Back to Journals » International Journal of General Medicine » Volume 17
Development and Validation of a Multivariable Predictive Model for the Risk of Histologic Chorioamnionitis in Patients with Premature Rupture of Membranes in the Late Preterm and Term
Received 26 October 2023
Accepted for publication 10 January 2024
Published 16 January 2024 Volume 2024:17 Pages 141—152
DOI https://doi.org/10.2147/IJGM.S445374
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Woon-Man Kung
Xinshui Wang,1 Zheren Huang,2 Yan Ma2
1Department of Endocrinology, The Third Affiliated Hospital of Soochow University, Changzhou, 213003, People’s Republic of China; 2Department of Obstetrics and Gynecology, The Third Affiliated Hospital of Soochow University, Changzhou, 213003, People’s Republic of China
Correspondence: Yan Ma, Department of Obstetrics and Gynecology, The Third Affiliated Hospital of Soochow University, Changzhou, 213003, People’s Republic of China, Tel +86-0-13813693377, Email [email protected]
Background: This study aimed to develop and validate a model to predict histologic chorioamnionitis (HCA) risk in late preterm and term premature rupture of membranes (PROM) patients using clinical and laboratory parameters.
Methods: We conducted a retrospective study on 116 late preterm and term PROM cases, divided into a training (n=81) and a validation set (n=35). A multivariable logistic regression model was developed using the training set. Performance was assessed via the area under the receiver operating characteristic curve (AUC) and net reclassification index (NRI). Decision curve analysis (DCA) evaluated the model’s clinical utility. Additionally, nomograms and a web version of the model were developed.
Results: In the training set, the combined model constructed using maternal BMI, gravidity, amniotic fluid characteristics, and prenatal white blood cell (WBC) count showed significantly higher AUC than WBC alone (0.859 vs 0.710, P=0.010), with improved accuracy and sensitivity. In the validation set, the AUC of the combined model remained higher than that of WBC, but the difference was not statistically significant (0.728 vs 0.584, P=0.173). NRI analysis indicated that the combined model improved the correct classification of HCA by 25.0% (P=0.012) compared to that of WBC alone. DCA demonstrated that the combined model had a higher net benefit than WBC in most cases. The nomograms and web version of the model provided convenient tools for clinicians to predict the risk of HCA.
Conclusion: This study successfully developed and validated a clinically feasible multivariable model to predict the risk of HCA in women with late preterm and term PROM.
Keywords: histologic chorioamnionitis, laboratory indicators, late pregnancy, predictive model, premature rupture of membranes
Introduction
Premature rupture of membranes (PROM) refers to the spontaneous rupture of membranes before the onset of labor and is a significant obstetric complication worldwide. Depending on the gestational age at which it occurs, PROM can be classified as term PROM or preterm premature rupture of membranes (PPROM).1 PPROM leads to 25–30% of preterm births and is closely associated with chorioamnionitis (CA).2,3 CA is an inflammatory condition that can occur at any stage of pregnancy, and infection can easily cause membrane rupture, while PROM can further exacerbate the infection.4 CA can be classified as clinical chorioamnionitis (CCA) and histologic chorioamnionitis (HCA). HCA typically lacks obvious early symptoms in clinical practice and is primarily diagnosed through placental pathology examination.5 However, it poses significant risks to pregnant women and fetuses, potentially resulting in uterine inertia, postpartum hemorrhage, preterm birth, neonatal sepsis, chronic lung disease in neonates, and brain injury.6–8 Therefore, there is an urgent need for early and accurate antenatal methods to identify whether pregnant women with PROM have concomitant HCA.
Numerous studies have investigated inflammatory proteins in amniotic fluid obtained through amniocentesis as potentially useful biomarkers for identifying pregnant women with PROM at high risk of HCA.7,9,10 However, amniocentesis is an invasive procedure associated with risks and potential complications, which limits its clinical application.11 Previous research has also suggested that non-invasive and convenient inflammatory markers in maternal blood, such as C-reactive protein (CRP), white blood cell (WBC) count, procalcitonin (PCT), and interleukin-6 (IL-6), may serve as useful indicators for predicting the presence of HCA in women with PROM. However, the practicality of these maternal serum inflammatory markers remains controversial.12,13 CRP lacks specificity for infection and is related to physiological changes in pregnancy.14 Pregnancy can cause physiological elevation of WBC count, which is also affected by steroid administration, resulting in a limited value of WBC.15 PCT levels may not show significant changes in the presence of local infection,16 and IL-6 measurement is time-consuming and requires specific assay kits.17 Literature reports have also indicated that factors such as gestational age at membrane rupture, parity, and amniotic fluid index are closely associated with HCA.18 However, these factors are not sufficiently sensitive or specific.19 Zhang et al20 proposed that a multivariable prediction model can improve diagnostic performance. Therefore, it is essential to integrate important parameters into an accurate and feasible clinical approach to identify PROM women with HCA.
Existing models for predicting HCA mostly focus on PPROM women (gestational age <37 weeks). There are few studies on women with late PROM. However, rupture of membranes in pregnant women at or after 34 weeks is the leading cause of early-onset neonatal infection and death in newborns.21 Many obstetricians believe that active management is the best strategy to prevent chorioamnionitis and early-onset neonatal infection, favoring infectious outcomes,22 but increasing the risk of early delivery.23 Therefore, it is still controversial whether pregnant women with late PROM should choose active management or expectant management.
Our study contributes to the existing body of research by developing an innovative multivariable model for predicting HCA in women with late preterm and term PROM. This model uniquely combines maternal clinical characteristics with laboratory indicators, offering a more accurate and comprehensive tool for early diagnosis and management of HCA in late preterm and term PROM cases. This approach aims to enhance clinical decision-making and improve pregnancy outcomes, addressing a critical gap in current obstetric diagnostic practices.
Methods
Clinical Information
We conducted a retrospective analysis of 198 pregnant women with PROM admitted to the Department of Obstetrics at the Third Affiliated Hospital of Soochow University from March 2018 to August 2021. The inclusion criteria were as follows: 1) pregnant women with PROM at ≥34 weeks of pregnancy, 2) singleton pregnancies, 3) viable fetuses, 4) availability of placental pathology diagnosis, and 5) absence of severe maternal diseases (such as preeclampsia, intrahepatic cholestasis of pregnancy, autoimmune diseases, placental abruption, cardiovascular diseases, kidney disease, cancer, and infectious diseases). The exclusion criteria were: 1) patients without placental pathology diagnosis, 2) pregnant woman with bloody amniotic fluid. General information about the enrolled patients, including age, gender, weight, gravidity, parity, gestational age, amniotic fluid volume and characteristics during delivery, was recorded. This study adhered to the principles of the Helsinki Declaration and was approved by our institutional ethics committee [(ethics number: (2023) KD 091)]. Since the data were anonymized to protect the privacy and confidentiality of the participants, informed consent was not required. This waiver of informed consent was approved by our institutional ethics committee. The study flowchart is shown in Figure 1, and a total of 116 women with late preterm and term PROM were finally included, divided into a training set (n=81) and a validation set (n=35) with a 7:3 stratified random sampling ratio. The absence of significant differences in baseline characteristics between included (n=116) and excluded (n=82) participants (see Table S1) suggested that the results were unlikely to be affected by selection bias.
 |
Figure 1 Flowchart of patient enrollment. |
Diagnosis
According to the standards of “Guidelines for the Diagnosis and Management of Premature Rupture of Membranes (2015)”,24 the diagnosis of PROM in pregnant women could be performed based on the patient’s medical history and physical examination. The diagnostic criteria were as follows: (1) preterm pregnant women complained of vaginal fluid leakage or external genitalia moisture; (2) visual examination with a speculum revealed fluid flowing out of the cervical os or the presence of a fluid pool in the posterior fornix; (3) ultrasound examination showed a decreased amount of amniotic fluid before the rupture of membranes; (4) the color change of the pH test paper was observed, with a normal vaginal pH ranging between 4.4 and 6.0, while the pH of amniotic fluid was 8.0;25 and (5) insulin-like growth factor testing yielded positive results. No antibiotics were used in the case before admission. After PROM was confirmed, the patients were placed in a hip-high lying position and absolutely bed rest were performed. Body temperature, heart rate, pulse, fetal heart rate, vaginal secretion characteristics, and uterine tenderness were monitored. Term PROM that has not been delivered for more than 12 hours should be managed with antibiotics. If PPROM complicated by group B Streptococcus (GBS) infection occurs, antibiotics will be given immediately upon admission, usually penicillin or cephalosporins. The subjects of our study were PROM women at ≥34 weeks of pregnancy. We recommend that all pregnant women terminate their pregnancies. If continued expectant treatment is required, the balance between benefits and risks should be carefully considered and discussed with the patient. Expectant treatment should not exceed 37 weeks. If fetal distress and CCA were found, the pregnancy should be terminated immediately, and the specific delivery method was determined by the obstetric situation.24
The Redline’s diagnostic criteria for HCA6 included pathological examination of placental and fetal membrane sections, with ≥ five neutrophil infiltrations in each high-power field considered the gold standard.
Laboratory Test
Blood samples were collected from the patient on the day of admission for analysis. Blood routine tests were performed using the Sysmex XN9000 hematology analyzer (Hyogo, Japan), which included WBC (3.5–9.5×109/L), neutrophil ratio (40.00–75.00%), hemoglobin (HGB, 115–150g/L), platelet count (PLT, 125–350×109/L). Blood glucose (GLU, 3.9–6.1mmol/L), lipid levels [total cholesterol (TC, <5.20mmol/L), triacylglycerol (TG, <1.70mmol/L)], and CRP (0–10mg/L) were measured using the Beckman Coulter AU5800 analyzer (Brea, CA, USA). PCT (0–0.05ng/mL) levels were determined using a Roche cobas 8000 analyzer (Indianapolis, IN, USA). All women were subjected to rectovaginal testing for GBS, as well as regular fetal heart rate electronic monitoring and review via B-ultrasound. Amniotic fluid is a clear and colorless liquid that can become slightly cloudy and less transparent during full-term pregnancy. The presence of meconium in the amniotic fluid during pregnancy causes discoloration, resulting in the development of meconium-stained amniotic fluid (MSAF). According to the grading system for meconium, MSAF can be classified into 3 grades: grade I (semi-transparent, light green or yellow flesh), grade II (milky white, dark green and light yellow, brown), and grade III (opaque and dark green). After membrane rupture, record the amniotic fluid characteristics (clear, grade I, grade II, and grade III). Postpartum records included measurements of amniotic fluid volume, mode of delivery, gender of the newborn, newborn weight, and Apgar scores at 1 minute and 5 minutes.
After delivery, the placenta were sent to the pathology department of our hospital for HCA diagnosis. The sampling and examination methods of pathological specimens were as follows. The tissue sample of 3 cm × 3 cm was taken from the placental and fetal membrane tissues around the rupture site, fixed in 10% formaldehyde, and embedded in paraffin as per standard protocol. All slides are reviewed by pathologists, who do not obtain clinical information about the specimens. Each slide was diagnosed by two experienced pathologists, and in case of disagreement, the final diagnosis was made by a third senior pathologist.
Statistical Analysis
Statistical analysis of the data was performed using R software (version 3.4.3; http://www.R-project.org/). Continuous variables were presented as mean ± standard deviation (normally distributed) or median (minimum-maximum) for skewed distributions. Categorical variables were presented as frequencies or percentages (%). Differences in general characteristics and laboratory parameters between the HCA and non-HCA groups were assessed using the chi-square test (for categorical variables), t-test (for normally distributed variables), or Mann–Whitney U-test (for skewed distributions).
In the training set, a multivariable logistic regression model for predicting PROM combined with HCA was constructed using stepwise regression and a Bootstrap-based approach. The initial independent variables considered in the model included a series of clinical and laboratory parameters with a significance level of P≤0.1. The listed independent variables were screened for collinearity (by calculating the variance inflation factor VIF), and variables with VIF>10 were eliminated. During the model-building process, 1000 rounds of Bootstrap resampling were used to select the most influential predictive variables. The receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC) with its 95% confidence interval (CI) was calculated. Pairwise comparisons of the AUC were performed using the DeLong test.26 The net reclassification index (NRI) was calculated to evaluate the model’s improvement in classification ability. The Hosmer-Lemeshow test and calibration curves were used to assess the consistency between the predicted probabilities of the model and the observed outcomes. Decision curve analysis (DCA) was performed to evaluate and compare the clinical utility of the predictive models. To facilitate clinical application, nomograms and a web version were developed for the model. The web version could directly output the risk of HCA by inputting the relevant variables associated with the model. All statistical tests were two-tailed, and a P<0.05 was considered statistically significant. All variables included in the analysis have been collected completely without missing values. Therefore, no further treatment for missing values was performed. Subsequent statistical analyses were conducted based on this complete dataset.
Results
Among the included, 116 pregnant women with late PROM showed no fever symptoms. The general clinical characteristics, laboratory test results, and delivery outcomes were comparable between the training set (n=81) and the validation set (n=35) (all P>0.05) (Table S2). The only significant difference observed was a higher WBC count in newborns from the training set compared to the validation set (P=0.016).
Comparison of General Data and Laboratory Indicators Between the HCA Group and Non-HCA Group
Table 1 presents the comparison of general characteristics and laboratory parameters between the training set and validation set. In the training set, out of 81 cases, 35 (43.2%) were found to have HCA. There were no statistically significant differences between HCA and non-HCA groups in terms of age, BMI, gestational diabetes, hypertension, gestational weeks, time of rupture of membranes, and several laboratory parameters (prenatal PCT, HGB, PLT, and TG) (all P>0.05). However, lower parity and gravidity were observed in the HCA group compared to the non-HCA group (P<0.05 for both). Significant differences were found in amniotic fluid volume and characteristics between the two groups (P<0.05), with the HCA group having a higher amniotic fluid volume and a higher proportion of grade I–II and grade III amniotic fluid characteristics. Group B Streptococcus (GBS) positivity rate was higher in the HCA group than in the non-HCA group (22.9% vs 6.5%, P=0.034). Regarding laboratory parameters, the HCA group showed substantially higher levels of prenatal WBC count, prenatal neutrophils, prenatal CRP, and GLU levels (P<0.05), while TC was lower (P=0.040).
 |
Table 1 Comparison of General Information and Laboratory Indicators Between Training and Validation Sets in Late Preterm and Term PROM |
In the validation set, most of the clinical characteristics and the aforementioned laboratory parameters did not show statistically significant differences between the two groups (P>0.05), except for amniotic fluid characteristics (P=0.010), with the HCA group having a higher proportion of grade I–II and III amniotic fluid characteristics.
Additionally, the delivery outcomes of pregnant women in the training and validation sets are presented in Table S3. There were no significant differences between the HCA and non-HCA groups in the training set regarding fetal weight, fetal gender, and Apgar scores at 1 minute and 5 minutes (P>0.05). However, the HCA group had a significantly higher rate of cesarean delivery (45.7% vs 15.2%, P=0.003), and newborns in the HCA group had higher WBC count, neutrophil ratio, and CRP levels (P<0.05). In the validation set, there were no significant differences in the aforementioned parameters (P>0.05).
Construction of a Multivariable Predictive Model
In the training set, a multivariable logistic regression analysis was performed with the presence or absence of HCA as the dependent variable. The following general clinical characteristics and laboratory parameters were included as initial independent variables (with a relaxed significance level of P=0.1): maternal age at delivery, BMI, gestational diabetes, hypertension, parity, gravidity, gestational weeks, time of rupture of membranes, amniotic fluid volume, amniotic fluid characteristics, GBS, prenatal WBC, prenatal neutrophil ratio, prenatal CRP, prenatal PCT, HGB, PLT, GLU, TC, and TG. The resulting combined predictive model is as follows:
Logit (P) = 4.83855–0.26394 × BMI – 1.02209 × gravidity + 2.28747 × amniotic fluid characteristics (0=clear, 1=I–II, 2=III) + 0.35048 × prenatal WBC, where P represents the probability of HCA. The statistical results of the final variables of the model are shown in Table 2. The post-hoc power analysis results for the multivariable logistic regression model using computer simulation for efficacy assessment can be found in Table S4.
 |
Table 2 Bootstrap Statistical Analysis of Combined Model Variables |
We chose to compare the WBC with the combined model because, based on our analysis, WBC serves as an independent inflammatory marker and is widely used in clinical practice. In the training and validation sets, ROC curves were plotted for the combined model and WBC (Figure 2A and B), and the comparison of diagnostic performance indicators is shown in Table 3. Delong’s test revealed that the combined model had a significantly higher AUC in the training set compared to WBC (0.859 vs 0.710, P=0.010), particularly demonstrating higher accuracy and sensitivity. In the validation set, both the combined model and WBC showed a decrease in AUC (indicating some degree of overfitting), but the AUC of the combined model remained higher than the WBC, although the difference was not statistically significant due to the small sample size (0.728 vs 0.584, P=0.173). Nevertheless, the combined model still exhibited higher accuracy and sensitivity.
 |
Table 3 Comparison of Diagnostic Performance Between WBC and Combined Model |
Since the difference in AUC between the combined model and WBC in the validation set was not statistically significant, we performed the NRI and found that the combined model correctly reclassified 25.0% (95% CI: 2.4–52.4%, P=0.012) of cases for the differentiation of HCA, while there was no significant difference in the differentiation of non-HCA cases (P=0.406). This finding indicated that the combined model was more effective in identifying true positive cases of HCA in late PROM. The Hosmer-Lemeshow test further confirmed that the model demonstrated good fit in both the training and validation sets, with chi-square values of 10.241 (P=0.249) and 11.083 (P=0.135), respectively. The calibration curves of the combined model are shown in Figure 2C and D, demonstrating better calibration in the training set compared to the validation set.
In the training set and validation set, further comparison of the combined model and WBC was conducted using DCA (Figure 3A and B). In most scenarios, the net benefit of the combined model was higher than that of the single marker WBC.
Model Display
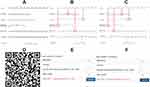
The nomograms for the combined model (Figure 4A) and the web version of the model (Figure 4D) were created. To access the web version, please scan the QR code below the figure The risk of HCA could be displayed by inputting the corresponding values of the four markers into the web version. The web-based model will not collect inputted patient information and is solely used for one-time calculation. To demonstrate its application, we selected two cases.
Case A: The patient was 26 years old (40+6), BMI = 30.1 kg/m2, gravidity = 1, clear amniotic fluid (0), and WBC = 16.7×109/L. The corresponding scores were 27.5, 67.5, 0, and 45.0, resulting in a total score of 140.0. According to the nomograms (Figure 4B), the estimated risk of HCA was approximately 0.8. The web version provided a result of 0.849 (Figure 4E). The postpartum pathology confirmed HCA.
Case B: The patient was 23 years old (37+6), BMI = 26.7 kg/m2, gravidity = 2, clear amniotic fluid (0), and WBC = 8.77×109/L. The corresponding scores were 37, 55.0, 0, and 17.0, resulting in a total score of 109.0. According to the nomograms (Figure 4C), the estimated risk of HCA was approximately 0.28. The web version provided a result of 0.235 (Figure 4F). The postpartum pathology confirmed non-HCA.
Discussion
We developed a multivariable combined model based on maternal BMI, gravidity, amniotic fluid characteristics, and prenatal WBC to predict the risk of HCA in pregnant women with late preterm and term PROM. The model was further validated, and the research findings indicated that the combined model exhibited higher accuracy and sensitivity than WBC alone, enabling more effective identification of true positive cases.
PROM has been regarded as a severe perinatal complication for many years, with approximately 50% to 60% of PPROM cases associated with HCA.3,27 HCA is believed to result from prenatal inflammation or infection and is a major cause of preterm birth.28 Preterm birth accounts for over 70% of perinatal mortality in developed countries.29 HCA is associated with various neonatal complications, including respiratory distress syndrome, bronchopulmonary dysplasia, and intraventricular hemorrhage.30–32 HCA often presents without symptoms, and clinical manifestations lack specificity and sensitivity. Therefore, early identification of HCA in PROM pregnancies is crucial, as it holds significant clinical value in preventing maternal and neonatal complications.
During late pregnancy, pregnant women experience physiological and metabolic changes, and their bodies require increased nutrient intake to meet the fetus’s needs, including proteins, carbohydrates, fats, and other essential nutrients.33,34 Adequate weight gain during pregnancy is necessary to ensure the fetus’s health. Insufficient maternal intake can lead to malnutrition, and lower weight gain is associated with an increased risk of adverse maternal and infant outcomes.35,36 Rudra et al37 have identified a lower BMI in pregnant women as a risk factor for PROM and an increased risk of HCA. Consistent with these findings, our study also showed a lower BMI in the HCA group, and it acted as a protective factor in the combined model, indicating that appropriate weight gain before delivery was associated with a lower incidence of HCA.
Amniotic fluid contamination with meconium and bacterial, viral, or fungal infections are risk factors for HCA.38 However, only 4–25% of HCA cases exhibit purulent or foul-smelling amniotic fluid or cervical secretions.39 Consistent with these findings, our results also showed that 20.0% of pregnant women with HCA had turbid amniotic fluid. Therefore, relying solely on the external characteristics of amniotic fluid cannot accurately determine whether a pregnant woman has HCA. Research also has indicated a strong association between aseptic intra-amniotic inflammation, unrelated to microorganisms, and HCA.40 Roberts et al41 have also suggested that HCA can occur without obvious intra-amniotic infection. Furthermore, our findings revealed that pregnant women in the HCA group had relatively lower gravidity. It has been reported that primiparity is an important risk factor for HCA,42,43 which may be related to the long interval from rupture of membranes to delivery and immature cervical conditions at membranes rupture. The longer the time of membrane rupture, the greater the risk of infection.44 It may also be attributed to the personal experiences and health education obtained by multiparous women during previous pregnancies and prenatal examinations. Pregnant women must be well-informed about their health status, learn to recognize warning signs and take early measures to prevent pregnancy complications.
WBC count in the blood is one of the most commonly used methods to assess systemic inflammatory response and its intensity. However, maternal WBC count varies widely during pregnancy, limiting its value.15,45 Nevertheless, much research has evaluated the association between maternal blood WBC count and HCA.46–48 Musilova et al46 have concluded that maternal WBC count cannot be used as a non-invasive screening tool to identify PROM pregnancies complicated by HCA. Asadi et al16 also found low WBC count diagnostic accuracy. Some studies have indicated an independent association between elevated WBC count and HCA, but its specificity and sensitivity, when used alone, are not high.48 Studies show that WBC of PPROM women is not an accurate predictor of chorioamnionitis, but CRP levels are more reliable and can be used for diagnosis.49 Xie et al50 proposed that prenatal CRP levels higher than 8 mg/L mean a significant increase in the HCA risk, which can lead to adverse neonatal outcomes. However, some studies have indicated that using CRP as a predictor for PROM combined with chorioamnionitis is still lacking sufficient support.12,13
The non-invasive prediction model constructed by Galletta et al,51 based on abdominal pain, uterine activity, fever, incubation period >3 days, and CRP, showed good diagnostic performance. Shi et al47 developed a nomogram model that utilizes maternal serum CRP, PCT, neutrophil-to-lymphocyte ratio, and gestational age at membrane rupture, which also showed good predictive ability. However, their models are only suitable for pregnant women with PROM between 20 and 37 weeks. Our previous research52 also found that CRP was the only blood indicator that could predict HCA, but its predictive efficiency was not ideal, whereas the complex model constructed based on hematological indicators and meaningful clinical parameters has higher diagnostic value, but it has not been verified. On the basis of increasing sample size, this study identified WBC count as the best laboratory indicator for predicting HCA. However, the diagnostic performance was significantly improved when combined with BMI, amniotic fluid characteristics, and gravidity in a composite model. Our internal validation results demonstrated the strong predictive capability of the combined model, effectively identifying true positive cases. Although there were differences in calibration curves between the training and validation sets, this might be attributed to the relatively small sample size in the validation set. Therefore, for clinical practitioners, considering multiple indicators together can provide a more accurate prediction of the risk of HCA in PROM pregnancies, enabling timely and appropriate therapeutic interventions.
This study has some limitations. It was a retrospective study, which may introduce selection bias. The sample size was relatively small, which may affect the statistical significance and generalizability of the results. This study focused on pregnant women with PROM at ≥34 weeks, including late PPROM and term PROM; no stratified research was conducted, and the results may not apply to pregnant women with PROM at <34 weeks. The study only explored certain clinical features and laboratory indicators, and did not document fetal tachycardia. Future research may need to assess additional risk factors, such as IL-6, IL-8, detection of related microorganisms in amniotic fluid, and fetal tachycardia. The model was validated using internal validation, and further validation with external data is required to evaluate its applicability.
Conclusions
Despite these limitations, this study developed and validated a clinically feasible multivariable model based on maternal BMI, gravidity, amniotic fluid characteristics, and prenatal WBC count, predicting the risk of HCA in women with late preterm and term PROM. The model demonstrated higher applicability and accuracy than WBC count. The nomograms and web version of the model provided convenient tools for clinicians to predict HCA risk. This might assist obstetricians in timely understanding the risk status of pregnant women and making personalized clinical decisions, thereby improving the health outcomes of both mothers and newborns.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval and Informed Consent
This study adhered to the principles of the Helsinki Declaration and was approved by ethics committee of the Third Affiliated Hospital of Soochow University [(ethics number: (2023) KD 091)]. Since the data were anonymized to protect the privacy and confidentiality of the participants, informed consent was not required. This waiver of informed consent was approved by our institutional ethics committee.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors of this article declare to have no conflict of interest related to this study.
References
1. Kuba K, Bernstein PS. ACOG practice bulletin No. 188: prelabor rupture of membranes. Obstetrics Gynecol. 2018;131(1):e1–e14. doi:10.1097/AOG.0000000000002455
2. Sae-Lin P, Wanitpongpan P. Incidence and risk factors of preterm premature rupture of membranes in singleton pregnancies at Siriraj Hospital. J Obstet Gynaecol Res. 2019;45(3):573–577. doi:10.1111/jog.13886
3. Menon R, Richardson LS. Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Semin Perinatol. 2017;41(7):409–419. doi:10.1053/j.semperi.2017.07.012
4. Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Clin Exp Obstet Gynecol. 2015;213(4 Suppl):5.
5. Kong X, Jiang L, Zhang B, Sun L, Liu K. Predicting chorioamnionitis in patients with preterm premature rupture of membranes using inflammatory indexes: a retrospective study. Taiwan J Obstet Gynecol. 2023;62(1):112–118. doi:10.1016/j.tjog.2022.11.006
6. Czikk MJ, McCarthy FP, Murphy KE. Chorioamnionitis: from pathogenesis to treatment. Clin Microbiol Infect. 2011;17(9):1304–1311. doi:10.1111/j.1469-0691.2011.03574.x
7. Cobo T, Kacerovsky M, Palacio M, et al. A prediction model of histological chorioamnionitis and funisitis in preterm prelabor rupture of membranes: analyses of multiple proteins in the amniotic fluid. J Matern Fetal Neonatal Med. 2012;25(10):1995–2001. doi:10.3109/14767058.2012.666592
8. Miyazaki K, Furuhashi M, Ishikawa K, et al. Impact of chorioamnionitis on short- and long-term outcomes in very low birth weight preterm infants: the neonatal research network Japan. J Matern Fetal Neonatal Med. 2016;29(2):331–337. doi:10.3109/14767058.2014.1000852
9. Park JW, Park KH, Jung EY, Terry J. Clinical significance of histologic chorioamnionitis with a negative amniotic fluid culture in patients with preterm labor and premature membrane rupture. PLoS One. 2017;12(3):e0173312. doi:10.1371/journal.pone.0173312
10. Oh KJ, Park KH, Kim SN, Jeong EH, Lee SY, Yoon HY. Predictive value of intra-amniotic and serum markers for inflammatory lesions of preterm placenta. Placenta. 2011;32(10):732–736. doi:10.1016/j.placenta.2011.07.080
11. Lee JE, Dan K, Kim HJ, Kim YM, Park KH. Plasma proteomic analysis to identify potential biomarkers of histologic chorioamnionitis in women with preterm premature rupture of membranes. PLoS One. 2022;17:7.
12. van de Laar R, van der Ham DP, Oei SG, Willekes C, Weiner CP, Mol BW. Accuracy of C-reactive protein determination in predicting chorioamnionitis and neonatal infection in pregnant women with premature rupture of membranes: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2009;147(2):124–129. doi:10.1016/j.ejogrb.2009.09.017
13. Popowski T, Goffinet F, Maillard F, Schmitz T, Leroy S, Kayem G. Maternal markers for detecting early-onset neonatal infection and chorioamnionitis in cases of premature rupture of membranes at or after 34 weeks of gestation: a two-center prospective study. BMC Preg Childbirth. 2011;11(1):26. doi:10.1186/1471-2393-11-26
14. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39(2):206–217. doi:10.1086/421997
15. Chang J, Streitman D. Physiologic adaptations to pregnancy. Neurol Clin. 2012;30(3):781–789. doi:10.1016/j.ncl.2012.05.001
16. Asadi N, Faraji A, Keshavarzi A, Akbarzadeh-Jahromi M, Yoosefi S. Predictive value of procalcitonin, C-reactive protein, and white blood cells for chorioamnionitis among women with preterm premature rupture of membranes. Int J Gynaecol Obstet. 2019;147(1):83–88. doi:10.1002/ijgo.12907
17. Cho HY, Jung I, Kwon JY, Kim SJ, Park YW, Kim YH. The delta neutrophil index as a predictive marker of histological chorioamnionitis in patients with preterm premature rupture of membranes: a retrospective study. PLoS One. 2017;12:3.
18. Park JW, Park KH, Lee JE, Kim YM, Lee SJ, Cheon DH. Antibody microarray analysis of plasma proteins for the prediction of histologic chorioamnionitis in women with preterm premature rupture of membranes. Reprod Sci. 2019;26(11):1476–1484. doi:10.1177/1933719119828043
19. Kim SA, Park KH, Lee SM. Non-invasive prediction of histologic chorioamnionitis in women with preterm premature rupture of membranes. Yonsei Med J. 2016;57(2):461–468. doi:10.3349/ymj.2016.57.2.461
20. Zhang L, Fang X, Li Z, et al. Establishment of a prediction model for histological chorioamnionitis and its association with outcomes of premature infants. Front Pediatr. 2023;11:1194563. doi:10.3389/fped.2023.1194563
21. Cohen-Wolkowiez M, Moran C, Benjamin DK, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28(12):1052–1056.
22. Neerhof MG, Cravello C, Haney EI, Silver RK. Timing of labor induction after premature rupture of membranes between 32 and 36 weeks’ gestation. Am J Obstet Gynecol. 1999;180(2 Pt 1):349–352. doi:10.1016/S0002-9378(99)70212-7
23. van Baar AL, Vermaas J, Knots E, de Kleine MJ, Soons P. Functioning at school age of moderately preterm children born at 32 to 36 weeks’ gestational age. Pediatrics. 2009;124(1):251–257. doi:10.1542/peds.2008-2315
24. Obstetrics Subgroup CSoOaG, Chinese Medical Association. Guidelines for the diagnosis and management of premature rupture of membranes (2015). Clin J Obstet Gynecol. 2015;50(1):3–8.
25. Adama van Scheltema PN, Anker PS I, Vereecken A, Vandenbussche FP, Deprest JA, Devlieger R. Biochemical composition of fluids for amnioinfusion during fetoscopy. Gynecol Obstetr Invest. 2008;66(4):227–230. doi:10.1159/000147168
26. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi:10.2307/2531595
27. Aviram A, Quaglietta P, Warshafsky C, et al. Utility of ultrasound assessment in management of pregnancies with preterm prelabor rupture of membranes. Ultrasound Obstet Gynecol. 2020;55(6):806–814.
28. Palmsten K, Nelson KK, Laurent LC, Park S, Chambers CD, Parast MM. Subclinical and clinical chorioamnionitis, fetal vasculitis, and risk for preterm birth: a cohort study. Placenta. 2018;67:54–60. doi:10.1016/j.placenta.2018.06.001
29. Stranak Z, Feyereisl J, Korcek P, Feyereislova S, Krofta L. Procalcitonin is more likely to be released by the fetus rather than placental tissue during chorioamnionitis. Biomed Pap. 2016;160(4):499–502. doi:10.5507/bp.2016.041
30. Sarno L, Della Corte L, Saccone G, et al. Histological chorioamnionitis and risk of pulmonary complications in preterm births: a systematic review and Meta-analysis. J Matern Fetal Neonatal Med. 2021;34(22):3803–3812. doi:10.1080/14767058.2019.1689945
31. Villamor-Martinez E, Alvarez-Fuente M, Ghazi AMT, et al. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: a systematic review, meta-analysis, and metaregression. JAMA Network Open. 2019;2(11):e1914611. doi:10.1001/jamanetworkopen.2019.14611
32. Villamor-Martinez E, Fumagalli M, Mohammed Rahim O, et al. Chorioamnionitis is a risk factor for intraventricular hemorrhage in preterm infants: a systematic review and meta-analysis. Front Physiol. 2018;9:1253. doi:10.3389/fphys.2018.01253
33. Mackeen AD, Young AJ, Lutcher S, et al. Encouraging appropriate gestational weight gain in high-risk gravida: a randomized controlled trial. Obes Sci Pract. 2022;8(3):261–271. doi:10.1002/osp4.565
34. Huang LT. Maternal and Early-Life Nutrition and Health. Int J Environ Res Public Health. 2020;17(21):7982. doi:10.3390/ijerph17217982
35. Voerman E, Santos S, Inskip H, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. 2019;321(17):1702–1715. doi:10.1001/jama.2019.3820
36. Gu C, Wu W, Lai K, et al. Maternal pre-pregnancy BMI, MTHFR polymorphisms, and the risk of adverse pregnancy outcomes in pregnant women from South China: a retrospective cohort study. BMC Pregnancy Childbirth. 2023;23(1):295. doi:10.1186/s12884-023-05605-6
37. Rudra CB, Frederick IO, Williams MA. Pre-pregnancy body mass index and weight gain during pregnancy in relation to preterm delivery subtypes. Acta Obstet Gynecol Scand. 2008;87(5):510–517. doi:10.1080/00016340801996838
38. Chapman E, Reveiz L, Illanes E, Bonfill Cosp X. Antibiotic regimens for management of intra-amniotic infection. Cochrane Database Syst Rev. 2014;12:CD010976.
39. Suzuki S. Association between clinical chorioamnionitis and histological funisitis at term. J Neonatal Perinatal Med. 2019;12(1):37–40. doi:10.3233/NPM-17155
40. Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43(1):19–36.
41. Roberts DJ, Celi AC, Riley LE, et al. Acute histologic chorioamnionitis at term: nearly always noninfectious. PLoS One. 2012;7(3):e31819. doi:10.1371/journal.pone.0031819
42. Alla S, Ramseyer A, Whittington JR, Peeples S, Ounpraseuth ST, Magann EF. Maternal features at time of preterm prelabor rupture of membranes and short-term neonatal outcomes. J Matern Fetal Neonatal Med. 2022;35(11):2128–2134. doi:10.1080/14767058.2020.1782376
43. Fowler JR, Simon LV. Chorioamnionitis. In: Treasure Island (FL) Ineligible Companies. Disclosure: Leslie Simon Declares No Relevant Financial Relationships with Ineligible Companies. StatPearls; 2023.
44. Rottenstreich A, Levin G, Tsur A, Shai D, Meyer R. Chorioamnionitis at latent phase more than doubles the risk for cesarean delivery compared to chorioamnionitis at active phase. Arch Gynecol Obstet. 2021;303(4):905–910. doi:10.1007/s00404-020-05815-9
45. Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37(2):339–354.
46. Musilova I, Pliskova L, Gerychova R, et al. Maternal white blood cell count cannot identify the presence of microbial invasion of the amniotic cavity or intra-amniotic inflammation in women with preterm prelabor rupture of membranes. PLoS One. 2017;12:12.
47. Shi H, Sun L, Wang Z, et al. Non-invasive prediction of histologic chorioamnionitis using maternal serum markers in women with preterm prelabour rupture of membranes. Am J Reprod Immunol. 2022;88(3):e13594. doi:10.1111/aji.13594
48. Cho I, Lee KN, Joo E, Kim YM, Kim TE, Park KH. Plasma E-selectin and kallistatin as predictive markers of histologic chorioamnionitis in women with preterm premature rupture of membranes. Am J Reprod Immunol. 2022;88:3.
49. Thornburg LL, Queenan R, Brandt-Griffith B, Pressman EK. Procalcitonin for prediction of chorioamnionitis in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2016;29(13):2056–2061. doi:10.3109/14767058.2015.1077224
50. Xie A, Zhang W, Chen M, et al. Related factors and adverse neonatal outcomes in women with preterm premature rupture of membranes complicated by histologic chorioamnionitis. Med Sci Monit. 2015;21:390–395. doi:10.12659/MSM.891203
51. Galletta MAK, Schultz R, Sartorelli M, et al. Clinical characteristics, complications, and predictive model of histological chorioamnionitis in women with preterm premature rupture of membranes. PLoS One. 2023;18:4.
52. Ma Y, Xu Y, Jiang L, Shao X. Application of a prediction model based on the laboratory index score in prelabor rupture of membranes with histologic chorioamnionitis during late pregnancy. Med Sci Monit. 2020;26:e924756. doi:10.12659/MSM.924756
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.