Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Development and Assessment of Prediction Models for the Development of COPD in a Typical Rural Area in Northwest China
Received 24 December 2020
Accepted for publication 7 February 2021
Published 26 February 2021 Volume 2021:16 Pages 477—486
DOI https://doi.org/10.2147/COPD.S297380
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Yide Wang,1 Zheng Li,1,2 Feng-sen Li1,2
1Department of Integrated Pulmonology, Fourth Affiliated Hospital of Xinjiang Medical University, Urumqi, People’s Republic of China; 2Xinjiang National Clinical Research Base of Traditional Chinese Medicine, Xinjiang Medical University, Ürümqi, People’s Republic of China
Correspondence: Zheng Li
Department of Integrated Pulmonology, Fourth Affiliated Hospital of Xinjiang Medical University, Urumqi, 830000, Xinjiang, People’s Republic of China
Tel +86-13999297797
Email [email protected]
Feng-sen Li
National Clinical Research Base of Traditional Chinese Medicine, Xinjiang Medical University, Urumqi, 830000, Xinjiang, People’s Republic of China
Tel +86-13999980996
Email [email protected]
Objective: This study aimed to construct and evaluate a clinical predictive model for the development of COPD in northwest China’s rural areas.
Methods: A cross-sectional study of a natural population was performed in rural northwest China. After assessing demographic and disease characteristics, a clinical prediction model was developed. First, we used the least absolute shrinkage and selection operator regression model to screen possible factors influencing COPD. Then construct a logistic regression model and draw a nomogram. The discriminability of the model was further evaluated by the calibration diagram, C-index and ROC curve system. Clinical benefit was analyzed using the decision curve. Finally, the 1000 bootstrap resamples and Harrell’s C-index was used for internal verification of the nomogram.
Results: Among 3249 patients in the local rural natural population, 394 (12.13%) were diagnosed with COPD. The LASSO regression model was used to find the optimal combination of parameters, and the screened influencing factors included age, gender, barbeque, smoking, passive smoking, energy type, ventilation system and Post-Bronchodilator FEV1. These predictors are used to construct a nomogram. C index is 0.81 (95% confidence interval:0.79– 0.83). The combination of the calibration curve and ROC curve indicates that the model has high discriminability. The decision curve shows benefits in clinical practice when the threshold probability is > 6% and < 58%, respectively. The internal verification results using Harrell’s C-Index were 0.80 (95% confidence interval: 0.78– 0.83).
Conclusion: Combining information such as age, sex, barbeque, smoking, passive smoking, type of energy, ventilation systems, and Post-Bronchodilator FEV1 can be easily used to predict the risk of COPD in local rural areas.
Keywords: COPD, predictive models, nomograms, China
Introduction
Chronic obstructive pulmonary disease (COPD), as chronic progressive lung disease, is an important cause of the global increase in morbidity and mortality.1 The high incidence of COPD, which is expected to rise to the third leading cause of death in the world by 2030, will impose a heavy social and economic burden.2 It is important to note that most of the world’s COPD morbidity and mortality occurs in low - and middle-income countries, and there are significant urban-rural differences—the rural situation is more severe than the urban one. There is even a growing body of research confirming that rural residence is an independent and important risk factor for COPD.3,4
Prognosis varies widely in different stages of diagnosis of COPD, and high mortality is likely associated with inadequate early diagnosis of the disease. Prevention strategies for early detection and early diagnosis are important for identifying people at increased risk of COPD or those with early disease, and for implementing early interventions in that population as much as possible, thereby reducing the disease burden.5 A clinical prediction model based on multiple risk factors plays an important role in efficiently and economically identifying high-risk groups.6–9 In addition to common risk factors include smoking, secondhand smoke exposure, sex, body mass index, age, socioeconomic status, history of asthma, and respiratory infections. Besides, biomass fuel, coal exposure, poverty, and low levels of education in rural areas are also important contributors to the high incidence and mortality of COPD.10,11 At present, Our understanding of the risk factors for the disease is still very limited.
Several clinical predictive models of COPD have been published, with problems of poor predictive ability and lack of model evaluation and validation.12 There is not even a predictive model based on rural areas of grimmer conditions. Therefore, in this study, we will develop and validate a risk prediction model for rural areas. Considering that Moyu County in northwest China is characterized by low urbanization level, prominent rural characteristics, small mobility of local personnel and high stability, this is taken as the investigation point.
Methods
Participants
To provide a scientific basis for accurate prevention of chronic diseases, China has carried out several natural population cohort studies and health follow-up studies in recent years. As one of the keys funded projects, “Natural Population Cohort Study in Northwest China” (SQ2017YFSF090013) aims to study the aetiology and disease burden of characteristic chronic diseases in Northwest China and analyze the relationship between chronic diseases and health in northwest China, including natural environment and behavioural patterns. “Study on cohort Construction and Health Follow-up of Xinjiang Multi-ethnic natural Population” (2017YFC0907203) is a sub-project of this project. Our study population is all from this project. We conducted a cross-sectional study of a natural population in rural northwestern China from June to December 2018.
Inclusion criteria:1. The age above 35 is not limited to gender; 2. Participate voluntarily and sign the informed consent;3. Able to communicate normally without obvious cognitive impairment;4. Permanent residents;5. Effective lung function tests and complete data. The criteria for COPD were: people with persistent respiratory symptoms or a history of risk factors such as smoke, combined with lung function test – post-bronchodilator FEV1/FVC ratio<0.70. Ethics Committee of Xinjiang Uygur Autonomous Region Chinese Medicine Hospital approved this study (IRB ID:2018XE0108), and all study participants signed written informed consent. Our study also complied with the Declaration of Helsinki.
Predictors and Definitions
Trained professionals performed the acquisition of data, questionnaires and lung function tests. Considering the Global Initiative for Chronic Obstructive Lung Disease (GOLD)13 and practical operability, we included candidates that include: height (a medical height meter with an accuracy of 0.1cm requires removal of shoes and caps during measurement); Bodyweight (using an electronic medical scale with an accuracy of 0.1kg, taking off the coat, shoes and hat during measurement); Waist circumference (using a soft tape measure, accurate to 0.1cm, with the lower edge of the tape measure placed at the highest point on either side of the hip bone and then around the waist horizontally for one week); Gross annual income, yuan (< 10,000, ≥10,000); Smoking is defined as daily or almost daily smoking; Passive smoking was defined as more than two times of passive tobacco per week. A ventilation system is defined as the installation of an exhaust fan or a range hood; Barbeque was defined as eating barbeque every day. Lung function was detected using MasterScreen Pneumo version2.13.
Statistical Analysis
R software (version3.6.3; https://www.R-project.org) was used for data analysis and mapping. Continuous variables were expressed as mean ± SD for T-test. The classified data were described as frequencies and percentages, and the chi-square test was performed. First of all, in order to screen the possible influencing factors of COPD, we took the presence or absence of COPD as the dependent variable, investigated relevant indicators as the independent variable, and carried out Univariate Logistic Regression Analyses. Indicators with statistically significant differences were incorporated into the LASSO model to screen potential predictors.14–16 The above candidate factors were incorporated into the logistic regression model, and their correlation with COPD was analyzed. The statistical significance level was bilateral, and the nomogram was further constructed.17,18 Then, we used the calibration curve, calibration C index and ROC curve to evaluate the discriminant performance of the model, respectively.19–21 To assess the usefulness of this model in clinical practice, we used the decision curve for evaluation.22 Finally, the internal validation of the model is carried out by using the random data extraction and C-index method.20
Results
Patients’ Characteristics
A total of 3249 patients from Local rural areas had complete data for model derivation. The mean age was 52.8±9.4 years. The rates of smoking, passive smoking, coal and biofuel use and ventilation system use were 14.2%, 7.9%, 95.5% and 17.3%, respectively. Besides, education is a mainly illiterate, semi-literate and primary school. Finally, a total of 394 cases (12.13%) were diagnosed with COPD (Supplemental Table 1). Compared with the control group, the COPD group had higher active smoking rate, passive smoking rate, daily barbeque consumption rate, furnace utilization rate and biofuel coal utilization rate.In addition, old age, male age, tuberculosis, lack of ventilation, emaciation, and low level of economic income may also be risk factors for COPD (Table 1).
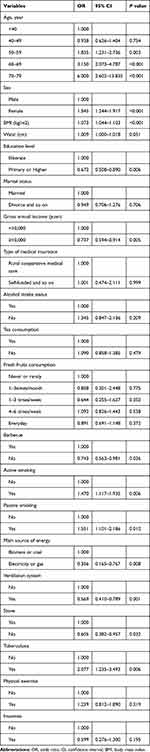 |
Table 1 Univariate Logistic Regression Analyses of COPD Influencing Factors |
Feature Selection
Among the many potential influencing factors, eight factors were screened out by the LASSO regression model. The above predictors are all non-zero coefficients in the regression model. These features include age, sex, barbeque, smoking, passive smoking, type of energy, ventilation systems, and Post-Bronchodilator (Figure 1).
Developing Nomogram
According to the LASSO Regression Model, the eight regression predictors screened were considered the best combination. We used these factors to construct logistic regression models. To visualize the model, we developed a nomogram (Figure 2).
Assessment of Nomogram
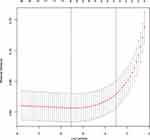
The calibration curve of the COPD prediction model is shown in Figure 3. C index was 0.81 (95% CI:0.79–0.83), combined with the ROC curve (Figure 4), the results showed that the model had high accuracy. We used the decision curve to analyze the nomogram’s clinical application. The results in Figure 5 show that using this model to predict COPD development is more beneficial when the threshold probability is >6% and <58%, respectively. Internally verified results show that the non-compliant nomogram C index is 0.80 (95% CI:0.78–0.83).
 |
Figure 4 ROC curve to predict the development of COPD. Abbreviations: ROC, receiver operator characteristics; AUC, area under the curve. |
 |
Figure 5 Decision curve of prediction models for the development of COPD. |
Discussion
To the best of our knowledge, this is the first based rural area COPD risk prediction model that compensates for the shortcomings of existing models. The prediction tool showed good resolution, calibration power and satisfactory clinical practicality.
As the largest developing country globally, China ranks among the top three in COPD mortality rates.23 Especially in rural areas of China, the prevalence of COPD is 13.7%,4,24 much higher than in urban areas. As global aging trends continue to intensify, COPD has become a major public health challenge that has drawn attention in many countries. In the latest global initiative for the diagnosis, treatment, and Prevention of Chronic obstructive pulmonary disease, COPD has been upgraded from an irreversible airflow limitation to a reversible disease that has been treated earlier.25 Studies have shown that early diagnosis of COPD patients is of great significance for improving prognosis, reducing disease progression, and reducing the economic burden.26 As a practical tool, clinical prediction models can be stratified according to the population’s risk characteristics, facilitating early detection and diagnosis of COPD, and facilitating effective individualized treatment and management.27–30 Therefore, it is necessary to construct a practical COPD prediction tool in typical rural areas.
This study shows that smoke exposure, including active and passive smoking, remains an important risk factor for COPD. Consistent with previously published predictive models,30–33 active smoking is an important predictor of our risk model. COPD is a result of the interaction between genes and the environment. Passive smoking, as an important environmental risk factor, is getting more and more attention.34 In this study, after screening by the LASSO regression model, passive smoking was also included in the prediction model. Age was a strong predictor of COPD in our model. The prevalence of COPD increased significantly with age compared to younger adults. However, it is not clear whether age-related aging contributes to COPD, or whether exposure to risk factors accumulates with age, leading to the disease. In addition, our study found a significantly higher prevalence in men than in women. It is consistent with most previous epidemiological studies.35,36 But some recent studies have shown the opposite. Recent studies in human pathology and animal studies have found that women have a greater burden of small-airway disease when exposed to the same amount of smoke.37–40
Surprisingly, of the four clinical prediction models for COPD published, only one study included lung function parameters.32 Several studies have suggested that low lung function predicts future COPD risk.41,42 The above evidence supports the use of lung function tests to predict the future risk of COPD in high-risk populations. The study also showed significant statistical differences in socioeconomic status factors such as education level and income level between the COPD and non-COPD populations. Combined with the research conclusions of Townend, J. et al, it is suggested that low socioeconomic levels will increase the risk of this disease.43 But it is not clear what factors of poverty are at work. However, our study shows that reducing Traditional fuel use, such as coal and biofuels, and increasing ventilation systems may significantly reduce COPD’s risk. Therefore, it is reasonable to speculate that the association between low socioeconomic level and increased risk of disease may reflect increased particle exposure caused by poverty, high biofuel utilization, lack of ventilation, etc.
Compared with other rural areas, the local diet is quite special. Barbeque is a common food eaten by local people. We included daily and occasional barbeque consumption as binary variables in the prediction model. The cooking process for local barbeques, which often involves burning traditional biofuels such as wood and crop stalks or coal, can cause high indoor pollution levels. We do not yet know whether this association between barbeques and increased COPD risk is related to increased exposure to particles or other causes for the high frequency of barbeque consumption.
The nomogram has good discrimination for COPD patients and other populations. This risk prediction tool can be used by health care professionals in clinical practice to provide opportunities for local high-risk populations in rural areas to correct possible risk factors and provide more cost-effective interventions. For example: promoting smoking cessation and tobacco control in public places; Reduce the use of coal and traditional bio-fuels such as wood, animal waste, crop stalks, etc. Add ventilation system and optimize barbeque cooking environment; A close look at people with low lung function. Simultaneously, this visual prediction tool can serve as a bridge between doctors and the general public: clinicians can scientifically disseminate more accessible, intuitive, and rational information about the prevention of COPD; Potential at-risk populations can refer to this model to avoid possible risk factors.
Inevitably, there are also several limitations to our current study. First of all, we only carried out internal verification of the model’s robustness, without external verification by other groups. Therefore, this nomogram needs to be externally evaluated in a wider population to further assess its universal applicability. Second, although we have included as many potential influencing factors as possible, there may still be some characteristics that have not been analyzed due to the limitations of realistic conditions. Finally, our findings are based on a cross-sectional study of a natural population, so prospective studies are needed to verify this tool before being widely used clinically.
Conclusion
We developed and validated a new COPD prediction model for rural northwest China. The influencing factors of this nomogram are easy to obtain and have good discrimination and internal verification. Our clinical prediction model complements existing research. Of course, this nomogram’s general applicability still needs to be further verified by more external people. Finally, we believe this model will be an important step in establishing early screening and diagnostic criteria for COPD in rural areas.
Data Sharing Statement
Additional data for this study can be available from the corresponding authors with reasonable request.
Acknowledgments
Major Science and Technology Special Project of Xinjiang Uygur Autonomous Region (Special Project No.: 2020A03004, Project No.: 2020A03004-1); The national key research and development plan “precision medicine research” key special sub-project (2017YFC0907203).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019
2. Nikolaou V, Massaro S, Fakhimi M, Stergioulas L, Price D. COPD phenotypes and machine learning cluster analysis: a systematic review and future research agenda. Respir Med. 2020;171:106093. doi:10.1016/j.rmed.2020.106093
3. Raju S, Brigham EP, Paulin LM, et al. The burden of rural chronic obstructive pulmonary disease: analyses from the national health and nutrition examination survey. Am J Respir Crit Care Med. 2020;201(4):488–491. doi:10.1164/rccm.201906-1128LE
4. Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430. doi:10.1016/S2213-2600(18)30103-6
5. Hill K, Goldstein RS, Guyatt GH, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182(7):673–678. doi:10.1503/cmaj.091784
6. Chuchalin AG, Khaltaev N, Antonov NS, et al. Chronic respiratory diseases and risk factors in 12 regions of the Russian federation. Int J Chron Obstruct Pulmon Dis. 2014;9:963–974. doi:10.2147/COPD.S67283
7. Siddharthan T, Grigsby MR, Goodman D, et al. Association between household air pollution exposure and chronic obstructive pulmonary disease outcomes in 13 low- and middle-income country settings. Am J Respir Crit Care Med. 2018;197(5):611–620. doi:10.1164/rccm.201709-1861OC
8. Salvi S, Barnes PJ. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138(1):3–6. doi:10.1378/chest.10-0645
9. Zha Z, Leng R, Xu W, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in Anhui Province, China: a population-based survey. BMC Pulm Med. 2019;19(1):102. doi:10.1186/s12890-019-0864-0
10. Mushtaq A. COPD and rural health in the USA. Lancet Respir Med. 2018;6(5):330–331. doi:10.1016/S2213-2600(18)30142-5
11. Croft JB, Wheaton AG, Liu Y, et al. Urban-rural county and state differences in chronic obstructive pulmonary disease - United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(7):205–211. doi:10.15585/mmwr.mm6707a1
12. Matheson MC, Bowatte G, Perret JL, et al. Prediction models for the development of COPD: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1927–1935. doi:10.2147/COPD.S155675
13. Mirza S, Clay RD, Koslow MA, Scanlon PD. COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc. 2018;93(10):1488–1502. doi:10.1016/j.mayocp.2018.05.026
14. Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26(30):5512–5528. doi:10.1002/sim.3148
15. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. doi:10.18637/jss.v033.i01
16. Kidd AC, McGettrick M, Tsim S, Halligan DL, Bylesjo M, Blyth KG. Survival prediction in mesothelioma using a scalable lasso regression model: instructions for use and initial performance using clinical predictors. BMJ Open Respir Res. 2018;5(1):e000240. doi:10.1136/bmjresp-2017-000240
17. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–180. doi:10.1016/S1470-2045(14)71116-7
18. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi:10.1200/JCO.2007.12.9791
19. Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the Hosmer-Lemeshow test revisited. Crit Care Med. 2007;35(9):2052–2056. doi:10.1097/01.CCM.0000275267.64078.B0
20. Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–2123. doi:10.1002/sim.1802
21. Zhang D, Zhang B. Semiparametric empirical likelihood confidence intervals for the difference of areas under two correlated ROC curves under density ratio model. Biom J. 2014;56(4):678–696. doi:10.1002/bimj.201300058
22. Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8(1):53. doi:10.1186/1472-6947-8-53
23. Zhu B, Wang Y, Ming J, Chen W, Zhang L. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1353–1364. doi:10.2147/COPD.S161555
24. Chan KY, Li X, Chen W, et al. Prevalence of chronic obstructive pulmonary disease (COPD) in China in 1990 and 2010. J Glob Health. 2017;7(2):020704. doi:10.7189/jogh.07.020704
25. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
26. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995–1008. doi:10.2147/COPD.S195382
27. Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the us preventive services task force. JAMA. 2016;315(13):1378–1393. doi:10.1001/jama.2016.2654
28. Han MK, Steenrod AW, Bacci ED, et al. Identifying patients with undiagnosed COPD in primary care settings: insight from screening tools and epidemiologic studies. Chronic Obstr Pulm Dis. 2015;2(2):103–121. doi:10.15326/jcopdf.2.2.2014.0152
29. Sakamoto Y, Yamauchi Y, Yasunaga H, et al. Development of a nomogram for predicting in-hospital mortality of patients with exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:1605–1611. doi:10.2147/COPD.S129714
30. Himes BE, Dai Y, Kohane IS, Weiss ST, Ramoni MF. Prediction of chronic obstructive pulmonary disease (COPD) in asthma patients using electronic medical records. J Am Med Inform Assoc. 2009;16(3):371–379. doi:10.1197/jamia.M2846
31. Guo YI, Qian Y, Gong YI, Pan C, Shi G, Wan H. A predictive model for the development of chronic obstructive pulmonary disease. Biomedical Rep. 2015;3(6):853–863. doi:10.3892/br.2015.503
32. Higgins MW, Keller JB, Becker M, et al. An index of risk for obstructive airways disease. Am Rev Respir Dis. 1982;125(2):144–151. doi:10.1164/arrd.1982.125.2.144
33. Kotz D, Simpson CR, Viechtbauer W, van Schayck OC, Sheikh A. Development and validation of a model to predict the 10-year risk of general practitioner-recorded COPD. NPJ Prim Care Respir Med. 2014;24(1):14011. doi:10.1038/npjpcrm.2014.11
34. Ukawa S, Tamakoshi A, Yatsuya H, Yamagishi K, Ando M, Iso H. Passive smoking and chronic obstructive pulmonary disease mortality: findings from the Japan collaborative cohort study. Int J Public Health. 2017;62(4):489–494. doi:10.1007/s00038-016-0938-1
35. Landis SH, Muellerova H, Mannino DM, et al. Continuing to Confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012–2013. Int J Chron Obstruct Pulmon Dis. 2014;9:597–611. doi:10.2147/COPD.S61854
36. Foreman MG, Zhang L, Murphy J, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPD gene study. Am J Respir Crit Care Med. 2011;184(4):414–420. doi:10.1164/rccm.201011-1928OC
37. Lopez Varela MV, Montes de Oca M, Halbert RJ. Sex-related differences in COPD in five Latin American cities: the PLATINO study. Eur Respir J. 2010;36(5):1034–1041. doi:10.1183/09031936.00165409
38. Silverman EK, Weiss ST, Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(6):2152–2158. doi:10.1164/ajrccm.162.6.2003112
39. Martinez FJ, Curtis JL, Sciurba F, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176(3):243–252. doi:10.1164/rccm.200606-828OC
40. Tam A, Churg A, Wright JL, et al. Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193(8):825–834. doi:10.1164/rccm.201503-0487OC
41. Bui DS, Burgess JA, Lowe AJ, et al. Childhood lung function predicts adult chronic obstructive pulmonary disease and asthma-chronic obstructive pulmonary disease overlap syndrome. Am J Respir Crit Care Med. 2017;196(1):39–46. doi:10.1164/rccm.201606-1272OC
42. Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–122. doi:10.1056/NEJMoa1411532
43. Townend J, Minelli C, Mortimer K, et al. The association between chronic airflow obstruction and poverty in 12 sites of the multinational BOLD study. Eur Respir J. 2017;49(6):1601880. doi:10.1183/13993003.01880-2016
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.