Back to Journals » Nature and Science of Sleep » Volume 14
Development and Validation of a Nomogram for Predicting Obstructive Sleep Apnea in Patients with Pulmonary Arterial Hypertension
Authors Hu M, Duan A, Huang Z , Zhao Z, Zhao Q, Yan L, Zhang Y, Li X, Jin Q , An C, Luo Q, Liu Z
Received 27 April 2022
Accepted for publication 22 July 2022
Published 9 August 2022 Volume 2022:14 Pages 1375—1386
DOI https://doi.org/10.2147/NSS.S372447
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Meixi Hu,1,* Anqi Duan,1,* Zhihua Huang,1,* Zhihui Zhao,1 Qing Zhao,1 Lu Yan,1 Yi Zhang,1 Xin Li,1 Qi Jin,2 Chenhong An,1 Qin Luo,1 Zhihong Liu1
1Center for Respiratory and Pulmonary Vascular Disease, Department of Cardiology, Fuwai Hospital, National Clinical Research Center for Cardiovascular Diseases, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 2Department of Cardiology, Shanghai Institute of Cardiovascular Disease, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qin Luo; Zhihong Liu, Center for Respiratory and Pulmonary Vascular Disease, Department of Cardiology, Fuwai Hospital, National Clinical Research Center for Cardiovascular Diseases, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, and Peking Union Medical College, No. 167 Beilishi Road, Xicheng District, Beijing, 100037, People’s Republic of China, Tel/Fax +86-10-88396589, Email [email protected]; [email protected]
Purpose: Patients with pulmonary arterial hypertension (PAH) are at high risk for obstructive sleep apnea (OSA), which may adversely affect pulmonary hemodynamics and long-term prognosis. However, there is no clinical prediction model to evaluate the probability of OSA among patients with PAH. Our study aimed to develop and validate a nomogram for predicting OSA in the setting of PAH.
Patients and Methods: From May 2020 to November 2021, we retrospectively analyzed the medical records of 258 patients diagnosed with PAH via right-heart catheterization. All participants underwent overnight cardiorespiratory polygraphy for OSA assessment. General clinical materials and biochemical measurements were collected and compared between PAH patients with or without OSA. Lasso regression was performed to screen potential predictors. Multivariable logistic regression analysis was conducted to establish the nomogram. Concordance index, calibration curve, and decision curve analysis were used to determine the discrimination, calibration, and clinical usefulness of the nomogram.
Results: OSA was present in 26.7% of the PAH patients, and the prevalence did not differ significantly between male (29.7%) and female (24.3%) patients. Six variables were selected to construct the nomogram, including age, body mass index, hypertension, uric acid, glycated hemoglobin, and interleukin-6 levels. Based on receiver operating characteristic analysis, the nomogram demonstrated favorable discrimination accuracy with an area under the curve (AUC) of 0.760 for predicting OSA, exhibiting a better predictive value in contrast to ESS (AUC = 0.528) (P < 0.001). Decision curve analysis and clinical impact curve analysis also indicated the clinical utility of the nomogram.
Conclusion: By establishing a comprehensive and practical nomogram, we were able to predict the presence of OSA in patients with PAH, which may facilitate the early identification of patients that benefit from further diagnostic confirmation and intervention.
Keywords: Epworth sleepiness scale, pulmonary hypertension, biomarkers, clinical prediction model
Introduction
Obstructive sleep apnea (OSA) is a common sleep breathing disorder characterized by repetitive upper airway obstruction, associated with a variety of cardiovascular diseases.1 As a global public health problem, OSA is estimated to occur in 1 billion adults aged 30–69 years, resulting in a huge economic and medical burden.2 Recently, increasing evidence has demonstrated a high prevalence and adverse impacts of comorbid OSA on pulmonary hypertension (PH).3,4 Pulmonary arterial hypertension (PAH), classified as group 1 PH, is a relatively rare but life-threatening condition. In patients with PAH, the presence of OSA can induce a series of pathophysiological consequences including intermittent hypoxia, chronic inflammation, sympathetic stimulation, and alterations in intrathoracic pressure, which potentially contributes to the deterioration of pulmonary hemodynamics and ventricular function. Therefore, it is crucial to refer patients with suspected OSA to a sleep center for diagnostic examination and timely intervention. However, OSA is still largely under-recognized and under-treated.4
Polygraphy (PG) and polysomnography (PSG) currently constitute the major diagnostic tools for OSA in clinical settings. However, their routine use is limited in less developed countries due to the high price, time-consuming nature, and lack of compliance.5 It is, therefore, not surprising that about 90% of OSA patients are inappropriately diagnosed or treated.6 According to the latest clinical practice guidelines published by the American Academy of Sleep Medicine (AASM), the efficiency and feasibility of diagnosis of OSA can be improved with proper clinical screening tools.7 Simple-to-use questionnaires, such as the Epworth sleepiness scale (ESS), are widely used to screen patients at high risk for OSA, yet their performance has not been adequately validated in PAH patients. Recently, a number of clinical prediction models have been developed to identify the risk of OSA across different populations, including patients with type 2 diabetes or ischemic stroke, bariatric surgery candidates, and pregnant women.8–11 To accurately evaluate the risk of OSA in different clinical settings, comprehensive consideration of patients’ characteristics, comorbidities, traditional risk factors, and biochemical investigations are required. To our knowledge, a clinical prediction model for OSA has not yet been developed in patients with PAH.
Therefore, in this study, we sought to explore potential risk factors for OSA in PAH patients and to construct a clinically feasible nomogram for identifying patients at high risk of OSA.
Materials and Methods
Patient Population
Patients diagnosed with PAH via right-heart catheterization (RHC) and having complete cardiorespiratory PG data were consecutively enrolled between May 2020 and November 2021 at Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences. The cardiorespiratory PG study was performed either prior to or following the RHC procedure within seven days. The diagnosis of PAH complied with the latest definition.12 Patients were excluded according to the following criteria: (1) patients who were classified as other groups (II–V) of PH; (2) patients under the age of 18; (3) patients with central sleep apnea (CSA); (4) patients suffering from severe cardiorespiratory conditions and chronic obstructive pulmonary disease (COPD) requiring nocturnal oxygen supplementation; (5) individuals with a sleep duration of fewer than four hours. The study was approved by the ethical committee of Fuwai Hospital, and informed consent for study participation was obtained from each patient.
Basic Data Collection
Anthropometric and Clinical Characteristics
The demographic data and physical examination of each patient were collected. Histories of smoking and alcohol drinking were recorded, and the presence of comorbidities, such as hypertension, hyperlipidemia and diabetes was also obtained. The degree of daytime sleepiness of the patients was assessed with the ESS questionnaire.5 The ESS questionnaire contains eight questions of sleepiness assessment in specific situations during the day; 0, 1, 2, and 3 points indicated no, mild, moderate, and severe sleepiness, respectively, with a total score of 24 points. ESS scores of more than 10 points indicate excessive daytime sleepiness.
Biochemical Measurements
Fasting venous blood samples were collected for laboratory tests on the first day of admission. The following biochemical parameters were measured by standard laboratory methods: uric acid (UA), homocysteine, C-reactive protein (CRP), interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), serum creatinine, plasma glucose and lipid profiles, glycated hemoglobin (HbA1c), and N-terminal pro-B-type natriuretic peptide (NT-proBNP). Medication, such as diuretics and inotropes, was initiated after blood samples collection to maximally reduce its effect on the baseline level of biochemical parameters.
Nocturnal Respiratory Events Evaluation
All enrolled PAH patients underwent full-night cardiorespiratory PG monitoring via an Embletta device (Medcare, Flaga, Reykjavik, Ireland) as previously described13 and nocturnal cardiorespiratory signals were recorded. Nasal airflow, finger pulse oximetry, thoracic and abdominal movements, body position, and snoring were recorded to evaluate the respiratory events. Sleep apnea is defined according to AASM guidelines as a complete cessation of airflow during sleep, or a decrease in airflow of more than 90% lasting for ≥10 seconds. Hypopnea is defined as a sustained reduction in airflow intensity of more than 30% for ≥10 seconds, corresponding with an oxygen desaturation of at least 3%. Central apnea was defined when there was no respiratory movement in the chest and abdomen during a sleep apnea event; otherwise, it was considered an obstructive event. The apnea–hypopnea index (AHI) was calculated as the number of apnea and hypopnea events per hour. An AHI ≥5/h with obstructive apneic events, accounting for more than 50% of the respiratory events, was considered a diagnosis of OSA.
Statistical Analysis
According to the distribution, continuous variables were presented as means ± standard deviation or median (interquartile ranges), while categorical variables were as frequencies (percentages). Continuous data were analyzed using the Student’s t-test or Mann–Whitney test, whereas categorical data were analyzed by the chi-square test. Missing data account for less than 5% of our study, which is acceptable for us to ignore them, and we use average imputation to fill in the gaps. A least absolute shrinkage and selection operator (Lasso) regression procedure was utilized for reducing the dataset dimensions, in order to identify the most important predictors. Based on the predictors screened by Lasso regression, a multivariable logistic regression analysis was performed to establish a nomogram for detecting OSA. A calibration curve was drawn to evaluate the calibration of the model. After internally verified by bootstrapping (1000 bootstrap resampling), the relative corrected C-index was calculated to assess the discriminatory ability of the nomogram. Additionally, the receiver operating characteristic (ROC) curve was developed to assess the accuracy of the nomogram and ESS for predicting the risk of OSA. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LRP), and negative likelihood ratio (LRN) of each scale were calculated. According to the net benefit of the nomogram based on a variety of threshold probabilities in the population studied, decision curve analysis (DCA) was used to evaluate the clinical effectiveness.14 This nomogram, according to the DCA, is more effective in predicting OSA risk than the ESS, the treat-all-patients scheme or the treat-none method. A P<0.05 was considered to be statistically significant. All statistical tests were performed using SPSS software (version 22.0; IBM SPSS Statistics, IBM Corp., Armonk, NY, USA) and R (version 4.0.5, R Foundation for Statistical Computing, Vienna, Austria).
Results
Basic Characteristics of the Study Population
Between May 2020 and November 2021, 627 consecutive patients were referred for diagnostic RHC and overnight cardiorespiratory PG monitoring. A total of 316 patients were diagnosed with PAH, among which 14 patients were excluded due to age restriction, 24 patients were excluded due to diagnosis of CSA, 20 were excluded due to insufficient sleeping data of less than four hours, thereby the remaining 258 patients with PAH were included in the final analysis. Among the PAH patients, 41.8% are diagnosed with idiopathic PAH, 12.5% are diagnosed with connective tissue disease associated PAH, 44.2% are diagnosed with congenital heart disease associated PAH, and 1.5% are diagnosed with portopulmonary hypertension. The mean age was 43 ± 16 years and 140 (54%) of patients were female. Among all study participants, only 3.1% reported excessive daytime sleepiness (ESS >10). There are sixty-nine (26.7%) patients in accordance with the diagnostic criteria for OSA, among which 53 (76.8%) and 16 (23.2%) had mild and moderate-to-severe OSA, respectively. Patients with PAH were divided into two groups according to the presence of OSA. The OSA group was characterized by an older age, a higher BMI score, poorer respiratory characteristics, and a higher proportion of comorbidities, including hypertension, diabetes, and hyperlipidemia, as compared to the non-OSA group (P<0.05). The scores of ESS and World Health Organization functional class, however, were not significantly different between OSA and non-OSA groups (Tables 1 and 2).
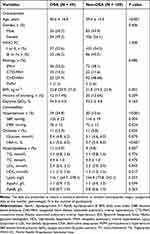 |
Table 1 Anthropometric and Clinical Characteristics of the Study Participants |
 |
Table 2 Laboratory and Nocturnal Respiratory Parameters of the Study Participants |
Factor Selection for Nomogram Construction
Based on the results of baseline comparison, univariable logistic analyses (Table S1), and clinical significance, the Lasso regression was performed on 17 potential variables. Using the linear combination of factors weighted by coefficients assigned to each subject, a risk score was calculated for each subject, from which a coefficient distribution curve was derived (Figure 1A). The cross-validation error graph for the Lasso regression model can be viewed in Figure 1B. For the most regularized and minimalistic model, the cross-validation error was within one standard error of the minimum. Finally, utilizing multivariable logistic regression (Table 3), a nomogram incorporating six factors (age, BMI, hypertension, HbA1c, UA, and IL-6) was developed (Figure 2).
 |
Table 3 Prediction Factors for OSA in Patients with Pulmonary Arterial Hypertension |
 |
Figure 2 Nomogram constructed to predict obstructive sleep apnea (OSA). Abbreviations: BMI, body mass index; HbAlc, glycated hemoglobin; IL-6, Interleukin-6; UA, uric acid. |
Validation of the Nomogram
A 1000-bootstrap analysis was used to validate the nomogram. The C-index and corrected C-index of the nomogram were 0.760 and 0.738, respectively, exceeding 0.7 in both cases, indicating a satisfactory performance. Besides, observations and predictions of OSA correlated well with the calibration plots (Figure 3). Based on ROC analysis (Table 4), when the cut-off value of AHI was set as 5 events/h, the AUC for the nomogram was 0.760. In contrast to ESS (AUC = 0.528), our nomogram showed a better predictive value (P < 0.001; Figure 4). The number of patients with moderate-severe OSA was too small to support a model with six predictors. Thus, the prediction value for moderate-severe OSA of this model was not validated in our study.
 |
Table 4 The Clinical Efficiency of the Nomogram and ESS for Detecting OSA |
 |
Figure 3 Calibration curves for the nomogram. Abbreviation: OSA, obstructive sleep apnea. |
Clinical Application
We assessed the clinical relevance using DCA (Figure 5). The results of the DCA on the visual basis confirmed that the nomogram had superior overall net benefits within a wide, practical threshold probability range.
Discussion
In this study, a nomogram for predicting OSA in PAH patients was developed and validated. This nomogram, incorporating six factors (age, BMI, hypertension, HbA1c, UA, and IL-6), demonstrated superior discrimination capability and predictive value to traditional screening questionnaire (ESS). To our knowledge, this is the first study to develop a predictive model of OSA in PAH. Our findings will facilitate OSA risk assessment for patients with PAH, and therefore assist clinicians in referring patients for further diagnostic confirmation.
While the prevalence of OSA is still uncertain in PAH patients, there is increasing evidence that PAH patients are at a greater risk of developing OSA than the general population of similar age. Badesch15 analyzed 2438 patients with PAH from REVEAL registry and found that OSA was present in 21% of patients. Dumitrascu16 performed overnight PG in 169 patients with precapillary pulmonary hypertension and demonstrated a 16% prevalence of OSA (defined as an AHI ≥10/h). Consistent with previous studies, we observed a 26.7% prevalence of OSA in 258 PAH patients. This phenomenon could be explained by several OSA predisposing factors that existed in patients with PAH. First, the rostral shift of fluid from the legs to the neck at night could contribute to the upper airway obstruction.17,18 Second, the skeletal and respiratory muscle weakness observed in PAH may also affect the upper airway.19 Third, the tolerance of ventilatory perturbations during sleep is likely to be impaired in patients with PAH.2 The presence and severity of OSA can contribute to the progression of PAH through deterioration in pulmonary hemodynamics and cardiac function. Therefore, the early identification of OSA is an important part of determining the optimal treatment regimen for PAH.
In our study, the lasso regression procedure is performed to analyze a wide variety of clinical variables and to avoid overfitting. Out of 17 potential variables with statistical significance and clinical importance, six variables were finally selected to construct a predictive nomogram. Among these parameters, age and obesity are two well-known risk factors for OSA. According to the reports from European PAH registries, the median age of newly diagnosed patients has been rising in these years;20,21 hence, the greater burden of comorbidities including sleep-disordered breathing should be noted. The prevalence of obesity in PAH is about 30%, and active weight management is crucial to improve the functional status as well as sleep disorders.22 Furthermore, our nomogram suggested the presence of systemic hypertension is one of the most significant predictors of OSA in PAH patients. PAH patients with risk factors for left-heart disease including hypertension, also known as atypical PAH, are considered to respond differently to PAH targeted therapies with attenuated treatment tolerance and efficacy.23 Considering the close relationship between OSA and cardiovascular diseases, one may speculate that OSA plays a role in the increased comorbid burden of PAH. On the other hand, the presence of hypertension also indicates an increased likelihood of OSA.1 Thus, it is reasonable to include age, BMI, and hypertension in our prediction model of OSA.
In patients with OSA, biochemical parameters can be altered as a result of the metabolic and endocrine dysfunction caused by nocturnal hypoxemia, inflammatory conditions, abnormal sleep patterns, and psychological stress. Our nomogram incorporated the results of routine laboratory tests, including HbA1c, UA, and IL-6, which also showed predictive value in previous studies for evaluating the risk of OSA.24 Xu25 established a nomogram encompassing glucose and insulin levels as a screening tool in the general population. They found that the presence of glucose metabolic disorder is a valuable predictor of OSA. Similarly, in our study, HbA1c, a diagnostic marker for diabetes and a golden standard for glucose management, was screened out to develop the nomogram. Furthermore, Shi8 constructed a prediction model for 280 patients with type 2 diabetes mellitus. Besides disordered glucose metabolism described by homeostasis model assessment 2 insulin resistance index, this model suggested that UA was also predictive of OSA. An association between high-level UA and the severity of PAH has been demonstrated previously, and UA was also independently associated with a higher risk of OSA.26 As well, previous research has found the levels of IL-6, one of the most important inflammatory markers, were significantly elevated in patients with OSA.27 Meanwhile, IL-6 has been implicated in the pathogenesis of PAH and can constitute a predictor of a worsening prognosis.28 Overall, these easily applicable parameters are not only useful in detecting the presence of OSA but also should be closely monitored in the management of PAH.
Among the existing screening tools for OSA, studies have found that the Berlin Questionnaire, STOP-Bang, STOP, and ESS, though having been validated in the general populations, were of limited sensitivity and specificity in disease-specific populations.29 Minic4 evaluated daytime sleepiness in 52 PAH patients and demonstrated an extremely poor sensitivity (19.4%) of ESS when predicting OSA (AHI ≥5 events/h). Similarly, only 3.1% of PAH patients reported excessive daytime sleepiness in our study, which could be explained by the overactivity of the sympathetic nervous system observed in PAH.30 Excessive sleepiness is not a sensitivity marker for OSA in our study, but that significant uncertainty still exists about its specificity. In addition, bias is known to be inevitable in questionnaires with self-report measures. Therefore, it is not surprising that our nomogram, consisting of only objective parameters, exhibited better performance. In contrast to ESS (AUC = 0.528), our nomogram showed a significantly higher predictive value in patients with PAH whether the AHI cut-off values were five events per hour (AUC = 0.760) (Table 4). In terms of clinical feasibility, the parameters included in our nomogram are all routinely collected in clinical settings without additional personnel and expense. Besides, the intuitive nature of the nomogram allows clinicians to make fast decisions with a clear understanding of a patient’s needs. After the screening process, those patients with a high pre-test probability of OSA should be transferred to a sleep center for PG or PSG monitoring as soon as possible. For PAH patients who finally confirmed the diagnosis of OSA, continuous positive airway pressure treatment is recommended to prevent the deterioration of pulmonary hemodynamics and improve the standard of living.
Limitation
There are a few limitations that warrant mentioning in our study. First of all, the sample size of the study may limit the precision of the study. Nonetheless, PAH is a rare condition, which qualifies this study as having the largest sample size to study the prevalence and risk factors of OSA in PAH patients. In addition, we use cardiorespiratory PG in the sleep evaluation rather than diagnostic polysomnography, which could lead to an underestimation of OSA prevalence. However, there is a substantial body of research today confirming that the differences between PSG and cardiorespiratory PG are acceptable, and considering clinical feasibility and cost-effectiveness, cardiorespiratory PG is widely used as the diagnostic procedure for OSA.31 Third, validation of the test was conducted on candidates derived from the same institution, which limits the ability to generalize the results to other PAH populations. Moreover, due to the single-center design and its retrospective nature, there is a certain degree of selection bias in our study. These limitations indicate that additional efforts should be undertaken to gather prospective information from multiple centers to further demonstrate the robustness of the nomogram.
Conclusions
Our study demonstrated that OSA is relatively more prevalent in the PAH population than in the general population. ESS questionnaires were proved to be less sensitive in predicting OSA in PAH. However, the combination of clinical characteristics and OSA-associated biochemical parameters provides a new approach to identifying OSA in PAH patients. The nomogram developed and validated in this study may facilitate an early identification of OSA in PAH patients with satisfactory performance and discrimination.
Abbreviations
AASM, American Academy of Sleep Medicine; AHI, apnea–hypopnea index; AUC, area under the curve; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CSA, central sleep apnea; ESS, Epworth Sleepiness Scale; HbAlc, glycated hemoglobin; IL-6, Interleukin-6; IL-8, Interleukin-8; LRN, negative likelihood ratio; LRP, positive likelihood ratio; NPV, negative predictive value; NT-proBNP, N-terminal pro-B-type natriuretic peptide; OSA, obstructive sleep apnea; PAH, pulmonary arterial hypertension; PG, polygraphy; PH, pulmonary hypertension; PPV, positive predictive value; PSG, polysomnography; RHC, right-heart catheterization; ROC, receiver operating characteristic; TC, total cholesterol; TNF-α, tumor necrosis factor-α; UA, uric acid.
Data Sharing Statement
The data will be shared on reasonable request to the corresponding author.
Ethics Approval and Informed Consent
This study was performed in line with the principles of the Declaration of Helsinki, and approval was granted by the Fuwai Hospital Ethics Committee. Informed consent was obtained from all patients included in the study.
Consent for Publication
The participant has consented to the submission of this article to the journal.
Acknowledgments
Meixi Hu, Anqi Duan and Zhihua Huang share the first authorship. The authors would like to express their gratitude to all the participants for their cooperation and acknowledge the technical support (Embletta system) provided by Fuwai hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Funding
This work was supported by National Natural Science Foundation of China (81370326, 81641005); Beijing Municipal Science and Technology Project [Z181100001718200]; Beijing Municipal Natural Science Foundation [7202168]; CAMS Innovation Fund for Medical Sciences (CIFMS) [2020-I2M-C&T-B-055, 2021-I2M-C&T-B-032]; “Double First-Class” Discipline Construction Fund of Peking Union Medical College and Chinese Academy of Medical Sciences [2019E-XK04-02]; the Capital’s Funds for Health Improvement and Research (CFH) [2020-2-4033, 2020-4-4035]; the National High Level Hospital Clinical Research Funding (2022-GSP-GG-35).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Yeghiazarians Y, Jneid H, Tietjens JR, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;144(3):e56–e67. doi:10.1161/CIR.0000000000000988
2. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi:10.1016/S2213-2600(19)30198-5
3. Sharma S, Stansbury R, Hackett B, Fox H. Sleep apnea and pulmonary hypertension: a riddle waiting to be solved. Pharmacol Ther. 2021;227:107935. doi:10.1016/j.pharmthera.2021.107935
4. Minic M, Granton JT, Ryan CM. Sleep disordered breathing in group 1 pulmonary arterial hypertension. J Clin Sleep Med. 2014;10(3):277–283. doi:10.5664/jcsm.3528
5. Veugen C, Teunissen EM, den Otter L, Kos MP, Stokroos RJ, Copper MP. Prediction of obstructive sleep apnea: comparative performance of three screening instruments on the apnea-hypopnea index and the oxygen desaturation index. Sleep Breath. 2021;25(3):1267–1275. doi:10.1007/s11325-020-02219-6
6. Watson NF. Health care savings: the economic value of diagnostic and therapeutic care for obstructive sleep apnea. J Clin Sleep Med. 2016;12(8):1075–1077. doi:10.5664/jcsm.6034
7. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi:10.5664/jcsm.6506
8. Shi H, Xiang S, Huang X, Wang L, Hua F, Jiang X. Development and validation of a nomogram for predicting the risk of obstructive sleep apnea in patients with type 2 diabetes. Ann Transl Med. 2020;8(24):1675. doi:10.21037/atm-20-6890
9. Šiarnik P, Jurík M, Klobučníková K, et al. Sleep apnea prediction in acute ischemic stroke (SLAPS score): a derivation study. Sleep Med. 2021;77:23–28. doi:10.1016/j.sleep.2020.11.022
10. Chen W, Feng J, Wang Y, Wang C, Dong Z. Development and validation of a nomogram for predicting obstructive sleep apnea in bariatric surgery candidates. Nat Sci Sleep. 2021;13:1013–1023. doi:10.2147/NSS.S316674
11. Bourjeily G, Chambers A, Salameh M, et al. Anthropometric measures and prediction of maternal sleep-disordered breathing. J Clin Sleep Med. 2019;15(6):849–856. doi:10.5664/jcsm.7834
12. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi:10.1183/13993003.01913-2018
13. Yan L, Huang Z, Zhao Z, et al. The prognostic impact of serum uric acid on disease severity and 5-year mortality in patients with idiopathic pulmonary artery hypertension. Front Med. 2022;9:805415. doi:10.3389/fmed.2022.805415
14. Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8(1):53. doi:10.1186/1472-6947-8-53
15. Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–387. doi:10.1378/chest.09-1140
16. Dumitrascu R, Tiede H, Eckermann J, et al. Sleep apnea in precapillary pulmonary hypertension. Sleep Med. 2013;14(3):247–251. doi:10.1016/j.sleep.2012.11.013
17. Jutant EM, Montani D, Sattler C, et al. Hypoxemia during sleep and overnight rostral fluid shift in pulmonary arterial hypertension: a pilot study. Pulm Circ. 2021;11(2):764550782. doi:10.1177/2045894021996930
18. Carvalho CG, Yadollahi A, Granton J, Ryan CM. Temporal shifts in fluid in pulmonary hypertension with and without sleep apnea. J Sleep Res. 2019;28(6):e12863. doi:10.1111/jsr.12863
19. Jilwan FN, Escourrou P, Garcia G, Jaïs X, Humbert M, Roisman G. High occurrence of hypoxemic sleep respiratory disorders in precapillary pulmonary hypertension and mechanisms. Chest. 2013;143(1):47–55. doi:10.1378/chest.11-3124
20. Hoeper MM, Huscher D, Pittrow D. Incidence and prevalence of pulmonary arterial hypertension in Germany. Int J Cardiol. 2016;203:612–613. doi:10.1016/j.ijcard.2015.11.001
21. Mueller-Mottet S, Stricker H, Domenighetti G, et al. Long-term data from the Swiss pulmonary hypertension registry. Respiration. 2015;89(2):127–140. doi:10.1159/000370125
22. Weatherald J, Huertas A, Boucly A, et al. Association between BMI and obesity with survival in pulmonary arterial hypertension. Chest. 2018;154(4):872–881. doi:10.1016/j.chest.2018.05.006
23. Opitz CF, Hoeper MM, Gibbs JS, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol. 2016;68(4):368–378. doi:10.1016/j.jacc.2016.05.047
24. Fleming WE, Holty JC, Bogan RK, et al. Use of blood biomarkers to screen for obstructive sleep apnea. Nat Sci Sleep. 2018;10:159–167. doi:10.2147/NSS.S164488
25. Xu H, Zhao X, Shi Y, et al. Development and validation of a simple-to-use clinical nomogram for predicting obstructive sleep apnea. Bmc Pulm Med. 2019;19(1):18. doi:10.1186/s12890-019-0782-1
26. Bouloukaki I, Mermigkis C, Tzanakis N, et al. Evaluation of inflammatory markers in a large sample of obstructive sleep apnea patients without comorbidities. Mediators Inflamm. 2017;2017:4573756. doi:10.1155/2017/4573756
27. Imani MM, Sadeghi M, Khazaie H, Emami M, Sadeghi Bahmani D, Brand S. Evaluation of serum and plasma interleukin-6 levels in obstructive sleep apnea syndrome: a meta-analysis and meta-regression. Front Immunol. 2020;11:1343. doi:10.3389/fimmu.2020.01343
28. Toshner M, Rothman AMK. IL-6 in pulmonary hypertension: why novel is not always best. Eur Respir J. 2020;55(4):2000314. doi:10.1183/13993003.00314-2020
29. Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi:10.1016/j.smrv.2016.10.004
30. Ciarka A, Doan V, Velez-Roa S, Naeije R, van de Borne P. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;181(11):1269–1275. doi:10.1164/rccm.200912-1856OC
31. Chiner E, Cánovas C, Molina V, et al. Home respiratory polygraphy is useful in the diagnosis of childhood obstructive sleep apnea syndrome. J Clin Med. 2020;9(7):2067. doi:10.3390/jcm9072067
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



