Back to Journals » Journal of Pain Research » Volume 15
Development and Validation of the Italian “Brief Five-Item Chronic Pain Questionnaire” for Epidemiological Studies
Authors Toccaceli V, Tenti M , Stazi MA , Fagnani C, Medda E, Gargiulo L , Burgio A , Sampaolo L , Ferri M, Raffaeli W
Received 15 February 2022
Accepted for publication 18 June 2022
Published 8 July 2022 Volume 2022:15 Pages 1897—1913
DOI https://doi.org/10.2147/JPR.S362510
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Twillman
Virgilia Toccaceli,1 Michael Tenti,2 Maria Antonietta Stazi,1 Corrado Fagnani,1 Emanuela Medda,1 Lidia Gargiulo,3 Alessandra Burgio,3 Letizia Sampaolo,4 Maurizio Ferri,1 William Raffaeli2
1Centre for Behavioural Sciences and Mental Health, Istituto Superiore di Sanità (Italian National Institute of Health), Rome, Italy; 2Fondazione ISAL, Institute for Research on Pain, Torre Pedrera, Italy; 3ISTAT, National Institute of Statistics, Rome, Italy; 4Centre for Disease Prevention and Health Promotion, Istituto Superiore di Sanità (Italian National Institute of Health), Rome, Italy
Correspondence: Michael Tenti, Fondazione ISAL, Via San Salvador, 204, Rimini, 47922, Italy, Tel +390541725166, Fax +390541725164, Email [email protected]
Background: Chronic pain (CP) prevalence estimates addressing a wide phenotype are still quite fragmented and may vary widely due to the lack of standardized tools of investigation. There is an urgent need to update general population CP estimates.
Methods: For this purpose, the Brief Five-item Chronic Pain Questionnaire was developed through experts’ consultations for design and content validity assessment; literature analysis of measures used to investigate CP for general population surveys; understandability evaluation through a survey on a convenience sample of affected and non-affected individuals; reliability assessment by means of two double-wave online surveys carried out by the Italian Twin Registry; criterion and construct validity assessment through the third wave of the 2019 European Health Interview Survey (Ehis).
Results: Key dimensions were defined to describe CP main aspects from a public health perspective. Literature analysis showed that validated questionnaires were rarely used to address important public health CP aspects. Understandability of the measure was good. Test-retest analyses showed adequate reliability of the measure: k values were at least “moderate” with highest values regarding CP “occurrence” and “intensity”. Correlations of CP with well-known comorbidities (cancer, depression), and specific traits (age, education) as well as of CP and its intensity with “physical pain occurrence and intensity” detected in the Ehis 2019, confirmed, respectively, a good construct and criterion validity. Construct validity was also evaluated through the correlation between “perceived treatment effectiveness” and “interference of pain in daily life activities” as recorded in the Ehis 2019.
Conclusion: The designed questionnaire is a brief self-administered measure, particularly suitable to detect persistent states of pain and related intensity in large-scale general population surveys by means of a first filtering item followed by four further items. It is, in fact, designed to detect CP possible underlying causes/triggers, drugs/treatments taking and frequency, and self-perceived effectiveness among CP sufferers. Further validation of the measure in different social and cultural contexts is desirable.
Keywords: chronic pain, epidemiology, methodology, questionnaire, survey, European Health Interview Survey
Introduction
Chronic pain (CP) is defined as persistent or recurrent pain lasting longer than 3 months and it refers to a wide range of clinical conditions that have recently been systematically categorized into the 11th version of the International Classification of Diseases (ICD-11).1,2 CP affects 1 in 5 individuals worldwide and has a heavy load on families, healthcare systems and national economies.3 CP has, in fact, detrimental biological, physical, psychological and social consequences, being among the major causes of “years lived with disability”.4
Despite the enormous burden of CP, its epidemiology is still quite fragmented and prevalence estimates vary widely, owing mainly to the lack of standardized tools of investigation and discrepancies of CP definitions used in epidemiological research. As recently reviewed by Steingrìmsdòttir and colleagues,5 indeed, standardized methods for ascertaining CP in epidemiological studies should be a priority, since these methods are insufficiently developed. In addition, clinical recorded data, one of the major sources of health information, are not structured with respect to CP, representing a further challenge to provide sound estimates for this phenomenon.
In Italy, the most recent CP prevalence estimates regarding the general population come from a pan-European study conducted more than 15 years ago which accounted for a prevalence of nearly 26% of CP-affected individuals.6 More recent estimates derive from a few surveys on very restricted samples in dissimilar settings or surveys focused on pain subtypes: a cross-sectional survey on adult population (N=1300) living in Narni, which revealed a CP prevalence of 28.4%;7 a retrospective population-based study on stroke patients (N=601) in the Rimini district, revealing a prevalence of central post-stroke pain of 11%.8
At the European level the situation is similar, the importance of CP is well-recognized, but prevalence data are quite outdated,6 and the whole picture cannot be easily discerned out of disparate small-scale studies.
From a public health perspective, there is a clear need to focus on CP as a general affliction occurring in many different conditions, and across diagnoses. CP major comorbidities, outcomes and psychosocial burden, in fact, frequently depend more on the severity and interference of pain than on the specific, underlying diagnosis in itself.5
In this framework, there are important reasons for the development of a short and effective CP questionnaire for large, national epidemiological studies addressing the general population. Since CP is an experience with high individual variability, we think, as many experts do,9,10 that a subjective measure of CP is likely to be more valuable than any other objective measure.
The study describes the design and validation of a short-self-administered questionnaire in Italy – The “Brief Five-item Chronic Pain Questionnaire” (“5-Item CP Quest”) – useful to detect CP sufferers in epidemiological surveys of the Italian general population, and among them, to assess a few public health-relevant CP dimensions.
Materials and Methods
The CP research project complies with the requirements of the Helsinki Declaration for research involving human beings (version 19 October 2013) and it was approved by the Ethics Board of Istituto Superiore di Sanità (ISS). A multidisciplinary group of experts was established with one CP clinician WR (algologist), four epidemiologists MAS, CF, EM, and VT, one psychologist experienced in CP MT, two statisticians LG and AB, one survey designer MF and one scientific information retrieval expert LS. They are co-authors of the present study and variously took part in the different phases described below.
The “5-Item CP Quest” was developed following the model proposed by Tsang and colleagues:11
- Experts’ consultations to frame the problem, from a public health perspective, of obtaining updated prevalence estimates of CP among the general population and to choose key dimensions of CP to be investigated (2017).
- Literature search to detect questionnaires which could have met the needs highlighted by the group of experts (2017).
- Based on literature search results, experts’ consultations to design the measure prototype and the assessment of its “content validity” and understandability (2018).
- “Reliability” assessment, across time, of the prototype measure through two distinct double-wave online surveys on adult twin-samples provided by the Italian Twin Registry (ITR) at ISS (July and October 2018).
- “Criterion validity” and “Construct validity” assessment through the results obtained with the administration of the “5-Item CP Quest” within the “European Health Interview Survey” carried out in Italy by the Italian Institute of Statistics (ISTAT) in 2019 (Ehis wave 3, 2019).12
Phase a
The first experts’ consultations were conducted in three face-to-face, not-recorded meetings that took place in 2017. Firstly, as the lack of validated tools to update data on CP among the general population was apparent for the two ISAL Foundation CP experts (the clinician and the psychologist), they contacted the group of epidemiologists at the Istituto Superiore di Sanità, and then a plenary discussion of the issue took place in the first meeting. After the problem was framed, CP experts and the epidemiologists agreed to invite two statisticians of ISTAT – who have experience in large population surveys, such as the Ehis, and who are in charge of the Italian section of this survey – to join the group of experts. They altogether took part to the second and third meetings.
The experts’ consultations addressed the following topics and worked to reach the necessary agreement on: (i) which dimensions to be investigated to highlight key aspects of CP from a public health perspective; (ii) maximum number of items to be used according to possible restraints imposed by large “health interview” investigations; (iii) analysis of the relationship between each dimension and the number of items necessary to frame the condition. Efforts were focused on the need to construct a brief questionnaire, as brevity represents a fundamental requirement when designing tools for general population broad surveys; questionnaire length may, in fact, improve quality of responses and response rates,13,14 and even contribute to diminish respondents’ burden.15 Moreover, the group of experts agreed that the questionnaire would essentially rely on a single question, the first one, with the function of a main filter to effectively discriminate between CP “affected” and “non-affected” individuals.
This means that, for individuals replying “no” to this filter-question, the questionnaire is over. Instead, for those replying “yes” the questionnaire expands to specific CP-related dimensions. As the questionnaire is not a psychometric scale that produces a total score with the contribution of all the items, the validation focused mainly on the first item. After testing for understandability of the whole measure, it was central to assess validity parameters for the first item.
Phase b
The experts’ consultations were supported by an extensive literature search and analysis of peer-reviewed articles regarding measures in use for general population surveys on CP, to assess what was already done to devise and improve methods for collecting reliable data and produce valid figures. A sensible search based on one main key question: “which are the measures used in the last 20 years to investigate CP as an overall phenotype for general population surveys?” was performed. A set of methodological search issues was discussed to define inclusion/exclusion criteria, and the appropriate keywords for building the search strategy. The search covered the highly structured peer-reviewed database PubMed/Medline. The main search filters, appropriately combined through Boolean operators, included all controlled terms, free-text terms, and synonyms related to “questionnaires” and “Chronic Pain”. Inclusion criteria were: epidemiological descriptive and/or analytical studies investigating CP as an overall phenotype on samples drawn from the general adult population, published in peer-reviewed journals as research articles, in English or Italian, and accessible in full text. Exclusion criteria were: studies focused exclusively on one (or more) CP subtypes, studies in which it was unspecified or unclear whether the condition investigated was acute or chronic pain, studies addressing the condition exclusively among children and/or adolescents. The full search string is included as Supplementary Data. The search was conducted by LS, and retrieved articles were jointly evaluated by VT, MT and LS according to inclusion and exclusion criteria; disagreements were resolved by five online discussion sessions.
Phase c
Two experts’ consultations, involving ISAL Foundation CP experts, ISTAT experts and ISS epidemiologists and survey designers, were organized after the literature search to outline the questionnaire, taking into consideration both the search results and the outcomes of phase “a”. Any discrepancies between the experts were resolved until a consensus was reached: two face-to-face discussions, with email exchanges in between, were necessary to reach the goal. Each expert reviewed the draft at various stages, providing comments until the development of a final prototype version, which was adopted by the group of experts in a plenary session. In particular, the meetings dealt with the following main topics: (i) the most suitable formulation of the first filter item, where CP definition was basically set on that provided by the ICD-11; (ii) the wording for each question and the items format to make them understandable in self-administered surveys of large numbers of individuals drawn from the general population and, at the same time, rigorous with respect to the phenomenon to be investigated. Content validity was then taken into account to evaluate the extent to which the measure would have been able to address the main aspects related to CP conditions among individuals in non-clinical settings. Content validity is, in fact, an indication of the degree to which the construct is comprehensively sampled by the items of the questionnaire.16 Moreover, the “5-Item CP Quest” understandability was assessed in two equally sized samples (n=26) of subjects with and without CP condition, drawn from a group of patients and volunteers randomly chosen within the ISAL Foundation networks. The understandability assessment was carried out using an ad-hoc self-report instrument that rated comprehension level on a 5-point Likert scale ranging from 1 (“the question is very difficult to understand”) to 5 (“the question is very easy to understand”).
Phase d
Two subsequent double-wave surveys were developed thanks to the Italian Twin Registry (ITR), an epidemiological research infrastructure operating at the ISS. The first survey used a first version of the developed prototype of the “5-Item CP Quest”, the second survey adopted a revised version of the prototype, approved after the evaluation of the first test-retest results by the experts’ group (see phase “d”). Each test-retest was aimed, in fact, to evaluate consistency of the prototype measure across two different time points. Test-retest reliability was calculated comparing individuals’ responses between two subsequent administrations. Test-retest reliability (often referred to as the stability of a scale) indicates whether the measure provides similar results on two (or more) subsequent administrations in the same sample of individuals.16 It is assumed, therefore, that there have been no actual changes in respondents regarding the outcome being measured during the interval between administrations. The first double wave survey administered in July 2018, was then repeated in October 2018, on a larger sample and an age group more similar to that targeted, in the meanwhile, for the “criterion” and “construct validity” assessment (see below, phase “e”). Potential individuals to be interviewed were drawn, as convenience samples, from the ITR, which enrolls general population twins resident in Italy, who voluntarily enter the Register and choose to participate – under specific informed consent procedures – to investigations and surveys promoted by the ITR research group.17 It is important to point out that Twin Registers have a high value in public health research as transferability of twin studies’ results to the whole general population has been widely demonstrated for several biomedical phenotypes.18–20 After the first test-retest analysis, appropriateness of the response option categories was evaluated by the group of experts, and two items response categories were expanded. After the second test-retest, the final version of the “5-Item CP Quest” was adopted.
Phase e
The developed CP questionnaire was finally subjected to “criterion” and “construct” validity assessment by using the representative sample of the Italian population that was targeted in the Ehis wave 3 (2019) (information regarding the survey methodology is available in English at https://www.istat.it/en/archivio/173452, and in Italian at https://www.istat.it/it/archivio/167485). A measure has “criterion validity” when its results approximate the results of another measure of interest assumed as a criterion, while it has “construct validity” when evidence supports the existence of the hypothetical construct that the scale purports to be measuring (i.e., CP occurrence and related dimensions, in our case).16 Criterion validation was assessed for items 1 and 2 of the “5-Item CP Quest”, which was hosted by the “Ehis wave 3 (2019)”, by analyzing the correlation between answers to these items and answers provided to the question on “physical pain occurrence and intensity in the last 4 weeks” in the Ehis wave 3 (adopted from the Short Form-36 Health Survey (SF-36)).21 For construct validation the group of experts set out a number of hypotheses about the relationship of CP with specific sociodemographic variables and other health measurable outcomes. As part of the construct validation process, in fact, a measure might be hypothesized to have stronger (positive or negative) relationships with variables that are known to be related to the construct (convergent validity) and weaker (positive or negative) relationships (or ideally no relationship at all) with variables that bear little relation to the construct (discriminant validity or divergent validity). Assessment of the construct validity of the measure was then based on the existing knowledge about CP comorbidities and CP prevalence estimates across different socio-demographic categories (i.e., age, education level), and was performed comparing the findings of the Italian Ehis wave 3 (2019) with the corresponding expected figures. In particular, the following hypotheses were tested for filter-item 1: (i) a positive association, and its size, between CP and cancer; (ii) a positive association, and its size, between CP and depression; (iii) a positive association between CP and age; (iv) a negative association between CP and education level; (v) a lack of association between CP and respondents’ geographical area of residence (taking into account income levels). Finally, (vi) a negative association between perceived treatment effectiveness and the interference of pain in daily life activities were tested for item 5.
Design and evaluation phases for the “5-Item CP Quest” are summarized in Figure 1.
 |
Figure 1 The flow-chart describes the phases for the design and evaluation of the “Brief Five-item Chronic Pain Questionnaire”. |
Recruitment, Data Collection and Measures
For ensuring reliability and validity respectively, different surveys, samples and analyses were used.
The ITR Surveys
Two surveys, each replicated twice, were conducted by means of an online version of the CP questionnaire to assess test-retest reliability. This assessment was based on the simple Cohen’s kappa statistic to estimate agreement beyond chance for nominal items and the Cohen’s weighted kappa statistic with quadratic weights for ordinal variables. According to the following cut-offs for kappa values, reliability level was defined: slight (kappa in the range 0.00–0.20); fair (kappa in the range 0.21–0.40); moderate (kappa in the range 0.41–0.60); substantial (kappa in the range 0.61–0.80); almost perfect or perfect (kappa in the range 0.81–1.00).22 We considered adequately reliable those items with kappa ≥ 0.40 (i.e., items with at least moderate agreement or at the upper limit of fair agreement); indeed, the 0.40 cut-off for kappa statistic is generally taken as the minimally acceptable level of reliability in studies of medical diagnoses and self-reported conditions, including questionnaire-based assessments of CP.23 The first survey was conducted in July 2018 on a convenience sample of 215 unmatched twins (age 50–60 years) enrolled by the ITR. The second survey was performed in October 2018 on a convenience larger sample of 1104 unmatched twins (age 40+ years). All the subjects were invited to participate by email. A link that gave access to the online questionnaire was sent by email to participants in both waves, after they underwent an informed consent procedure. No primary exclusion criteria were adopted.
Test-retest reliability analyses were performed using Stata statistical software (Release 16) (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
The European Health Interview Survey, Wave 3 (2019)
A national survey on CP was carried out in Italy in 2019 by ISTAT, within the Ehis wave 3 (2019). The “5-Item CP Quest” was included in this survey, in a specific set of questions for self-administration. The Ehis sampling design was in two stages: the primary stage units (PSUs) are municipalities (840) stratified by the population size and secondary stage units (SSUs) are households. Within each household, all the components were interviewed (Elementary Units, Eus). The final estimates cover people living in households, excluding people living in institutions (e.g., people stably living in long-term care facilities). The theoretical sample size was 30,142 households, while the number interviewed was 22,796. Moreover, due to the health information contained in the “5-Item CP Quest”, according to the Data Protection Authority recommendations who made the responses not compulsory, about 1 out of 10 individuals did not answer. The CP module was set as a self-administered questionnaire, using a paper form to be filled in by each respondent. The interviewers, selected by local statistical offices, trained by ISTAT researchers, and monitored in the field data collection, were available to give more information to help for filling in the questionnaire. The self-completed “5-Item CP Quest” forms were collected by the interviewers the same day or the day after the interview was administered to all household members.
Criterion validity was assessed by performing chi-square tests for the association of CP occurrence and intensity (first and second item of the “5-Item CP Quest”), detected by the “5-Item CP Quest”, and “physical pain within the last 4 weeks” occurrence and intensity detected by the SF-36 contained in the Ehis wave 3 (2019).
To verify construct validity, chi-square tests for the association of CP occurrence with well-known CP comorbidities and socio-demographic factors – depression, cancer, age, education level – as well as for the association between “perceived treatment effectiveness” (item 5 of the “5-Item CP Quest”) and “pain interference with daily activities”, recorded by the Ehis wave 3, were performed.
Since self-reported data on diseases can be sometimes overestimated or underestimated, the association analysis of CP with depression and cancer was carried out considering only individuals reporting that these diseases were diagnosed by a doctor. Descriptive statistical analysis of the Ehis data, and criterion and construct validity analyses were performed using SAS statistical software (version 15.1, https://www.sas.com).
Results
The State of the Art for CP Measures in General Population Surveys
The literature search strategy (time limits: 2000 – Nov 2017) yielded 883 studies, which were evaluated against relevance to our purpose and analyzed. According to the affinity with our main research question, the possibility to deploy also specific reviews emerging from the updated search was left open as it might be useful to detect further studies for the purposes of our work.
Of the 883 studies retrieved from the first search, 826 were excluded by title or abstract based on inclusion/exclusion criteria. The remaining 57 were evaluated in full-text and 44 studies met the inclusion/exclusion criteria and were included in the analysis. The search also retrieved the work of Steingrìmsdòttir et al,5 a systematic review on a topic parallel to ours. From the references included in Steingrìmsdòttir and colleagues’ work, 43 further studies were included in the analysis.
Among the included studies, 10 papers examined our target dimensions,6,23–31 but only 3 of these papers carried out a validation procedure of the questionnaire used.23,28,31
With respect to our purposes, Azevedo et al evaluated the five dimensions more extensively: the question on CP was divided into two sub-questions, many self-reported etiologies were proposed, and a whole questionnaire (i.e., the Brief Pain Inventory [BPI]) was used to measure pain intensity.23 Walters et al also used the entire BPI to measure pain intensity;31 while Elzahaf et al used multiple questions to evaluate CP occurrence, use of drugs or other treatments and the perception of their effectiveness, hindering the shortness of the survey procedure.28 Ng et al evaluated CP treatments and perceived effectiveness with multiple questions, and the cause of pain with an open question,29 even if the questionnaire was based on a previously validated measure.32
Moreover, Breivik et al6 used multiple questions to evaluate CP occurrence, use of drugs or other treatments and perception of their effectiveness. De Souza et al investigated CP occurrence and the use of drugs or other treatments with multiple questions.26 Toblin et al30 and Dureja et al27 used two questions for investigating CP and an extensive list of self-report causes, hence a too-long procedure for the purpose of investigating the presence and the main dimensions of CP in general population surveys. Conversely, De Moraes Vieira et al asked only whether the cause of pain was known or not, and what specific treatment the person received (e.g., NSAIDs, analgesics, corticosteroids) without investigating frequency of taking.25 Chrubasik and colleagues evaluated pain intensity with the use of a 3-ranks VRS, the cause of pain with an open question, and type of treatments without any question regarding the frequency of assumption.24 The literature search sustained the rationale of our work and the need of a new brief questionnaire, providing hints for its outlook.
Table 1 reports the studies which considered the same key dimensions as the proposed “5-Item CP Quest” and their characteristics.
 |
Table 1 Studies Which Took into Account the Key Dimensions of the Proposed “5-Item CP Quest” and Their Characteristics |
(The search was updated in July 2021, to control for the state of the art in the construction of similar measures, and it yielded 325 citations. Three hundred and thirteen were excluded by title or abstract based on inclusion/exclusion criteria. The remaining 12 were evaluated in full-text and 8 studies met the inclusion/exclusion criteria. The complete list of the total 95 studies (years 2000–2021) that met the criteria is provided in “Supplementary material”, together with an extensive analysis regarding how each of them investigated the dimensions addressed by the “5-Item CP Quest”, with a check of the presence of a validity assessment procedure for the measure.)
Design of the “5-Item CP Quest”, Content Validity and Understandability Assessment
Content validity was evaluated through the work of the experts and, progressively, also through the assessment of the test-retest results. The following considerations can be provided for the construction of the five items.
For the formulation of the first questionnaire item, the definition of CP provided by the Task Force of the International Association for the Study of Pain (IASP) for the ICD-11 was mainly relied on,1 taking into account the dimension of “persistency” of CP which widely characterizes the affliction, and the limit of “more than 3 months” to indicate time duration. The concept of “recurrency” was not included in item 1 to avoid possible mis-detection of acute and sub-acute pain conditions different from CP. This choice was intended to minimize the number of respondents suffering from acute pain and, at the same time, to maximize detection of effective CP sufferers. The term “recurrent”, in fact, may also refer to an episodic or intermittent pain whose occurrence is not well-established. The IASP Task Force definition does not provide explanation about time definition for “recurrency”, while several studies operationalized “recurrency” in different ways, based also on specific CP clinical subtypes taken into account.23,33–35 Moreover, some authors suggest that it is unclear whether multiple pain episodes lasting several days within 3 months meet the CP criterion.36 Consequently, the term “recurrency” would have required thoughtful explanations addressing different CP phenotypes, making the specific term unsuitable to be adopted in this kind of self-administered measure. For these reasons, it was decided to privilege the simplest question formulation, desirable for general population surveys. The second item regarding CP “intensity” was formulated by the use of a 5-rank Verbal Rating Scale (VRS) consisting of a list of adjectives that describe different levels of pain. The least severe descriptor is “very mild”, which was associated to a score of 1; the most severe descriptor is “very severe”, associated to a score of 5. The VRS was, therefore, the same as that included within the SF-36 adopted in Ehis wave 3 2019.12 VRS has proven to be a valid, reliable and appropriate measure of pain intensity like the Numerical Rating Scale,37 and it was chosen for its strengths, i.e., ease of administration, high intelligibility, and high compliance rates.38,39 These features were considered particularly useful in large self-administered epidemiological surveys, containing several other measures, to increase response rates while reducing missing data and respondents’ burden.
The third item was aimed to address possible CP causes or triggers, that are important to distinguish, in a public health perspective, different groups of pathologies’ burden, care and costs for treatments. It includes 5 possible causes/triggers: “surgery”, “trauma”, “cancer”, “a disease which received a diagnosis”, and “a disease not yet diagnosed”. For what concerns item 3 response categories, “Cancer” was taken into account as cancer-related pain represents a specific sector; it would be extremely useful to obtain data on “cancer-related CP” prevalence and treatment considering the high prevalence and the suboptimal management it receives.40 Moreover, “post-surgical” and “post-traumatic” CP were both included as they are recognized as relevant and often neglected groups of CP conditions.2 Obtaining data on these conditions may provide useful insights for prevention activities. They are among the major CP causes relevant from a public health perspective (e.g., they are involved in loss of work days). From the same perspective, the distinction provided between “diagnosed” and “undiagnosed” CP may assume a great relevance as an individual with an undiagnosed CP condition could have greater diagnostic and treatment difficulties compared with an individual already diagnosed with CP, with higher costs for both the individual and the health system (e.g., more healthcare utilization).41,42 Although different pain conditions may produce different burdens,4 a trade-off between shortness of the list of causes/triggers and disease specification was adopted; therefore, instead of definite CP diagnoses (e.g., low-back pain, osteoarthritis), two wide group-related conditions were included as possible answers, namely “diagnosed” and “undiagnosed” CP related conditions. It is worth highlighting that the item was designed to detect, among potentially “affected individuals”, outside clinical contexts, the best possible cause or trigger for CP onset, relying less on the concept of specificity of the diagnosis, whose use is more useful in clinical contexts.
The fourth item referred to treatments and drugs assumption. It investigates whether the individual has taken or is taking medications or other therapies due to his/her persistent physical pain. Both time-based condition of the assumption (i.e., past and present) were possible because, if the first item had to be consistent with all the others, we could not overlook the dimension of a past assumption within the “last three months” period. The fourth item reached a good test-retest reliability also with a dichotomous (Yes/No) version in the initial draft questionnaire. However, the group of experts decided to use a more structured version of response categories to maximize the potential provided by the fifth item (addressing self-perceived effectiveness of the treatments and/or drugs). Thus, the fourth item was finally articulated in 4 response categories: “Yes, with continuity”; “Yes, with cycles of treatment”; “Yes, when needed”; “No”.
The fifth item regards respondents’ self-perception of treatments or drugs’ effectiveness. Although a dichotomous response category (Yes/No) was initially adopted to foster the simplicity of the questionnaire, during the experts’ consultations a three categories formulation of answers (“treatments/drugs allow me to feel good again”; “treatments/drugs allow me to resolve only part of my pain”; “treatments/drugs do not have any effect”) was proposed to detect possible situations of partial treatment effectiveness.
For what concerns “understandability”, the two samples of affected and non-affected respondents did not differ for demographic characteristics (chi-square tests: p=0.21 for gender, p=0.70 for education, p=0.12 for geographical area of residence; t-test for age: p=0.47). Results showed a high item understanding, with at least 95% of respondents cumulatively reporting the categories “4” and “5” for all items, both in CP-affected and in CP-free subjects. The maximum comprehension level (i.e., “5”) was reported by at least 85% of CP-free subjects and by at least 77% of CP-affected subjects. The lowest observed comprehension level was “3”, which was reported by only one CP-free subject for each of the items 1, 3 and 4. Item understanding appeared to be independent from the presence/absence of CP (chi-square tests: p=0.60 for item 1, p=0.31 for item 2, p=0.35 for item 3, p=0.20 for item 4, p=0.64 for item 5).
Reliability Assessment
First Test-Retest
Of the 215 twins contacted in July 2018 for the first test-retest evaluation, 114 twins (53%) replied. Only 70 of these 114 subjects gave their consent to participate to the test and were recontacted in a follow-up survey; of them, 53 subjects (76%) completed the online questionnaire for the second time. Median time between the two waves was 15 days. The observed kappa values were: 0.85 (Item 1); 0.74 (Item 2); 0.52 (Item 3); 0.60 (Item 4); 0.40 (Item 5). These values were significant (p<0.001) for all items except Item 5 (p=0.07).
These results were preliminary and were to be confirmed in larger samples. The group of experts proposed to modify the response categories of those questions that showed moderate reliability (i.e., Items 4 and 5), and a new version of the questionnaire underwent a second test-retest analysis.
Second Test-Retest
The twin sample for the second test-retest spanned a larger age-range and, consequently, included a higher number of participants. Of the 1104 twins contacted in October 2018 for the second test-retest evaluation, 317 subjects (29%) replied; of these, 273 subjects gave their consent and participated to the test. At the second wave, 185 subjects out of the 273 (68%) replied and completed the online questionnaire. Because 139 of the 185 subjects expressed their formal consent to participate, only data from these 139 subjects were used for the test-retest analysis. Median time between the two waves was 3 weeks. The observed kappa values were: 0.75 (Item 1); 0.50 (Item 2); 0.54 (Item 3); 0.60 (Item 4); 0.40 (Item 5). These values were significant (p<0.001) for all items.
The level of items’ reliability remained generally stable, but a few aspects have to be pointed out. We decided to consider a slightly longer between-wave time for the second test-retest in order to maximize the number of respondents at the retest survey. Moreover, as regards Item 2, we hypothesized that the longer time between the two waves of the second test-retest might have lowered the reliability compared with the previous test, due to a possible higher recall bias. As regards item 5, the reliability level remained exactly the same as in the previous test, but it became higher (0.54) when the response format was recoded as binary (i.e., 1=the treatment had an effect; 0=the treatment had no effect); however, we decided to maintain this new version of the item response categories because they would have allowed measuring a more complex dimension of CP effectiveness.
The procedures described produced a self-administered questionnaire to detect CP as a whole phenotype, among large samples drawn from the general population. The final “5-Item CP Quest” is, therefore, a brief questionnaire which takes into account the following dimensions: (i) occurrence of CP on the basis of persistency and time duration (first filtering item); (ii) CP intensity addressed with a 5-rank VRS; (iii) potential underlying CP causes or triggers; (iv) use of drugs or other treatments and modality of assumption; (v) self-perceived effectiveness of these treatments/drugs. The final “5-Item CP Quest” is reported as supplementary data.
Criterion and Construct Validity Assessment of the “5-Item CP Quest” Through the Results of the Ehis Wave 3 (2019)
For what concerns the Ehis wave 3 (2019), in general, the response rate, net of the not-eligible households (i.e., 927 households, e.g., those that moved in another town), was 78%. The number of respondents >18 years for the “5-Item CP Quest” was 38,775, out of an overall number of interviewed subjects of 44,492 (response rate 87%). Specifically, in our analyses we took into account only respondents aged 18 years and over, even if the admitted age to respond was >15 years; for what concerns the analysis of “education”, the age taken into account was 25 and over, to give stability to the education level.
Firstly, to further evaluate the properties of the questions, we examined the proportion of missing data and the distribution of responses. A percentage of 13.0% of people aged 18 and over did not answer to questions on CP (13.2% for men, 12.7% for women). The percentage of non-respondents rises to 14.0% among young people aged 18–34, especially in males (15.3% aged 18–34). However, the analysis of characteristics of non-respondents compared with respondents did not show any significant distortions; in particular, the distributions by gender, age group, geographical area, educational level and living arrangement were similar between respondents and non-respondents, providing at least partial reassurance against possible selection effects in the two groups.
Considering “Criterion validity”, we focused on the first and second items of the “5-Item CP Quest” and compared their response frequencies with the frequencies gathered by the Ehis wave 3 (2019) question regarding “physical pain occurrence and intensity within the last 4 weeks”, present within the principal module of the Ehis. “Physical pain during the last 4 weeks” was significantly (P<0.0001) associated with CP, with almost all (99.8%) the subjects affected by CP declaring to have experienced physical pain within the last 4 weeks.
CP intensity was significantly (P<0.0001) associated with the intensity of “physical pain in the last 4 weeks”. On average, 56% and 70% of respondents declared the same level of pain intensity both for CP and for “physical pain during the last 4 weeks”, respectively “very severe” and “very mild” [data not shown].
According to the well-known association of CP with a few specific pathologies and traits, we considered ascertained knowledge regarding the following comorbidities and traits as reference for comparison with the results of the Ehis wave 3 (2019). A significant association between CP and these pathologies/traits might indicate a good construct validity.
As predicted, CP occurrence investigated through our questionnaire in the Ehis wave 3 (2019) was significantly (P<0.0001) associated with the presence of depression diagnosed by a specialist, with 12.2% of CP sufferers having received a diagnosis of depression. Moreover, Ehis wave 3 (2019) results showed a significant (P<0.0001) association between CP and cancer, with a proportion of 56.4% of people with cancer suffering from CP. CP was also significantly (P<0.0001) and positively associated with age; in particular, the prevalence of CP was 11% in the 25–44 age group, 24.5% in the 45–64 age group and 42.8% over the age of 65. A significant (P<0.0001) negative association between CP and education level was found (Table 2).
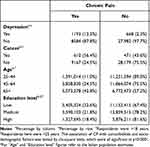 |
Table 2 Association Between CP and Well-Known Comorbidities and Socio-Demographic Factors from the Ehis Wave 3 (2019) |
For what concerns item 5, a significant (P<0.0001) negative association emerged between the perceived effectiveness of treatments/drugs among CP-affected individuals and the declared pain interference in daily activities; indeed, a reduction in pain interference with increasing effectiveness perception was observed (Table 3).
 |
Table 3 Association Between CP Affected Individuals’ “Perceived Effectiveness of Treatments/Drugs” and “Declared Pain Interference in Daily Activities” from the Ehis Wave 3 (2019) |
For what concerns divergent validity, a lack of association emerged between CP occurrence and the respondents’ geographical area of residence (North, Centre, South and Islands), taking into account income differences among them (low income: χ2=56.52, P=0.06; middle income: χ2 =12.87, P=0.53; high income: χ2=18,57, P=0.40). All these comparisons provide support for construct validity of the “5-Item CP Quest”.
Discussion
In this study, we developed and validated, in the Italian context, a 5-item questionnaire named “Brief Five-item Chronic Pain Questionnaire” with the acronym “5-Item CP Quest”.
Throughout the working phases, we have progressively strengthened the aim to pursue the design and validation of a brief measure for CP investigations among the general population, coping with the need (or the urgency) for validated and standardized tools for ascertaining CP in epidemiological studies,5 and prevent a too easy approach of transferring figures from clinical to general population settings.
Literature search findings confirmed that validated questionnaires were infrequently used and important CP dimensions regarding public health aspects had seldom been encompassed.
The analysis of the literature confirmed that questionnaires used so far to measure CP as an overall phenotype were, for the great majority, not validated in epidemiological settings, or were substantially derived from tools used in clinical fields, as the Brief Pain Inventory (BPI).43 Whereas, the Chronic Pain Grade,44 one of the few validated measures of CP severity for general population postal surveys, was not deemed suitable for our purposes as it does not take into account most CP dimensions we were interested in; moreover, the pain intensity detection modality it adopted would have affected the briefness of our measure. Generally, the 10 studies retrieved from the literature,6,23–31 presented measures that were not really suitable for self-administered surveys in terms of length and complexity of their numerous items.
The validation process encompassed content validity, that required a long consultation among experts, and understandability evaluation of the “5-Item CP Quest”. As far as we know, no methodological articles on CP measures have provided so far clear assessment of their understandability.
Moreover, results of the test-retest analyses showed, under some limitations, an adequate reliability of the “5-Item CP Quest”. Particularly, for the first filtering-item the values for kappa statistic were similar or even higher than those obtained in other studies (e.g., Azevedo and colleagues).23 The highest reliability performance observed for CP “intensity” suggests that the measure may be particularly suitable to detect persistent states of pain and related intensity in the general population. Since CP in itself is an enduring experience, we have tried to decrease the risk of obtaining “inflated” correlations, repeating the reliability test two times and, above all, on a larger sample of individuals (also in terms of a wider age range), three months later from the first test-retest. This has allowed to exert a certain control on the risk of “recall bias” whatever its direction. One of the problems with reliability across time, in fact, is that individuals may remember their previous responses and repeat them at the second administration, thus increasing the correlation between the two administrations.
We had the great opportunity to be hosted by the National representative Ehis survey, that provided valuable comparisons to ascertain criterion and construct validity of our measure. For what concerns criterion validity, the almost complete overlapping between subjects suffering from CP and those suffering from a “physical pain within the last 4 weeks” detected in the Ehis wave 3 (2019) is quite reassuring, considering that the item chosen for comparison comes from the SF-36, a questionnaire widely used in studies and clinical trials regarding several medical and psychiatric disorders and also recommended and validated for chronic pain conditions.45,46 Moreover, the comparison between intensity levels reported by respondents to the two questionnaires contributes to strengthen as well this conclusion; the overlap regards, in fact, a considerable percentage of individuals (ranging from 56% to 70%).
Considering construct validity assessment, we deem that well-known CP comorbidities or CP correlations with specific traits (age, education level) are well confirmed in the Ehis, providing a satisfying result for what concerns consistency of the whole construct. In particular, the findings of the Ehis wave 3 (2019) confirmed CP association with depression, providing results similar to those obtained by other epidemiological studies: Azevedo and colleagues found a prevalence of diagnosed depression among CP-affected subjects of 13% in a study conducted in Portugal,23 while in the Irish study of Raftery and colleagues,47 15% of CP sufferers met the criteria for clinically relevant depression. It is noteworthy that other studies found even higher prevalence of depression among people with CP. A frequently cited survey of Breivik et al found a European prevalence of 21%,6 while Miller and Cano,48 in a study on a representative US community sample, found that approximately 35% of participants with CP also had comorbid depression. It has to be pointed out, however, that assessing depression in CP-affected subjects is complex and presents several critical aspects; furthermore, the prevalence of depression among pain sufferers may vary widely due to variability in the definition and assessment of depression across studies, as well as in samples and setting considered.49
Regarding cancer, pain is the main symptom characterizing the pathology that affects in a persistent manner nearly 55% of all cancer patients, at least 66% of patients at an advanced stage of the disease, and 33–40% of individuals who survive from cancer.50 The Ehis wave 3 (2019) shows that, among people with cancer, CP involved 56.4% of respondents. This figure is consistent with several international estimates; for example, Breivik et al found a prevalence of moderate-severe cancer pain of 56% out of a sample of 5084 cancer patients from all over Europe,40 while a more recent systematic review including 117 studies, totaling 63,533 patients, found prevalence ranging from 39 to 66% (M = 52.5%).51
With respect to age, there is consistent evidence that CP and age are positively correlated. Reasons for this correlation are mainly based on the co-occurrence in older age of several pathologies such as arthrosis, diabetes, etc. which, in advanced stages, are characterized by persistent presence of pain.52 Considering age, our results are coherent with estimates from other epidemiological studies conducted in Portugal,23 Sweden,53 and Denmark,54 which found higher prevalence rates of CP with increasing age. Moreover, the National Health Interview Survey conducted in the USA in 201653 and an epidemiological study in 18 countries around the world, both in developed and developing countries,56 found an increase in the prevalence of CP with increasing age as well. It has also to be underlined that an opposing result comes from the European survey by Breivik and colleagues,6 which found no differences in the prevalence of CP in different age groups.
For what concerns education, there is clear evidence that CP is inversely correlated with education level from several studies conducted in Europe as well as in the USA and in Eastern countries, mainly because education is a strong marker of socioeconomic level.57–60 Our results in the Ehis wave 3 (2019) indicate a lower prevalence of CP with increasing educational level. This is, as well, particularly in line with a few epidemiological studies conducted in England,61 Norway,62 Denmark,54 and Portugal,23 and according to the National Health Interview Survey conducted in the USA in 2016, they all found a negative association between CP and educational levels.55
Finally, the findings of the Ehis wave 3 (2019) also confirmed a negative association between “pain interference in daily activities” (item from the SF-36) and “perceived effectiveness of treatments or drugs” as detected by our questionnaire. These results are in accordance with findings coming from other studies, such as an observational study conducted in Ukraine,63 that found increased odds of impairing pain in subjects who reported low/moderate effectiveness from treatments, and results recorded by a longitudinal intervention study by Tan and colleagues,64 showing that changes in pain interference were associated with treatment satisfaction.
One issue has to be addressed: the “5-Item CP Quest” does not include any measures of the interference of CP with daily activities. The disability induced by CP is a relevant aspect both from patient and public health perspectives; however, the Ehis general population survey, which hosted our questionnaire, had already adopted a measure of this dimension. The “5-Item CP Quest” is, therefore, particularly suitable to be included within large epidemiological surveys, which can make use of previously validated measures of pain-induced disability or other important dimensions. Researchers may consider this aspect and complement the “5-Item CP Quest” with other validated measures of CP impacting conditions. At the same time, the dimensions of “treatments” and “perceived efficacy” we have addressed are not yet investigated at population level in our country, and they can be particularly useful, from a public health perspective, to (a) better understand how many chronic pain individuals use CP treatments; (b) start dimensioning the neglect of treatments at population level, knowing how many CP sufferers might have remained outside the health-care service. Furthermore, both these dimensions may be useful to check the state of the national pain therapy network, in line with the provision of article 11 of the Italian law 38/2010 regarding citizens’ right to access pain therapy (Italian Parliament; available from: http://www.parlamento.it/parlam/leggi/10038L.htm; 2010). The law, in fact, introduces annual reports to the Parliament on the goals achieved and the critical issues emerged in the implementation and development of the health-care networks in palliative care and pain therapy.
Other valuable aspects of CP experience (e.g., emotional functioning, sleep disturbances) were not evaluated as the tool is meant to perform to the best in general population surveys rather than in clinical investigations, and its shortness can be appreciated for its adaptability to large, self-administered (also online) examinations.
As noted by Steingrímsdóttir et al,5 the increasing interest in CP and its load on affected individuals and societies call for adequate monitoring tools for this phenomenon. The “5-Item CP Quest” may be used in Italy for this purpose and may offer significant public health hints, particularly to the groups dealing with population statistics and policy making. The adoption of the questionnaire within a routine survey, may allow epidemiological monitoring of CP and provide data for interventions at population level (e.g., awareness campaigns, changes in CP network interventions). The use of our measure may also support the annual report to the Italian Parliament requested by law 38/2010, and offer the basis for the development of CP prevention and management programs targeting specific population subgroups.
Further work is needed to test reliability and external validity of the “Brief Five-Item CP Quest” both in Italy and in other countries, to take into account possible influences of cultural, social and health-policy related factors, especially for specific CP dimensions such as those addressing treatments and their self-perceived effectiveness. In this respect, a large twin study has already been started, and is currently under analysis, which makes use the “5-Item CP Quest” on a sample of more than 4000 Italian twins. The study is mainly aimed to disentangle the gene-environment architecture of the wide CP phenotype, but it will also allow us to further examine the validity of the questionnaire in the Italian general population. Similar research with the adoption of the “5-Item CP Quest” is being conducted by the Murcia Twin Registry in Spain, and a joint analysis is underway to investigate cross-cultural validity of the “5-Item CP Quest”.
We are well aware that the validation of a questionnaire is an ongoing process, thus we call epidemiologists and CP researchers to verify whether the “5-Item CP Quest” may be a valid measure of CP in general population studies, also in other countries. This process has to take into account that variables able to describe CP, particularly for public health purposes, may be differently weighted in different countries. We do invite researchers to adapt our questionnaire to different contexts and add, when necessary, further items and validate these versions within specific public health scenarios. Another interesting future direction may be to test whether our brief questionnaire allows no major loss of information compared with other validated and longer questionnaires used in previous epidemiological studies.
Conclusions
The “5-Item CP Quest” is designed as a brief self-administered measure to detect CP, investigating its intensity, possible underlying causes/triggers, treatments, and their perceived efficacy in general population surveys. Its content validity was deeply discussed by a group of experts and the measure shown to be understandable among both CP-affected and non-affected individuals. Reliability and construct validity assessment confirmed good psychometric properties of the questionnaire. The “5-Item CP Quest” focuses on a few public health-relevant CP dimensions and, being shorter than other validated CP questionnaires used in epidemiology, it may be complemented with other measures in wide epidemiological studies. The “5-Item CP Quest” may help in monitoring CP prevalence at a national level, thus contributing to the production of relevant data for health policies. CP is a public health priority, an affliction occurring in many different conditions, across age, gender and diagnoses. Further validation of the measure, in different cultural settings, is desirable.
Acknowledgments
The authors warmly thank all the Twins who kindly participated in the two test-retest surveys in 2018; all the individuals who participated in Italy in the Ehis wave 3 (2019) and accepted to answer the “5-Item CP Quest”; the Technical and Administrative Personnel of the Italian Twin Registry – under the expertise and personal guidance of Doctor Maria Antonietta Stazi, Director of the ITR, at the Istituto Superiore di Sanità – who made this work possible: Mrs. Sabrina Alviti, Mrs. Cristina D’Ippolito, Mr. Antonio Arnofi, Mrs. Miriam Salemi. The study was produced within the framework of an active 5 year-long scientific collaboration between Istituto Superiore di Sanità, Centre for Behavioural Science and Mental Health, and ISAL Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Treede R-D, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27. doi:10.1097/j.pain.0000000000001384
2. Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27. doi:10.1097/j.pain.0000000000001384
3. Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11:770. doi:10.1186/1471-2458-11-770 PMID: 21978149; PMCID: PMC3201926.
4. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi:10.1016/S0140-6736(15)60692-4
5. Óa S, Landmark T, Macfarlane GJ, Nielsen CS. Defining chronic pain in epidemiological studies: a systematic review and meta-analysis. Pain. 2017;158(11):2092–2107. doi:10.1097/j.pain.0000000000001009
6. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi:10.1016/j.ejpain.2005.06.009
7. Del Giorno R, Frumento P, Varrassi G, Paladini A, Coaccioli S. Assessment of chronic pain and access to pain therapy: a cross-sectional population-based study. J Pain Res. 2017;10:2577–2584. doi:10.2147/JPR.S136292
8. Raffaeli W, Minella CE, Magnani F, Sarti D. Population-based study of central post-stroke pain in Rimini district, Italy. J Pain Res. 2013;6:705–711. doi:10.2147/JPR.S46553
9. Smith BH, Penny KI, Purves AM, et al. The Chronic Pain Grade questionnaire: validation and reliability in postal research. Pain. 1997;71(2):141–147. doi:10.1016/s0304-3959(97
10. Sullivan MD, Turner JA, Romano J. Chronic pain in primary care. Identification and management of psychosocial factors. J Fam Pract. 1991;32(2):193–199.
11. Tsang S, Royse CF, Terkawi AS. Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi J Anaesth. 2017;11(Suppl 1):S80–S89. doi:10.4103/sja.SJA_203_17
12. Eurostat. European Health Interview Survey (EHIS wave 3). Methodological manual. 2018. Available from: https://ec.europa.eu/eurostat/web/products-manuals-and-guidelines/-/KS-02-18-240.
13. Iglesias C, Torgerson D. Does length of questionnaire matter? A randomised trial of response rates to a mailed questionnaire. J Health Serv Res Policy. 2000;5(4):219–221. doi:10.1177/135581960000500406
14. Galesic M, Bosnjak M. Effects of questionnaire length on participation and indicators of response quality in a web survey. Public Opin Q. 2009;73(2):349–360.
15. Rolstad S, Adler J, Rydén A. Response burden and questionnaire length: is shorter better? A review and meta-analysis. Value Health. 2011;14(8):1101–1108. doi:10.1016/j.jval.2011.06.003
16. Salkind NJ. Encyclopedia of Research Design (Vols. 1-0). Thousand Oaks, CA: SAGE Publications; 2010. doi:10.4135/9781412961288
17. Medda E, Toccaceli V, Fagnani C, et al. The Italian Twin Registry: an Update at 18 Years From Its Inception. Twin Res Hum Genet. 2019;22(6):572–578. doi:10.1017/thg.2019.75
18. Klemmensen R, Hobolt SB, Dinesen PT, Skytthe A, Nørgaard AS. The Danish political twin study: political traits in Danish twins and the general population. Twin Res Hum Genet. 2012;15(1):74–78. doi:10.1375/twin.15.1.74
19. Kyvik KO. Generalisability and assumptions of twin studies. In: Spector TD, Snieder H, MacGregor AJ, editors. Advances in Twin and Sib-Pair Analysis. London, United Kingdom: Greenwich Medical Media; 2000:67–77.
20. Öberg S, Cnattingius S, Sandin S, Lichtenstein P, Morley R, Iliadou AN. Twinship influence on morbidity and mortality across the lifespan. Int J Epidemiol. 2012;41(4):1002–1009. doi:10.1093/ije/dys067
21. Ware JE, Sherbourne CD, The MOS. 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483.
22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174.
23. Azevedo LF, Costa-Pereira A, Mendonça L, Dias CC, Castro-Lopes JM. Epidemiology of chronic pain: a population-based nationwide study on its prevalence, characteristics and associated disability in Portugal. J Pain. 2012;13(8):773–783. doi:10.1016/j.jpain.2012.05.012
24. Chrubasik S, Junck H, Zappe HA, Stutzke O. A survey on pain complaints and health care utilization in a German population sample. Eur J Anaesthesiol. 1998;15(4):397–408. doi:10.1046/j.1365-2346.1998.00317.x
25. de Moraes Vieira EB, Garcia JB, da Silva AA, Mualem Araújo RL, Jansen RC. Prevalence, characteristics, and factors associated with chronic pain with and without neuropathic characteristics in São Luís, Brazil. J Pain Symptom Manage. 2012;44(2):239–251. doi:10.1016/j.jpainsymman.2011.08.014
26. de Souza JB, Grossmann E, Perissinotti DMN, de Oliveira Junior JO, da Fonseca PRB, Posso IP. Prevalence of Chronic Pain, Treatments, Perception, and Interference on Life Activities: Brazilian Population-Based Survey. Pain Res Manag. 2017;2017:4643830. doi:10.1155/2017/4643830
27. Dureja GP, Jain PN, Shetty N, et al. Prevalence of chronic pain, impact on daily life, and treatment practices in India. Pain Pract. 2014;14(2):E51–E62. doi:10.1111/papr.12132
28. Elzahaf RA, Johnson MI, Tashani OA. The epidemiology of chronic pain in Libya: a cross-sectional telephone survey. BMC Public Health. 2016;16(1):776. doi:10.1186/s12889-016-3349-6
29. Ng KF, Tsui SL, Chan WS. Prevalence of common chronic pain in Hong Kong adults. Clin J Pain. 2002;18(5):275–281. doi:10.1097/00002508-200209000-00001
30. Toblin RL, Mack KA, Perveen G, Paulozzi LJ. A population-based survey of chronic pain and its treatment with prescription drugs. Pain. 2011;152(6):1249–1255. doi:10.1016/j.pain.2010.12.036
31. Walters JL, Baxter K, Chapman H, et al. Chronic Pain and Associated Factors in India and Nepal: a Pilot Study of the Vanderbilt Global Pain Survey. Anesth Analg. 2017;125(5):1616–1626. doi:10.1213/ANE.0000000000002360
32. Sternbach RA. Survey of pain in the United States: the Nuprin pain report. Clin J Pain. 1986;2(1):49–53.
33. Dueñas M, Salazar A, Ojeda B, et al. A nationwide study of chronic pain prevalence in the general Spanish population: identifying clinical subgroups through cluster analysis. Pain Med. 2015;16(4):811–822. doi:10.1111/pme.12640
34. Marconi E, Pecchioli S, Nica M, et al. Epidemiology and determinants of chronic migraine: a real-world cohort study, with nested case-control analysis, in primary care in Italy. Cephalalgia. 2020;40(5):461–469. doi:10.1177/0333102419889351
35. Ohayon MM, Schatzberg AF. Chronic pain and major depressive disorder in the general population. J Psychiatr Res. 2010;44(7):454–461. doi:10.1016/j.jpsychires.2009.10.013
36. Ballantyne JC, Fishman SM, Rathmell JP. Bonica’s Management of Pain. Philadelphia, PA: Lippincott Williams & Wilkins; 2018.
37. Karcioglu O, Topacoglu H, Dikme O, Dikme O. A systematic review of the pain scales in adults: which to use? Am J Emerg Med. 2018;36(4):707–714. doi:10.1016/j.ajem.2018.01.008
38. Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine. 2000;25(24):3140–3151. doi:10.1097/00007632-200012150-00009
39. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. doi:10.1111/j.1365-2702.2005.01121.x
40. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20(8):1420–1433. doi:10.1093/annonc/mdp001
41. Cromeens MG, Carey ET, Robinson WR, Knafl K, Thoyre S. Timing, delays and pathways to diagnosis of endometriosis: a scoping review protocol. BMJ Open. 2021;11(6):e049390. doi:10.1136/bmjopen-2021-049390
42. Külekçioğlu S. Diagnostic difficulty, delayed diagnosis, and increased tendencies of surgical treatment in fibromyalgia syndrome. Clin Rheumatol. 2022;41(3):831–837. doi:10.1007/s10067-021-05970-7
43. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23(2):129–138.
44. Smith BH, Elliott AM, Chambers WA, Smith WC, Hannaford PC, Penny K. The impact of chronic pain in the community. Fam Pract. 2001;18(3):292–299. doi:10.1093/fampra/18.3.292
45. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi:10.1016/j.pain.2004.09.012
46. LoMartire R, Äng BO, Gerdle B, Vixner L. Psychometric properties of Short Form-36 Health Survey, EuroQol 5-dimensions, and Hospital Anxiety and Depression Scale in patients with chronic pain. Pain. 2020;161(1):83–95. doi:10.1097/j.pain.0000000000001700
47. Raftery MN, Sarma K, Murphy AW, De la Harpe D, Normand C, McGuire BE. Chronic pain in the Republic of Ireland–community prevalence, psychosocial profile and predictors of pain-related disability: results from the Prevalence, Impact and Cost of Chronic Pain (PRIME) study, part 1. Pain. 2011;152(5):1096–1103. doi:10.1016/j.pain.2011.01.019
48. Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk? J Pain. 2009;10(6):619–627. doi:10.1016/j.jpain.2008.12.007
49. Tenti M, Raffaeli W, Gremigni P, Narrative A. Review of the Assessment of Depression in Chronic Pain. Pain Manag Nurs. 2021. doi:10.1016/j.pmn.2021.03.009
50. Bennett MI, Kaasa S, Barke A, et al. The IASP classification of chronic pain for ICD-11: chronic cancer-related pain. Pain. 2019;160(1):38–44. doi:10.1097/j.pain.0000000000001363
51. van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on Prevalence of Pain in Patients With Cancer: systematic Review and Meta-Analysis. J Pain Symptom Manage. 2016;51(6):1070–1090.e9. doi:10.1016/j.jpainsymman.2015.12.340
52. Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17(3):417–v. doi:10.1016/s0749-0690(05
53. Bergman S, Herrström P, Högström K, Petersson IF, Svensson B, Jacobsson LT. Chronic musculoskeletal pain, prevalence rates, and sociodemographic associations in a Swedish population study. J Rheumatol. 2001;28(6):1369–1377.
54. Kurita GP, Sjøgren P, Juel K, Højsted J, Ekholm O. The burden of chronic pain: a cross-sectional survey focussing on diseases, immigration, and opioid use. Pain. 2012;153(12):2332–2338. doi:10.1016/j.pain.2012.07.023
55. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi:10.15585/mmwr.mm6736a2
56. Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–891. doi:10.1016/j.jpain.2008.05.005
57. Wong SSC, Choi SW, Cheung CW. A comparison of chronic pain with and without neuropathic characteristics in a Hong Kong Chinese population: an analysis of pain related outcomes and patient help seeking behaviour. PLoS One. 2018;13(10):e0204054. doi:10.1371/journal.pone.0204054
58. Elliott AM, Smith BH, Hannaford PC, Smith WC, Chambers WA. The course of chronic pain in the community: results of a 4-year follow-up study. Pain. 2002;99(1–2):299–307. doi:10.1016/s0304-3959(02)00138-0
59. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230–1239. doi:10.1016/j.jpain.2010.07.002
60. Yu HY, Tang FI, Kuo BI, Yu S. Prevalence, interference, and risk factors for chronic pain among Taiwanese community older people. Pain Manag Nurs. 2006;7(1):2–11. doi:10.1016/j.pmn.2005.12.002
61. Torrance N, Elliott AM, Lee AJ, Smith BH. Severe chronic pain is associated with increased 10 year mortality. A cohort record linkage study. Eur J Pain. 2010;14(4):380–386. doi:10.1016/j.ejpain.2009.07.006
62. Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. Pain. 2011;152(10):2241–2247. doi:10.1016/j.pain.2011.04.029
63. Xu A, Hilton E, Arkema R, Tintle NL, Helming LM. Epidemiology of chronic pain in Ukraine: findings from the World Mental Health Survey. PLoS One. 2019;14(10):e0224084. doi:10.1371/journal.pone.0224084
64. Tan G, Jensen MP, Thornby JI, Anderson KO. Are patient ratings of chronic pain services related to treatment outcome? J Rehabil Res Dev. 2006;43(4):451–460. doi:10.1682/jrrd.2004.10.0128
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
