended to eat all the food provided. They also had to maintain an upright posture of 45 degrees or more during daytime (7 AM to 11 PM) and remain lying down on the bed during nighttime (11 PM to 7 AM).
The PD parameters included decrease from baseline in the integrated gastric acidity, time to reach pH >4, percentage of time with gastric pH >4 and mean gastric pH after the single- and multiple-dose. The integrated gastric acidity was calculated using the following method.8
Acid concentration (mmol/L) = 1000 * 10−pH
Acidity (mmol * h/L) =
(“t” - “t-1”) * (acid in mmol/L at time “t” + acid in mmol/L at time “t-1”)/2 Integrated gastric acidity (mmol*h/L) = Acidity summed cumulatively for every second Percent decrease from baseline in the integrated gastric acidity for a 24-hour interval after a single- or multiple-dose (%) = (Baseline – single- or multiple-dose)/Baseline * 100.
Safety and Tolerability Evaluation
Safety and tolerability were evaluated on subjects who were administered the test or reference treatment at least once. Throughout the study, adverse events (AEs), physical examinations, vital signs, 12-lead ECG and clinical laboratory tests were analyzed. The investigators assessed AEs by severity and causality.
Sample Size and Statistical Analysis
Sample size was calculated based on the intra-subject variability of Cmax and AUCtau of rabeprazole described in a previous study.9 It was calculated supposing an intra-subject variability of 32.83% and 90% confidence interval (CI) for the geometric mean ratio (GMR) of the test to reference treatment to fall within 0.8 to 1.25 with a 5% level of confidence and 80% power. Considering the possibility of a 10% dropout rate, 44 subjects were determined as the final sample size.
Analysis of variance (ANOVA) considering the sequence, period and treatment effects as the fixed effect and subjects nested within a sequence as the random effect was used to compare the primary PK and PD parameters between the treatments. The primary PK and PD endpoints were the AUCtau and the decrease from baseline in the integrated gastric acidity after the multiple-dose, respectively. The parameters were regarded equivalent if the 90% CI of the GMR fell within 0.8 to 1.25. The decrease from baseline in the integrated gastric acidity and percentage of time with a gastric pH >4 up to 4 hours after dose between treatments were compared using the paired t-test or Wilcoxon’s signed rank test after assessing the normality based on Shapiro–Wilk test.
Results
Due to the confidentiality issue, raw data will not be presented.
Study Population
The first subject was screened on January 15, 2021, and the last subject finished the study schedule on June 18, 2021. A total of 150 subjects were screened and 44 subjects were designated for each dose group. In each dose group, subjects were allocated to either sequence 1 (T → R) or sequence 2 (R → T) randomly. The mean age, height, weight and BMI of the 10-mg dose group were 30.30 years, 171.59 cm, 69.12 kg and 23.43 kg/m2. The corresponding values in the 20-mg dose group were 25.84 years, 169.68 cm, 67.78 kg and 23.48 kg/m2. There was no statistically significant difference in the demographic characteristics between the sequences in both dose groups.
One subject in each dose group withdrew his/her consent voluntarily before any treatment. Three subjects in the 10-mg dose group and 4 subjects in the 20-mg dose group failed to complete the study and were excluded from the PK and PD analysis. Therefore, the safety analysis was evaluated in 43 subjects in each dose group who received either treatment at least once. The PK and PD analyses were evaluated in 40 subjects in the 10-mg dose group and 39 subjects in the 20-mg dose group from whom the PK and PD data were collected after the single- and multiple-dose in both periods (Figure 1).
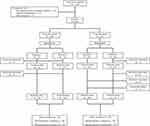 |
Figure 1 Study disposition. |
Pharmacokinetics
The absorption of rabeprazole was faster in the test treatment compared to the reference treatment (Figure 2). The Tmax of the test treatment ranged from 0.25 to 3.5 hours compared to 1.5 to 5 hours in the reference treatment after the multiple-dose in the 10-mg dose group (Table 1). Likewise, the Tmax of the test treatment in the 20-mg dose group ranged from 0.25 to 1.5 hours compared to 2 to 8 hours in the reference treatment after the multiple-dose. Furthermore, the concentration of rabeprazole was quantifiable in nearly all the subjects on the first PK sampling point of the test treatment with a median tlag of 0 after the single- and multiple-dose in both dose groups (Table 1).
 |
Table 1 Summary of the Pharmacokinetic Parameters of Rabeprazole by Treatment and Dose Groups |
The systemic exposure of rabeprazole was similar between the treatments. The GMR (90% CI) of the test to reference treatment in terms of the AUCtau was 1.0766 (1.0212–1.1350) and 1.0951 (1.0329–1.1610) in the 10- and 20-mg dose groups, respectively, after the multiple-dose (Table 1).
Pharmacodynamics
The onset of anti-secretory effect of the test treatment was faster compared to the reference treatment based on the mean gastric pH-time profiles and PD parameters at an earlier time after the administration (Figures 3 and 4 and Supplementary Table 1). Especially, the time to reach pH >4 in the test treatment was shorter after the single- and multiple-dose in both dose groups (Table 2). When the PD parameters were analyzed in the early phase of the gastric pH monitoring, the test treatment showed a better acid suppression activity. Both the percent decrease from baseline in the integrated acidity and the percentage of time with a gastric pH >4 up to 4 hours after the administration were significantly higher in the test treatment (Supplementary Table 1).
 |
Table 2 Summary of Pharmacodynamic Parameters by Treatment and Dose Groups |
Percent decrease from baseline in the integrated acidity for a 24-hour interval between two treatments met the predefined equivalence criteria after the multiple-dose for both doses. The GMR (90% CI) of the test to reference treatment after the multiple-dose was 0.9041 (0.8045–1.0159) in the 10-mg dose group and 1.0321 (0.9725–1.0954) in the 20-mg dose group. The other PD parameters were also similar between the treatments after the multiple-dose in both dose groups.
Safety and Tolerability
All the AEs were mild, and there were no serious AEs. There were 10 AEs from 21 subjects in the 10-mg dose group and 45 AEs from 18 subjects in the 20-mg dose group recorded after the administration of the treatments (Supplementary Table 2). The most prevalent type of AE in terms of system organ class was gastrointestinal disorders in both dose groups. There were no clinically significant changes in clinical laboratory tests, 12-lead ECG, physical examination and vital signs.
Discussion
As expected, the absorption of rabeprazole was faster in the test treatment compared to the reference treatment. The tlag and Tmax were shorter in the test treatment without changes in the PK parameters related to elimination such as t1/2. In our previous study comparing the FDC of esomeprazole with CaCO3 to the conventional enteric-coated esomeprazole, a quicker absorption of esomeprazole in the former treatment was also observed.10 Because the PPI is more easily disintegrated in the non-enteric coated PPI, a faster transition to the intestine could have promoted the absorption of the particular PPI. In some parts, neutralization of intragastric pH by NaHCO3 could have contributed to an increased permeation of the PPI through the gastric mucosa and consequently, increased the systemic absorption.11
PPIs inhibit acid secretion by binding to the H+, K(+)-ATPase after systemic absorption.12 Therefore, the systemic exposure of PPIs is closely associated with the anti-secretory activity, and the PK-PD relationship of PPIs is well described in previous studies.13 As a result, the acid suppression effect of the test treatment was expected to be faster than the enteric-coated rabeprazole. To evaluate the pharmacological effects of the treatments, integrated gastric acidity was calculated as the integral of the acid concentration with respect to time. This marker indicated the overall intragastric acid exposure, and the change from baseline has been used as a marker to explain the PD effects in similar studies.10,14,15 The baseline integrated acidity for a 24-hour interval between two treatments was not significantly different in both doses when assessed by Wilcoxon’s signed rank test (p-value = 0.1376 for 10-mg dose group and p-value = 0.0835 for 20-mg dose group). The decrease from baseline in the integrated gastric acidity after the multiple-dose was equivalent between the treatments in a 24-hour interval but was significantly greater up to 4 hours after the administration in the test treatment. The percentage of time with a gastric pH >4 was also higher in the test treatment until 4 hours post dose. This implies that the pharmacologic action of the test treatment was concentrated during the earlier time while maintaining the overall acid-suppression activity. Altogether, the test treatment successfully advanced the absorption and pharmacologic action of rabeprazole. A faster symptom relief for GERD patients has been one of the main targets for the development of PPIs.16 The faster onset of the pharmacological action of rabeprazole of the test treatment would be suitable for on-demand use.
The rapid absorption of rabeprazole resulted in a higher Cmax in the test treatment. PPIs have long been used safely, and it is reported that a higher exposure of PPIs in poor metabolizers (PM) is not necessarily associated with an increased risk of toxicity.17 Furthermore, according to the guideline from Clinical Pharmacogenetics Implementation Consortium (CPIC) in 2021, dose adjustment of PPIs for the PM was not recommended due to a lack of evidence.18 The efficacy parameters of PPIs such as percentage of time with a gastric pH >3 or 4 are known to be related to their AUC,13 and the possibility of AEs after long-term exposure is reported to be caused by the AUC rather than Cmax.19 The frequency and characteristics of the AEs were similar between the treatments. Conclusively, the higher Cmax of the test treatment is less likely to affect the safety and tolerability considering that the systemic exposure of rabeprazole between treatments after the multiple-dose was similar.
The test treatment was approved by the Korean MFDS by proving that the systemic exposure of rabeprazole and the overall acid inhibitory effect after the multiple-dose at steady state were equivalent with the conventional enteric-coated rabeprazole while presenting a faster onset of action at the same time. This procedure is based on the idea that the safety, tolerability and efficacy of the IP can be bridged from the data of the conventional PPI if the overall PK and PD characteristics are equivalent. This process is noteworthy in that if the primary PD parameter can be appropriately assessed in healthy subjects, and the pharmacological target between healthy individuals and patients is not clinically different, the approval of the new medication may not require unnecessary trials. This enables GERD patients to benefit from the newly developed medication without further trials and lowers the overall costs in drug development for pharmaceutical companies. YPI-011 is the first drug in this type of medication with rabeprazole as the PPI component.
While the idea of protecting PPIs through antacids might seem simple, matching the appropriate type with a sufficient amount of antacid is key in developing the IP. That is, the neutralizing capacity of the antacid should be sufficient to protect the PPI from degradation while the amount should be as small as possible for patients to administer it more comfortably. Especially, rabeprazole is known to be particularly susceptible in acidic conditions.20 In our previous study, a smaller of amount CaCO3 was needed to protect esomeprazole from gastric acid compared to NaHCO3.10,14 This could be due to the larger acid-neutralizing capacity (mEq HCl/g) of CaCO3, which is nearly double that of NaHCO3.21 However, it took less time for the NaHCO3 to meet the predefined PK/PD equivalence criteria than CaCO3, and this could be explained by its superior solubility and hence, neutralization of gastric acid even after the first dose. This study is the first study comparing the PK/PD characteristics of the FDC of rabeprazole with an antacid to the conventional enteric-coated rabeprazole, and NaHCO3 was used as the antacid salt. Interestingly, the first “immediate-release omeprazole”, Zegerid®, also used NaHCO3 as the antacid counterpart. More studies should be implemented to determine whether other types of antacids can be used for the “immediate-release rabeprazole”.
Conclusion
The systemic exposure and the suppression of gastric acid in the combination of rabeprazole with NaHCO3 were estimated to be equivalent to conventional rabeprazole at steady state. The absorption of rabeprazole and the acid-suppressive action was faster in the test treatment. The faster antacid effect of the test treatment would be suitable for on-demand use.
Acknowledgments
This study was sponsored by Yungjin Pharmaceutical Corp, Korea. The investigation was conducted at the Clinical Trials Center, Seoul National University Hospital.
Disclosure
Mi-Hye Lee is an employee of Yungjin Pharmaceutical. The other authors report no conflicts of interest associated with this work.
References
1. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. ACG. 2006;101(8):1900–1920.
2. Chen J, Brady P. Gastroesophageal reflux disease: pathophysiology, diagnosis, and treatment. Gastroenterol Nurs. 2019;42(1):20–28. doi:10.1097/sga.0000000000000359
3. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. 2020;324(24):2536–2547. doi:10.1001/jama.2020.21360
4. Yadlapati R, Gyawali CP, Pandolfino JE. AGA clinical practice update on the personalized approach to the evaluation and management of GERD: expert review. Clin Gastroenterol Hepatol. 2022;20(5):984–994.e1. doi:10.1016/j.cgh.2022.01.025
5. Howden CW. Review article: immediate-release proton-pump inhibitor therapy--potential advantages. Aliment Pharmacol Ther. 2005;22(Suppl 3):25–30. doi:10.1111/j.1365-2036.2005.02709.x
6. Engevik AC, Kaji I, Goldenring JR. The physiology of the gastric parietal cell. Physiol Rev. 2020;100(2):573–602. doi:10.1152/physrev.00016.2019
7. Shin JM, Munson K, Vagin O, Sachs G. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 2009;457(3):609–622. doi:10.1007/s00424-008-0495-4
8. Gardner JD, Perdomo C, Sloan S, et al. Integrated acidity and rabeprazole pharmacology. Aliment Pharmacol Ther. 2002;16(3):455–464. doi:10.1046/j.1365-2036.2002.01158.x
9. Keller GA, Czerniuk P, Bertuola R, et al. Relative bioavailability of a 5 mg mosapride/10 mg rabeprazole fixed dose combination tablet versus separate single tablets in healthy volunteers: a single-dose randomized open-label crossover study. Curr Med Res Opin. 2011;27(11):2203–2211. doi:10.1185/03007995.2011.624088
10. Bae S, Kwon J, Lee SB, Jang IJ, Yu KS, Lee S. Comparative pharmacokinetics/pharmacodynamics of fixed-dose combination of esomeprazole and calcium carbonate (AD-206) to the conventional esomeprazole. Drug Des Devel Ther. 2021;15:5099–5108. doi:10.2147/dddt.S341271
11. Benetti C, Flammini L, Vivo V, et al. Esomeprazole immediate release tablets: gastric mucosa ex vivo permeation, absorption and antisecretory activity in conscious rats. J Control Release. 2016;239:203–210. doi:10.1016/j.jconrel.2016.08.032
12. Proton Pump Inhibitors. LiverTox: clinical and research information on drug-induced liver injury. National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
13. Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol. 2008;64(10):935–951. doi:10.1007/s00228-008-0538-y
14. Kim D, Park MS, Yoo BW, Hong T, Park SJ, Kim CO. The safety, pharmacodynamics, and pharmacokinetics of immediate-release formulation containing esomeprazole 20 mg/sodium bicarbonate 800 mg in healthy adult male. Drug Des Devel Ther. 2019;13:3151–3159. doi:10.2147/dddt.S212491
15. Jing S, Zhu Y, Liu W, et al. Pharmacokinetics and pharmacodynamics of esomeprazole/sodium bicarbonate immediate-release capsules in healthy Chinese volunteers: a cross-over, randomized controlled trial. Adv Ther. 2021;38(3):1660–1676. doi:10.1007/s12325-021-01644-7
16. Bruley Des Varannes S, Coron E, Galmiche JP. Short and long-term PPI treatment for GERD. Do we need more-potent anti-secretory drugs? Best Pract Res Clin Gastroenterol. 2010;24(6):905–921. doi:10.1016/j.bpg.2010.09.004
17. Gardiner SJ, Begg EJ. Pharmacogenetics, drug-metabolizing enzymes, and clinical practice. Pharmacol Rev. 2006;58(3):521–590. doi:10.1124/pr.58.3.6
18. Lima JJ, Thomas CD, Barbarino J, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2C19 and proton pump inhibitor dosing. Clin Pharmacol Ther. 2021;109(6):1417–1423. doi:10.1002/cpt.2015
19. Jaynes M, Kumar AB. The risks of long-term use of proton pump inhibitors: a critical review. Ther Adv Drug Saf. 2019;10:2042098618809927. doi:10.1177/2042098618809927
20. Shin JM, Cho YM, Sachs G. Chemistry of covalent inhibition of the gastric (H+, K+)-ATPase by proton pump inhibitors. J Am Chem Soc. 2004;126(25):7800–7811. doi:10.1021/ja049607w
21. Brunton L, Chabner BA, Knollman B. Goodman and Gilman’s the Pharmacological Basis of Therapeutics.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



