Back to Journals » International Journal of General Medicine » Volume 17
Determinants and Prognoses of Visual-Functional Mismatches After Mechanical Reperfusion in ST-Elevation Myocardial Infarction
Authors Liu J, Jin J , Yu B, Zhang S, Lu X, Chen G, Yang Y, Dong H
Received 24 October 2023
Accepted for publication 9 February 2024
Published 27 February 2024 Volume 2024:17 Pages 693—704
DOI https://doi.org/10.2147/IJGM.S444933
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Jieliang Liu,1,* Junguo Jin,1,* Bingyan Yu,2 Shanghong Zhang,2 Xiaoqi Lu,1 Guoqiang Chen,2 Yi Yang,2 Haojian Dong1,3
1Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong, 510080, People’s Republic of China; 2Department of Cardiology, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, Guangdong, 510080, People’s Republic of China; 3Nyingchi People’s Hospital, Nyingchi, Tibet, 860000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haojian Dong, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong, 510080, People’s Republic of China, Email [email protected] Yi Yang, Department of Cardiology, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, Guangdong, 510080, People’s Republic of China, Email [email protected]
Background: Discordance between the anatomy and physiology of the coronary has important implications for managing patients with stable coronary disease, but its significance in ST-elevation myocardial infarction has not been fully elucidated.
Methods: The retrospective study involved patients diagnosed with ST-elevation myocardial infarction (STEMI) who underwent percutaneous coronary intervention (PCI), along with quantitative coronary angiography (QCA) and quantitative flow ratio (QFR) assessments. Patients were stratified into four groups regarding the severity of the culprit vessel, both visually and functionally: concordantly negative (QCA-diameter stenosis [DS] ≤ 50% and QFR > 0.80), mismatch (QCA-DS > 50% and QFR > 0.80), reverse mismatch (QCA-DS ≤ 50% and QFR ≤ 0.80), and concordantly positive (QCA-DS > 50% and QFR ≤ 0.80). Multivariable logistic regression analyses were conducted to identify the clinical factors linked to visual-functional mismatches. Kaplan‒Meier analysis was conducted to estimate the 18-month adverse cardiovascular events (MACE)-free survival between the four groups.
Results: The study involved 310 patients, with 68 presenting visual-functional mismatch, and 51 exhibiting reverse mismatch. The mismatch was associated with higher angiography-derived microcirculatory resistance (AMR) (adjusted odds ratio [aOR]=1.016, 95% CI: 1.010– 1.022, P< 0.001). Reverse mismatch was associated with larger area stenosis (aOR=1.044, 95% CI: 1.004– 1.086, P=0.032), lower coronary flow velocity (aOR=0.690, 95% CI: 0.567– 0.970, P< 0.001) and lower AMR (aOR=0.947, 95% CI: 0.924– 0.970, P< 0.001). Additionally, the mismatch group showed the worst 18-month MACE-free survival among the four groups (Log rank test p = 0.013).
Conclusion: AMR plays a significant role in the occurrence of visual-functional mismatches between QCA-DS and QFR, and the mismatch group showed the worst prognosis.
Keywords: ST-elevation myocardial infarction, quantitative coronary angiography, quantitative flow ratio, angiography-derived microcirculatory resistance
Introduction
Mismatches between visual and functional coronary lesion severity have been commonly observed in previous studies. In a previous study, visual-functional mismatches were observed in more than one-third of the study population with moderate coronary stenosis.1 Such mismatches have consistently been a crucial element in patient management, who undergoing percutaneous coronary intervention (PCI). And the identified visual-functional mismatches could potentially inform risk stratification and guide prognostic and therapeutic decisions for patients with coronary disease in the future. According to previous studies, many factors play significant roles in visual-functional discordance, including age, sex, lesion location, plaque burden, presence/absence of plaque rupture, angiographical lesion-related indices, and physiological function.2–4 Besides clinical features and laboratory indicators, microvascular function potentially also has a significant influence on visual-functional discordance, underscoring the cruciality of physiological assessment of coronary flow.5 However, Tomoyo Sugiyama MD et al found that microvascular function was not related to the prevalence of visual-functional discordance in intermediate-to-severe stable lesions.6 At present, the mechanism behind this discordance remains to be completely clarified, especially concerning the importance of microvascular function.
Additionally, previous studies on visual-functional mismatches have mostly focused on stable coronary disease, with few investigating its role in ST-elevation myocardial infarction (STEMI). STEMI is often accompanied by complex coronary pathophysiological changes and is correlated with a poor prognosis, especially coronary microvascular dysfunction.7 Therefore, we believe that visual-functional discordance studies focused on STEMI may be highly clinically relevant and particularly valuable, in which combined quantitative coronary angiography (QCA) and quantitative flow ratio (QFR) assessments may contribute to stratifying at-risk patients for targeted therapy and hold promise for future research. Therefore, this study aimed to investigate clinical factors that contribute to visual-functional discordance utilizing a comprehensive database that includes QFR, QCA, and microvascular function data, with a specific emphasis on microvascular function.
Methods
Study Population
This retrospective cohort study was conducted involving three medical centers. From June 2016 to April 2021, we enrolled patients who were diagnosed with onset of STEMI and then underwent PCI during interventional procedures at Guangdong Provincial People’s Hospital, Zhuhai Golden Bay Hospital and Jiexi City People’s Hospital. Inclusion criteria are as follows: Patients with STEMI; and patients who underwent PCI with valid angiography data to assess QFR after pre-dilation and then accepted stent implantation. The criteria of exclusion in the study were as follows: patients with left-main culprit lesions; patients with intravenous thrombolysis before PCI; and patients with known rheumatic diseases. Initially, 663 patients were enrolled, of which 353 lacked valid angiography data. Eventually, 310 patients who met the criteria outlined in this study were chosen for analysis (Figure 1).
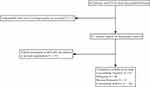 |
Figure 1 Flow chart of patients’ enrollment. |
Laboratory Assessment and Definition
Coronary artery narrowing was deemed visually obstructive if the QCA-diameter stenosis (DS) > 50%.8 We defined functionally significant stenosis as a QFR ≤ 0.8.9 Patients were stratified into four groups regarding the combined significance of culprit vessel’s visual and functional stenosis severity. Visual-functional mismatch was defined as QCA-DS > 50% and QFR > 0.80, whereas reverse mismatch was defined as QCA-DS ≤ 50% and QFR ≤ 0.80. Concordantly negative group was defined as QCA-DS ≤ 50% and QFR > 0.80, and concordantly positive group was defined as QCA-DS > 50% and QFR ≤ 0.80. STEMI diagnosis was based on the presence of at least two of the following three criteria: (1) electrocardiography (ECG) criteria: the presence of typical ST-segment elevation in at least two contiguous ECG leads; (2) biomarker criteria: evidence of proportionally elevated cardiac biomarkers, including troponin, creatine kinase (CK), and creatine kinase-myocardial band (CK-MB); (3) symptom criteria: typical chest pain symptoms. Major adverse cardiovascular events (MACE) included cardiac death (CD), recurrent myocardial infarction (MI), hospitalization for heart failure (HF), target vessel revascularization (TVR), and stroke.
The definition of hypertension was based on the guidelines of the European Society of Cardiology,10 and Diabetes mellitus was diagnosed based on the European Society of Cardiology guidelines.11 Smoking was defined as the current habit of smoking tobacco products. Dyslipidemia was diagnosed according to the Chinese guidelines for the management of dyslipidemia in adults.12 A history of MI was determined from the patient’s medical history. To facilitate comparison, cardiac troponin T (cTnT) and I (cTnI) levels were categorized into two groups based on whether the values were 100 times the upper limit of detection (cTnI/cTnT > 100 times and cTnI/cTnT < 100 times), while N-terminal pro-B-type natriuretic peptide (NT-proBNP) and BNP levels were grouped based on whether the values were 10 times the upper limit of detection (NT-proBNP/BNP > 10 times and NT-proBNP/BNP < 10 times). The estimated glomerular filtration rate (eGFR) was calculated in the study using the modification of diet in renal disease (MDRD) formula, measured in mL⋅min−1⋅1.73 m2.
Percutaneous Coronary Intervention and Coronary Angiography Analysis
Cardio-angiography (CAG) was conducted via the radial or femoral approach. Diagnostic catheters, either 5-Fr or 6-Fr Judkins catheters, were employed for left and right coronary angiography, respectively. Immediately before the procedure, patients were given heparin (70–100 IU/kg) or bivalirudin (1.75 mg/kg/h) to maintain an activated clotting time > 250s. All enrolled patients received pretreatment with mechanical reperfusion based on the condition of the lesion. The choice of PTCA/thrombus aspiration and the use of platelet glycoprotein IIb/IIIa inhibitor (GPI), drug-eluting stent (DES), and drug-coated balloon (DCB) depended on the judgment of the operators. The thrombolysis in myocardial infarction (TIMI) flow grade of all culprit lesions was ≥ 2 after pretreatment with mechanical reperfusion.
Coronary angiogram data were transmitted to the Angiography Laboratory of Guangdong Provincial People’s Hospital, where the analysis was anonymously conducted by two skilled observers. To estimate interobserver error, an additional observer was involved in the process. After successful mechanical reperfusion, QCA analysis was conducted on each culprit lesion utilizing QAngio XA 7.2 software (Medis Medical Imaging Systems, Leiden, the Netherlands). Standard techniques were used to collect QCA parameters, including percentage diameter stenosis (DS), minimum lumen diameter (MLD), percentage area stenosis (AS), minimum lumen area (MLA), and lesion length (LL).
The assessment of coronary physiological indices after mechanical reperfusion took place at the CardHemo Core Lab, affiliated with the Med-X Research Institute at Shanghai Jiao Tong University. This evaluation employed Murray’s law-based QFR calculation, which was conducted on a single coronary angiogram run using recently developed software (AngoPlus Core, Pulse Medical Imaging Technology, Shanghai, China). Firstly, adequately contrast-filled end-diastolic images were loaded. Two angiographic images separated by at least 25° were required to perform a three-dimensional reconstruction of the target vessel. This three-dimensional reconstruction, with the proposed analytic model, was based on geometrical features derived from the entrance angle of the coronary stenosis, the angularity of the center line, and reference points of the lumen diameter. The distal and proximal landmarks in the target vessel were registered as the regions of interest. Subsequently, the contrast flow model, by TIMI frame count of the proximal to the distal portion of the analyzed segment of the vessel was used as an estimation of coronary flow velocity and was used for final QFR computation.13 To ensure accurate QFR calculation, the analysis was performed on angiographic images with minimal foreshortening and overlap. During the same procedure as the QFR analysis mentioned above, angiography‑derived microcirculatory resistance (AMR) and coronary flow velocity (CFV) data were also obtained.
Statistical Analysis
We conducted descriptive analyses. Continuous variables were described as the mean ± standard deviation (SD) or median (interquartile range), and the categorical variables were shown as count data (percentages). The differences between the four groups (concordantly negative, mismatch, reverse mismatch, concordantly positive) were analyzed to compare employed one-way ANOVA, chi-square test/Fisher’s exact test, and Kruskal‒Wallis test depending on the circumstances. To identify the factors related to positive functional ischemia (QFR ≤ 0.80) and visual-functional discordance between QCA-DS and QFR, we performed multivariable logistic regression analyses in our study. Variables that showed significance in univariable analyses (P < 0.10) were selected in the multivariable model. To estimate the prognosis of patients with STEMI after PCI across different groups in the study, the Kaplan–Meier method and Log rank tests were conducted to assess the 18-month adverse cardiovascular events (MACE)-free survival. Statistical significance was defined as a P-value < 0.05. All analyses in our study were conducted using SPSS Statistics 26.0 software (IBM Corporation, Armonk, NY, USA) and R version 3.6.1 (The R Project for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics and Angiographic results
Totally 310 patients were enrolled in the present study. Among them, 118 (38.39%) patients exhibited visual-functional discordance between QCA and QFR, including 68 patients (21.94%) with a mismatch and 51 patients (16.45%) with a reverse mismatch. Concordantly negative QCA-QFR was observed in 67 patients, constituting 21.61% of the total cohort, whereas concordantly positive QCA-QFR was exhibited in 124 patients, accounting for 40.00% of the total enrolled patients (Figure 2). Their baseline characteristics are provided in Table 1. The four groups were well balanced concerning clinical characteristics except for a higher level of max CK-MB in the concordantly negative QCA-QFR group (p=0.028). The angiographic findings are demonstrated in Table 2. There were higher AMR and lower CFV when QFR>0.80. QCA analysis revealed the presence of graded differences in DS, AS, MLD, and MLA.
 |
Table 1 Baseline Information |
 |
Table 2 Angiographic Results |
 |
Figure 2 Comparison of the occurrence of QCA-QFR concordance/discordance after mechanical reperfusion. |
Factors Related to Visual‑functional Discordance
Our logistic regression analyses revealed that factors related to positive functional ischemia, defined by QFR ≤ 0.80, were lower CFV (adjusted odds ratio [aOR]=0.906, 95% CI: 0.843–0.973, P=0.007) and lower AMR (aOR=0.975, 95% CI: 0.967–0.982, P<0.001) (Supplemental Table 1). For culprit lesions appearing visually nonsignificant (DS ≤ 50%), we found reverse mismatch was related to greater AS (aOR=1.044, 95% CI: 1.004–1.086, P=0.032), lower CFV (aOR=0.690, 95% CI: 0.567–0.838, P<0.001) and lower AMR (aOR=0.947, 95% CI: 0.924–0.970, P<0.001) (Table 3). Conversely, for visually significant lesions (DS > 50%), visual-functional mismatch was related to higher AMR (aOR=1.016, 95% CI: 1.010–1.022, P<0.001) (Table 4).
 |
Table 3 Determinants of Reverse Mismatch (QFR ≤ 0.80) in Visually Nonsignificant Lesions (DS ≤ 50%) |
 |
Table 4 Determinants of Mismatch (QFR > 0.80) in Visually Significant Lesions (DS > 50%) |
Prognoses of Visual-Functional Mismatches
Significant difference has not been observed between the four groups based on physiological indices and QCA analysis after stent implantation, which suggests that the immediate outcome after stent implantation is similar between the four groups (Supplemental Table 2). In the comparison of prognoses between patients with concordant and discordant QCA-QFR, the discordant QCA-QFR group showed the worse 18-month MACE-free survival (Log rank test p = 0.040) (Supplemental Figure 1). Another analysis of Kaplan–Meier curves revealed that the mismatch exhibited the worst prognosis (Log rank test p = 0.013) (Figure 3).
Discussion
The primary findings of this study revealed that discordance between QCA-defined visual and QFR-derived functional stenosis severity after mechanical reperfusion in STEMI was common in clinical practice. The frequency of visual-functional mismatch and reverse mismatch was 21.94% and 16.45%, respectively; A higher AMR emerged as a significant predictor of mismatch, while lower AMR was a significant predictor of reverse mismatch. Notably, the mismatch group showed the most adverse prognosis among the studied groups.
Angiography-derived visual assessment has traditionally been the gold standard for decision-making in revascularization. However, as research on coronary stenosis has advanced, assessing its severity is no longer limited to visual assessments based on angiography. Functional assessment has become indispensable. The integration of visual and functional modalities enhances our understanding of coronary lesions and supports decision-making in managing coronary disease. The QFR is a recently developed angiography-derived physiological index validated against FFR.9 While both visual and functional indices have their utility in revascularization decisions, they do not always yield concordant results in clinical practice. Previous studies have reported common visual-functional mismatches in coronary artery disease. For example, in the CVIT-DEFER registry, a prospective multicenter study enrolling patients with moderate coronary lesions for whom clinical indications called for FFR analysis, visual-functional mismatch occurred in 43.4% of the lesions, with reverse mismatch occurring in 23.2%.1 Additionally, a separate investigation involving stable coronary disease patients found similar visual-functional discordance between QCA and QFR in one-third of the study population.6 It has been recognized that various factors contribute significantly to visual-functional mismatches in prior studies, including age, sex, lesion location, plaque burden, presence/absence of plaque rupture, angiographical lesion-related indices, and physiological function.2–4 Among these factors, microcirculatory resistance has been identified as a critical contributor.14–16 However, previous studies on visual–functional discordance were primarily conducted in stable coronary disease, and its role in STEMI has rarely been investigated. STEMI is often associated with complex coronary pathophysiological changes and is linked to a poor prognosis, particularly related to coronary microvascular dysfunction.7 We believe that examining visual-functional discordance in STEMI is clinically relevant and valuable. A combined assessment using QCA and QFR may contribute to stratifying at-risk patients for targeted therapy and offer future promise.
In our study on the determinants of QCA-QFR mismatches, we found that microvascular function played an important role in STEMI patients. We observed a discordance between QCA and QFR in 38.39% of the study population. Additionally, our results indicated that AMR was significantly associated with visual-functional discordance in STEMI. This finding may be attributed to the influence of coronary flow through the stenosis on QFR. A decrease in microvascular resistance means that coronary flow increases for a specific stenosis and a given driving pressure, potentially resulting in a low QFR value. Conversely, an increase in microvascular resistance causes a decrease in coronary flow through the given fixed stenosis, leading to an elevated QFR. Liang et al found that the QCA-QFR discordance was independently related to angiography-based microcirculatory indices in intermediate coronary lesions.17 Taishi Yonetsu MD et al also demonstrated that the microcirculatory resistance served as an essential role in visual-functional mismatch and reverse mismatch in patients with stable angina pectoris.5 The findings in our study are in line with previous research and further provide additional evidence emphasizing the essential role of microvascular function in the visual-functional discordance of STEMI. However, another study about visual-functional discordance suggested that the index of microcirculatory resistance (IMR) was not related to the prevalence of either mismatch or reverse mismatch.6 The role of microvascular function in visual-functional discordance in stable coronary disease is still controversial. Compared with the above studies, our study targeted the STEMI population, which demonstrates highly complex coronary pathophysiological changes, especially coronary microvascular dysfunction, an important difference from stable coronary artery disease. Our results provide stronger evidence that the dissimilarities in coronary microvascular function may be an important cause of visual-functional mismatches in STEMI.
The AMR was higher in lesions with QFR > 0.8 but lower in lesions with QFR ≤ 0.8 in our study, which is consistent with previous studies.18,19 Interestingly, the mismatch group was accompanied by a higher AMR after mechanical reperfusion in the STEMI patients, which implied that the mismatch group might be accompanied by worse coronary microvascular function. The findings indicated that the mismatch group may include patients with lesions characterized by low-pressure gradients, a phenomenon resulting from the inclined coronary flow due to impaired microvascular resistance.20 This indicates that the QFR > 0.80 and QCA-DS > 50% also identify those with impaired and nonimpaired coronary flow.
Furthermore, the analysis of the 18-month MACE-free survival showed that the mismatch group, accompanied by the higher AMR after mechanical reperfusion, had the worst prognosis. Additionally, one notable finding was that the mismatch group also presented with a higher QFR, which was related to a potentially better prognosis in previous studies. It is not difficult to explain this finding, however. There is a potential challenge associated with considering the impact of microvascular dysfunction in the functional assessment of culprit vessel territories. In a porcine microvascular damage model, Lee et al revealed that significant localized microvascular damage could lead to an increased FFR based on the IMR in a vessel supplying the target myocardial territory.21 It’s crucial to emphasize that impaired myocardial reperfusion resulting from coronary microvascular dysfunction (CMD) affects approximately half of all patients suffering from acute myocardial infarction (AMI). This, despite the high success rate of standard coronary reperfusion, stands as a significant adverse prognostic factor.22 Impaired microvasculature has been investigated as an important pathological change in patients with STEMI and is always correlated with a poor prognosis.23
Current strategies for the prevention and treatment of perioperative CMD in PCI include drug-containing strategies, nondrug strategies, and optimization of surgical procedures. Examples include intravenous or intracoronary application of GPI, thrombus aspiration, and postoperative oral medication aimed at improving coronary microcirculation and prognosis.24–27 However, there is insufficient evidence to substantiate the use of these treatments for treating the disease. As a frequent complication of AMI, CMD has recently underscored an unmet therapeutic demand. Looking ahead, we endorse the adoption of targeted strategies to identify patients who are likely to benefit from effective treatments.
In addition to AMR, our research found that larger AS and lower CFV were also associated with QCA-QFR reverse mismatch. A previous study showed that smaller intravascular ultrasound (IVUS)-MLA correlated with visual-functional reverse mismatch.2 This finding indicates that the size of lumen area plays an important role in the visual-functional discordance. However, a study conducted by Liang et al found that higher hyperemic flow velocity links to visual-functional reverse mismatch in intermediate coronary lesions,17 which is inconsistent with our result. We speculate that the reason may result from the difference in the study population. It was been demonstrated that there is impaired coronary flow after mechanical recanalization in AMI in response to elevated levels of endothelin-1 (ET-1), which was not shown in patients with unstable or stable angina pectoris.28 In other clinical research, the discordance between anatomy and physiology is also found to be related to complex plaque morphology.29 These findings indicate that therapeutic decision-making relying on visual estimation solely is not a reliable method. In the future, further large studies are needed to explore the benefits of QFR-guided revascularization and the outcome of STEMI.
The combination of angiography parameters and QFR for assessing microvascular dysfunction used in our study could better reflect the severity of coronary lesions in STEMI, possibly contributing to stratifying at-risk patients for targeted therapy and holding future promise. All these studies suggest that visual estimation may be an unreliable method for assessing functional severity. Even though angiography offers more accurate lumen contours, substantial visual-functional mismatches were still observed. When the QFR measurement in a culprit STEMI lesion indicates physiological suboptimal conditions, QCA optimization and assessment of microvascular function can be employed for therapeutic decision-making, thereby improving the prognosis.30 Our findings support the novel notion that microvascular function could significantly contribute to the discordance between QFR and QCA evaluation. The present study highlighted the crucial role of integrating anatomical and physiological assessment in identifying clinically significant coronary lesions and could provide additional diagnostic and prognostic insights into STEMI with visual-functional discordance.
Limitations
The present study has some limitations. Firstly, the relatively small size of the population involved in our study could theoretically result in a selection bias. Secondly, the study population consisted entirely of Chinese individuals, which means our findings may not be generalizable for the treatment of other ethnic populations. Finally, we employed QFR as the reference to identify angiography-derived visual stenosis severity and subsequently to evaluate the factors contributing to the visual-functional discordance. This approach may introduce significant inherent bias. In future studies, it would be advisable to consider employing an alternative, independent reference standard, such as the FFR, coronary flow reserve (CFR) or IMR, and some other invasive flow measurements or microcirculatory parameters to investigate discrepancies between angiography parameters and QFR.
Conclusions
Substantial visual-functional mismatches are present in STEMI after mechanical reperfusion during coronary angiography. The mismatch group (QCA-DS > 50% and QFR > 0.80) showed a poor prognosis after stenting. Combining angiography parameters and QFR for assessing microvascular dysfunction could better reflect the severity of coronary lesions in STEMI, possibly contribute to the stratification of at-risk patients for targeted therapy and hold future promise.
Ethics Approval and Consent to Participate
The present study and oral informed consent obtained from all patients was approved by the Ethics Committee of Guangdong Provincial People’s Hospital. Given the retrospective design of the study, the written informed consent was waived. We declare that the research on participants was conducted in accordance with the principles of the Declaration of Helsinki. All experimental data were extracted from electronic medical records of the patients and recorded by the authors.
Acknowledgments
We thank American Journal Experts for its linguistic assistance during the preparation of this manuscript.
Funding
Our research was supported by the Natural Science Foundation of China (No.82373529, No.81903287, No.82170259); the Guangzhou Municipal Science and Technology Grant (202002030112) and the Science and Technology Program of Tibet Grant (No. XZ202201ZY0051G).
Disclosure
The authors declare no conflicts of interest related to this article.
References
1. Nakamura M, Yamagishi M, Ueno T, et al. Prevalence of visual-functional mismatch regarding coronary artery stenosis in the CVIT-DEFER registry. Cardiovasc Interv Ther. 2014;29(4):300–308. doi:10.1007/s12928-014-0259-3
2. Park SJ, Kang SJ, Ahn JM, et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC. 2012;5(10):1029–1036. doi:10.1016/j.jcin.2012.07.007
3. Ahn SG, Suh J, Hung OY, et al. Discordance between fractional flow reserve and coronary flow reserve: insights from intracoronary imaging and physiological assessment. JACC. 2017;10(10):999–1007. doi:10.1016/j.jcin.2017.03.006
4. van de Hoef TP, Nolte F, EchavarrÍa-Pinto M, et al. Impact of hyperaemic microvascular resistance on fractional flow reserve measurements in patients with stable coronary artery disease: insights from combined stenosis and microvascular resistance assessment. Heart. 2014;100(12):951–959. doi:10.1136/heartjnl-2013-305124
5. Yonetsu T, Murai T, Kanaji Y, et al. Significance of microvascular function in visual-functional mismatch between invasive coronary angiography and fractional flow reserve. J Am Heart Assoc. 2017;6(6):e005916. doi:10.1161/JAHA.117.005916
6. Sugiyama T, Kanno Y, Hamaya R, et al. Determinants of visual-functional mismatches as assessed by coronary angiography and quantitative flow ratio. Catheter Cardiovasc Interv. 2021;98(6):1047–1056. doi:10.1002/ccd.29388
7. Konijnenberg LSF, Damman P, Duncker DJ, et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res. 2020;116(4):787–805. doi:10.1093/cvr/cvz301
8. Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging. 2009;2(8):1009–1023. doi:10.1016/j.jcmg.2009.06.004
9. Xu B, Tu S, Qiao S, et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J Am Coll Cardiol. 2017;70(25):3077–3087. doi:10.1016/j.jacc.2017.10.035
10. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi:10.1093/eurheartj/ehy339
11. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi:10.1093/eurheartj/ehz486
12. Joint committee for guideline revision. 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15(1):1–29. doi:10.11909/j.issn.1671-5411.2018.01.011
13. Tu S, Ding D, Chang Y, Li C, Wijns W, Xu B. Diagnostic accuracy of quantitative flow ratio for assessment of coronary stenosis significance from a single angiographic view: a novel method based on bifurcation fractal law. Catheter Cardiovasc Interv. 2021;97(Suppl 2):1040–1047. doi:10.1002/ccd.29592
14. Echavarria-Pinto M, Escaned J, Macías E, et al. Disturbed coronary hemodynamics in vessels with intermediate stenoses evaluated with fractional flow reserve: a combined analysis of epicardial and microcirculatory involvement in ischemic heart disease. Circulation. 2013;128(24):2557–2566. doi:10.1161/CIRCULATIONAHA.112.001345
15. Lee JM, Layland J, Jung JH, et al. Integrated physiologic assessment of ischemic heart disease in real-world practice using index of microcirculatory resistance and fractional flow reserve: insights from the international index of microcirculatory resistance registry. Circ Cardiovasc Interv. 2015;8(11):e002857. doi:10.1161/CIRCINTERVENTIONS.115.002857
16. Toth G, Hamilos M, Pyxaras S, et al. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014;35(40):2831–2838. doi:10.1093/eurheartj/ehu094
17. Geng L, Yuan Y, Du P, et al. Association of quantitative flow ratio-derived microcirculatory indices with anatomical-functional discordance in intermediate coronary lesions. Int J Cardiovasc Imaging. 2021;37(10):2803–2813. doi:10.1007/s10554-021-02292-2
18. Nijjer SS, de Waard GA, Sen S, et al. Coronary pressure and flow relationships in humans: phasic analysis of normal and pathological vessels and the implications for stenosis assessment: a report from the Iberian-Dutch-English (IDEAL) collaborators. Eur Heart J. 2016;37(26):2069–2080. doi:10.1093/eurheartj/ehv626
19. Park SD, Lee MJ, Woo SI, et al. Epicardial artery stenosis with a high index of microcirculatory resistance is frequently functionally insignificant as estimated by Fractional Flow Reserve (FFR). Intern Med. 2016;55(2):97–103. doi:10.2169/internalmedicine.55.4080
20. Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62(18):1639–1653. doi:10.1016/j.jacc.2013.07.076
21. Lee JM, Kim HK, Lim KS, et al. Influence of local myocardial damage on index of microcirculatory resistance and fractional flow reserve in target and nontarget vascular territories in a porcine microvascular injury model. JACC. 2018;11(8):717–724. doi:10.1016/j.jcin.2017.11.028
22. De Maria GL, Cuculi F, Patel N, et al. How does coronary stent implantation impact on the status of the microcirculation during primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction? Eur Heart J. 2015;36(45):3165–3177. doi:10.1093/eurheartj/ehv353
23. Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2016;37(13):1024–1033. doi:10.1093/eurheartj/ehv484
24. Ma Q, Ma Y, Wang X, et al. Intracoronary compared with intravenous bolus tirofiban on the microvascular obstruction in patients with STEMI undergoing PCI: a cardiac MR study. Int J Cardiovasc Imaging. 2020;36(6):1121–1132. doi:10.1007/s10554-020-01800-0
25. Jolly SS, James S, Džavík V, et al. Thrombus aspiration in ST-segment-elevation myocardial infarction: an individual patient meta-analysis: thrombectomy trialists collaboration. Circulation. 2017;135(2):143–152. doi:10.1161/CIRCULATIONAHA.116.025371
26. Wang S, Duan Y, Feng X, et al. Sustained nicorandil administration reduces the infarct size in ST-segment elevation myocardial infarction patients with primary percutaneous coronary intervention. Anatol J Cardiol. 2019;21(3):163–171. doi:10.14744/AnatolJCardiol.2018.57383
27. Velibey Y, Guvenc TS, Demir K, et al. Effects of bailout tirofiban on in-hospital outcomes and long-term mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous intervention. Angiology. 2019;70(5):431–439. doi:10.1177/0003319718808911
28. Schäfer U, Kurz T, Jain D, et al. Impaired coronary flow and left ventricular dysfunction after mechanical recanalization in acute myocardial infarction: role of neurohumoral activation? Basic Res Cardiol. 2002;97(5):399–408. doi:10.1007/s003950200049
29. Scarsini R, Terentes-Printzios D, De Maria GL, Ribichini F, Why BA. When and how should clinicians use physiology in patients with acute coronary syndromes? Interv Cardiol. 2020;
30. Niccoli G, Montone RA, Ibanez B, et al. Optimized treatment of ST-elevation myocardial infarction. Circ Res. 2019;125(2):245–258. doi:10.1161/CIRCRESAHA.119.315344
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

