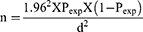Back to Journals » Veterinary Medicine: Research and Reports » Volume 14
Detection of Antibodies Against Brucellosis and Associated Risk Factors in Cross Breed Dairy Cattle in Smallholder Farmers, Southern Ethiopia
Authors Shurbe M, Wondimu A, Eshetu N, Seyoum W , Tora E , Simeon B, Rufael T, Sombo M
Received 26 November 2022
Accepted for publication 6 March 2023
Published 15 March 2023 Volume 2023:14 Pages 23—33
DOI https://doi.org/10.2147/VMRR.S389738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Young Lyoo
Mesfin Shurbe,1 Abreham Wondimu,1 Nebiyu Eshetu,1 Wasihun Seyoum,1 Ephrem Tora,1 Bekahegn Simeon,1 Tesfaye Rufael,2 Melaku Sombo2
1Department of Animal Science, College of Agriculture Sciences, Livestock and Fishery Research Center, Arba Minch University, Arba Minch, Ethiopia; 2Department of Molecular Biology, National Animal Health Institute, Sebeta, Ethiopia
Correspondence: Mesfin Shurbe, Department of Animal Science, College of Agriculture Sciences, Livestock and Fishery Research Center, Arba Minch University, P.O. Box, 21, Arba Minch, Ethiopia, Tel +251910145280, Email [email protected]
Background: A cross-sectional study was conducted in selected districts of Gamo zone, southern Ethiopia over a period of November 2019 and September 2020 to estimate seroprevalence and associated risk factors and assess knowledge and practices of smallholder farmers about bovine brucellosis. Two districts and four kebeles from each district were purposively selected, and individual animals were sampled using a simple random sampling technique. A total of 384 sera samples were collected, and concurrently, 236 dairy cattle owners were interviewed. The samples were tested for antibodies against Brucella using both Rose Bengal Plate test and Complement Fixation test following OIE standard protocol. Risk factors associated with bovine brucellosis were analyzed using univariate and multivariate logistic regressions.
Results: The survey result has shown that 95% and 97% of the small holder farmers did not know the cause and symptoms of bovine brucellosis, respectively, and the majority have engaged in risky practices. Eight (2.08%) of the collected serum samples were positive with screening test (RBT) and only six (1.5%) were positive with confirmatory test (CFT). Multivariable logistic regression analysis showed a statistically significant association between herd sizes and the disease. The seropositivity of the disease is higher in small-sized herds followed by medium-sized herds.
Conclusion: The seroprevalence of bovine brucellosis was found to be at a low percentage with confirmatory tests even if there was a presence of associated risk factors for the disease in the study area. Again, the results suggest that smallholder farmers have poor knowledge and risky practices, which expose them to the disease. Awareness creation about the disease is of paramount importance even if the prevalence was low in this serological study. The implementation of a test and slaughter program before the disease becomes widespread, along with the testing of new stock before introduction to the farms is recommended.
Keywords: brucellosis, seroprevalence
Introduction
Ethiopia has the largest livestock population in Africa with an estimate of 60.9 million cattle, 31.3 million sheep, and 32.7 million goats.1 However, livestock are not performing well as food producers in Ethiopia due to wide spread animal diseases. In particular, brucellosis has a significant socioeconomic and zoonotic impact, but has a low mortality rate, and has received little attention.2
Brucellosis in cattle affects reproductive performance and is an infectious disease of sexually mature cattle in Africa.3 The infection is mainly caused by Brucella abortus, to a lesser extent by Brucella melitensis and rarely by Brucella suis. Abortion and retained fetal membrane in cows and orchitis and epididymitis in bulls are characteristic signs of the disease.5 Animals with a high susceptibility to the disease contract it directly from aborted cows and fetuses, or indirectly via contaminated fomites.6
The disease remains a major public and animal health concern in many developing countries.7 Yet, Brucella infections are common in cattle populations in sub-Saharan Africa, with seroprevalences ranging from 0.3% to 45%8 and the prevalence of each of the countries associated with a risk of factors related to production systems and environmental factors.5
Despite their tight eradication programme, most developed countries have made significant progress but know the disease in Africa exerts the most serious problem.9 A number of serological reports have shown that Brucella infection among cattle is common in Ethiopia, particularly in pastoral areas.10 In Ethiopia, the previous seroprevalence rates stated were 7.62%,11 8–11%,12 and 1.8%.13 A seroprevalence of 50% for Brucella was reported by14 in the Borena Zone. Furthermore, Tolosa et al15 reported 0.77% in Jimma zone and Haile Selassie et al16 reported 1.2% in Tigray region. The results of another investigation in south and east Ethiopia revealed that 3.5% of cattle were positive for Brucella antibodies.17 Smallholder farmers in the study areas probably raised a small number of crossbreed dairy animals under a zero grazing system to produce milk for their own consumption and sale. However, reproductive failure due to abortion is a critical issue and creates serious problems in the present study area. In addition, information on the extent of the disease is not well known in the recent study area. Therefore, the present study aimed to estimate the serological prevalence of bovine brucellosis, identify its associated risks, and assess informant knowledge and practices related to bovine brucellosis as well as reproductive disorders associated with seropositive cows.
Materials and Methods
Study Area Profile
The study was undertaken in two purposely selected districts, namely, Chencha and Bonke of Gamo zone, Southern Ethiopia from November 2019 to September 2020 (Figure 1).
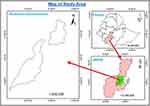 |
Figure 1 The study area landscape. |
Chencha District is situated between 1300m and 3250m above sea level. The location of Chencha district in astronomical terms is between 37o 29’57” East to 37o 39’36” West and between 6°8’ 55” North and 6o25’ 30” South. The district has two agroecological zones, namely, Dega and Weyna Dega, which account for approximately 82% and 18% of the total area, respectively. The rainfall regime in the district is bimodal. The first round of rain occurs between March and April. The second round of rain occurs from June to August. The annual rainfall distribution in the district varies between 900 and 1200 mm. The minimum and maximum temperatures in the district range from 11°C to 13°C and 18°C to 23°C, respectively. In the district, crop and livestock subsystems are practiced. Chencha has 67,269 cattle, 106,594 sheep, 11,870 goats, and 22,554 equines.18
Bonke is one of the 15 districts in Gamo Zone and lies between 5°55N latitude and 37°15E longitude with an altitude ranges from 600 to 4200 masl. The land area of the district is estimated to be 85,940 km2 and bordered on the south by the Derashe and Alle district, on the west by the Weito River, which separates it from Kemba, on the northwest by Deramalo, on the north by Dita, and on the east by Arba Minch Zuria district. The agro-ecology of the district is classified into three zones: Dega (covers 46% of the land area), Woina Dega (30%), and Kola (24%). The mean annual average rainfall and temperature of the district are 1400 mm and 13.05°C, respectively. The estimated livestock populations of the district are 137,171 cattle, 189,557 sheep, 65,758 goats, 36,566 equines, and 226,026 poultry.19
Study Design
A cross-sectional type of study was employed between November 2019 and September 2020 to screen dairy cattle for bovine brucellosis. Furthermore, smallholder farmers were surveyed regarding their knowledge and practice concerning bovine brucellosis using semi-structured questionnaires.
Study Animal
A study was conducted in purposively selected districts of the Gamo zone on cross-breeds of Jersey and Holstein Friesian exotic dairy cattle managed either under an extensive or semi-intensive management system. The animals in the study were no any previous vaccination history against bovine brucellosis. All crosses of jersey and Holstein Friesian aged more than 2 years were included in the study. In the sample collection period, age, parity, breed, and pregnancy status were collected and recorded as animal-level risk factors. The age of each study animal was determined by consulting the owners of the cattle.
Inclusion Criteria
All cross-breed dairy cattle with jersey and Holstein Friesian greater than two years old, as well as cattle owners who expressed a willingness to participate in the study, were included.
Sample Size and Sampling Method
Two districts, Chencha and Bonke, and five kebeles from each district were selected based on their potential for cross-breed dairy cattle. The study animals were then randomly selected from each kebele using simple random sampling. The sample size was calculated based on the formula given below as described by Thrusfield.20
Where n = sample size, Pexp = expected prevalence, and d = absolute precision.
Based on the expectation that disease prevalence would be 50% with 95% confidence level, and 5% absolute precision, a sample size of 384 exotic crossbreed dairy cows was calculated. A proportionate number of animals were then sampled from each district based on its crossbreed dairy cattle population. Thus, 236 and 148 individual animals were sampled from Bonke and Chencha districts, respectively.
Smallholder farmers’ knowledge and practices related to bovine brucellosis were surveyed in two districts along with blood sample collection. Thus, 236 dairy cattle owners were interviewed with semi-structured questionnaires following a pretest. Accordingly, 163 dairy cattle owners from Bonke and 73 from Chencha districts were interviewed.
Study Methodology
Questionnaire Survey
An assessment of farmers’ knowledge and practice of bovine brucellosis was conducted through a semi-structured questionnaire. A pre-tested semi-structured questionnaire was designed and administered to individual dairy cattle owners to assess the informants’ knowledge of bovine brucellosis, its symptoms and the unhygienic practices that lead to the spread of the disease.
Sample Collection and Preparation
Blood samples were collected from individual animals using plain 10mL vacutainer tubes from each animal’s jugular vein. The collected samples were labeled and left at room temperature for a moment to allow coagulation, then transferred to another sterile cryovial tube and transported respecting the cold chain and finally stored at −20°C until serological testing. The seropositivity of individual animals was determined using both screening (Rose Bengal plate test) and confirmatory test (Complement fixation test).
Screening and Confirmatory Testing
Rose Bengal Plate Test (RBPT)
The collected serum samples were screened for antibodies to brucellosis using the Rose Bengal plate test following the OIE protocol.21 The sera and antigen were removed from the refrigerator 30 min before the test and allowed to reach room temperature. Equal volumes of test sera were added to 30 µl of RBPT antigen. The mixture was thoroughly mixed, shaken for 4 min and finally agglutination was observed for positive sera. In the event of ambiguous results, a repeat test was conducted. The complement fixation test was performed on all positive RBPT reactor sera to confirm the diagnosis.
Complement Fixation Test (CFT)
RBPT positive samples were confirmed with a complement fixation test using the OIE protocol22 at the National Veterinary Institute (NVI) in Bishoftu, Ethiopia. Standardizing the antigen was done with a dilution (strength) of 1:20. Brucella antigen, complement, and 3% sensitized sheep red blood cells are added to serially diluted test sera on microtitre plates after serial dilution. The plates were incubated at 37°C for 30 min. At 1:10 dilution, positive results are considered for partial fixation (50% hemolysis) or complete fixation (no hemolysis). The negative control serum exhibited complete hemolysis, while the positive control serum exhibited inhibition of hemolysis.
Case Definition
Samples positive for the Rose Bengal plate test and Complement fixation test were considered positive for brucellosis. Because there had been no history of brucellosis vaccination in Ethiopia over the past few years, the observed seropositive animals were considered to be natural infections.
Associated Risk Factors
During the study period, age, breed, parity, and pregnancy status of study animals were considered intrinsic risk factors for bovine brucellosis, but management system, herd composition, herd size, history of previous abortions, history of previous retained fetal membranes, breeding practices, and source of replacement stock were considered as extrinsic risk factors. A data sheet was prepared for each animal containing this information, and individual animal biodata and management practices were recorded in line with blood sample collection from respective animals. Herd sizes can be divided into three categories: small herds under 10, medium herds between 10 and 50 animals, and large herds over 50 animals.4
Statistical Data Analysis
A Microsoft Excel spreadsheet for Windows 2010 (Microsoft Corporation) was used to record and code data generated by laboratory investigations and the questionnaire survey and STATA version 14.0 was employed to analyze the data (Stata Corporation, College Station, TX, USA). The survey results and the proportion of bovine brucellosis-related risk factors were presented using descriptive statistics. A seroprevalence calculation was conducted by dividing the number of seropositive samples by the total number of samples. Univariate and multivariate logistic regression analyses were used to show the degree of association between those risk factors and the disease (brucellosis). An odds ratio (OR) was calculated to measure the relationship between risk factors and brucellosis. Analysis output was interpreted as statistically significant (p≤ 0.05) at 95% confidence level and 5% precision.
Results
Knowledge and Practice of Smallholder Farmers About Brucellosis
Approximately, 95% (225/236) and 97% (97/236) of small holder farmers surveyed did not know the cause and symptoms of bovine brucellosis, respectively. In this survey, 70% of respondents (166/236) reported poor management of their farming operations. As a result of lack of knowledge about the disease, a comparable number of smallholder farmers introduce naive animals without further investigation. It was reported that 77% of respondents disposed of dead fetuses in open dumps in the setting, and 23% burr aborted material immediately after release from dairy animals. Most smallholder farmers performed assisted parturition without using safety materials. In addition, there were limited provisions for separate rooms for delivery and aborted dairy animals. Furthermore, 86% of smallholder farmers consume raw milk without pasteurization or boiling (Table 1).
 |
Table 1 A Survey of Smallholder Farmers’ Knowledge and Practices Regarding Bovine Brucellosis |
Overall Seroprevalence of Bovine Brucellosis in Cross Breed Dairy Cattle
In the serological tests, 8 (2.08%) animals were found to be positive by RBPT, which were then subjected to CFT. On the basis of the confirmatory CFT test, the overall seroprevalence rate was 1.5% (95% CI: 0.70–3.44) at the individual animal level (Table 2).
 |
Table 2 An Overview of the Associated Risk Factors for Bovine Brucellosis |
Risk Factors Associated with Bovine Brucellosis Seropositivity
Out of nine potential risk factors, four were statistically significant (P≤0.05) by univariable logistic regression analysis and subjected to multivariable logistic regression.
The management system of the study population was categorized into two groups: extensive and semi-extensive. Higher seroprevalence of bovine brucellosis was seen in extensively managed animals (3.06%), followed by semi-extensively managed animals (1.05%). A multivariate logistic regression revealed that extensively managed dairy animals have a 68.7% (OR = 0.31, CI 0.05–1.82) lower probability of showing seropositivity than semi-extended managed dairy animals (Table 3).
 |
Table 3 Analysis of Risk Factors for Bovine Brucellosis Using Univariate and Multivariate Logistic Regression |
One of the other hypothesized extrinsic factor was the breeding practices of dairy animals, which were divided into two categories, natural and artificial mating. The prevalence of bovine brucellosis is higher on farms mating dairy animals through natural methods (3.05%) than on farms, which use artificial insemination (0.45%). An analysis of multivariable logistic regression revealed that the odds of being seropositive are 82% (OR = 0.18, CI 0.02–1.65) less likely in artificial insemination practice than in natural breeding practice.
An analysis of multivariable logistic regression was conducted to determine the association between seropositivity and hypothesized extrinsic risk factors like herd sizes and herd composition. Study dairy farms with less than 10 animals were 8.59 times more likely to be seropositive for bovine brucellosis than study dairy farms with between 10 and 50 animals (OR=8.59, CI 1.18–62.5). The highest seroprevalence (10.52%) was observed in small-sized herds followed by medium-sized herds (1.01%). Moreover, study dairy animals not mixed with other herds of animals in the home were 8.89 times more likely to be seropositive (OR=8.89 CI 0.08–93.3) for bovine brucellosis than herds composed of other animals (Table 3).
The Association Between Brucella Seropositivity and Abortion and Retained Fetal Membranes in Cattle
A one-sided Fisher’s exact test result revealed a positive and significant correlation between abortion and seropositivity for brucellosis (P<0.05). Conversely, occurrence of retained fetal membranes was not significantly (P>0.05) associated with seropositivity of bovine brucellosis (Table 4).
 |
Table 4 An Overview of the Association Between Seropositivity to Bovine Brucellosis and Abortion and Retained Fetal Membranes in Cattle |
Discussion
Farmers’ Knowledge and Practices Concerning Brucellosis
Smallholder dairy farmers can greatly reduce the number of zoonotic infections, including brucellosis, if they change their habits and practices. Most respondents (97%) were not aware of the disease bovine brucellosis based on the results of the present study. The findings are consistent with those from studies in Tajikistan conducted by Lindahl et al23 and Kenya conducted by Kang’ethe et al,24 in which most respondents had never heard of brucellosis. In contrast to this finding, the findings of Kansiime et al25 and Holt et al26 suggest that community members in Uganda and Egypt, respectively, are highly aware of bovine brucellosis. The low seroprevalence of bovine brucellosis in dairy cattle resulting in low awareness of smallholder farmers about brucellosis in the current study area compared to study conducted in Egypt and Uganda in endemic situation of brucellosis.
A number of high-risk behaviors were prevalent among smallholder farmers. Participating in animal parturition without personal protective materials, disposing of aborted fetuses in an open setting, and consuming raw milk are very common practices posing a high risk of contracting Brucella infection. The reason may be lack of understanding of the disease, the risks of transmission, and insufficient resources, which may be contributing factors. The results of the study are in agreement with the findings of Edao et al,27 who reported that most farm workers in the study areas handled parturition animals in an unhygienic manner, disposed of aborted fetuses in an open setting, and consumed raw milk frequently. Furthermore, similar results were reported by Holt et al.26
Seroprevalence and Associated Risk Factors of Bovine Brucellosis
The current study result revealed that the overall animal-level seroprevalence based on the confirmatory CFT test was 1.5% (95% CI: 0.70–3.44). This finding is in agreement with those of other studies in the country,3,28 whose overall prevalence was 1.5% and 2.2%, in Addis Ababa dairy farms, respectively. A similar study by Geresu et al29 reported 1.4% in Asella and Bishoftu towns using the card test (CT), RBPT, indirect Enzyme-Linked Immuno Sorbant Assay (i-ELISA), and Complement Fixation Test (CFT). Furthermore, comparable overall seroprevalence reportspresented in the Jigjiga zone of Somali region (1.92%),30 Sidama zone (1.92%),31 South eastern Ethiopia (3.5%),17 Guto Gida district of East Wollega zone (1.97%), Central Oromia (2.9%),32 Jimma (0.77%),15 Tigray region (3.19%),10 Tigray region (1.2%),16 and Jimma (0.61%)33 have been documented. Elemo and Geresu,34 on the other hand, observed 4.95% seroprevalence levels in smallholder farms in Agarfa and Berbere districts of the Bale zone using RBPT and CFT tests. Furthermore, the seroprevalence rate was reported to be 11.0% by Kebede et al,35 in Wuchale Jida district, 4.9% by Alehegn et al36 in and around Gondar Town, and 13.14% by Desalegn et al37 in Assela government dairy farms and 4.6% by Hailemelekot et al38 in selected Ethiopian sites. A lower sero prevalence of bovine brucellosis may be attributed to semi-intensive milking of dairy cows, which limits the exposure of dairy cows to disease risks.
Only herd sizes had statistically significant associations with brucellosis occurrence among the associated factors considered in the current study. Other extrinsic risk factors subjected to multivariate logistic regression including management system, breeding practice, and herd composition had no statistically significant association with the disease. It is in agreement with Mussie's39 research concerning the effects of age, pregnancy status, stage of pregnancy, parity number, and management system on mortality in extensive systems in northwest Ethiopia. A different finding was reported by Berhe et al,10 who reported a higher sero-prevalence in transhumance systems compared to sedentary systems.
The present study found that seroprevalence varied statistically significantly from farm to farm with different herd sizes. These findings are consistent with those reported by Berhe et al10 and Ahmad et al,40 but differ from those reported by Kebede et al.35 The significant statistical variation among herd sizes was attributed to the fact that maintaining a hygienic condition of the farm becomes more difficult to handle efficiently by smallholder farmers as the stocking density of the farm increases.
An analysis of one-sided Fisher’s exact test results showed that abortion likelihood was positively and significantly correlated with brucellosis seropositivity (P<0.05). Nevertheless, bovine brucellosis seroprevalence was not significantly related to retained fetal membranes (P>0.05). This finding is consistent with Kebede et al35 regarding retained fetal membranes, but differs in terms of abortion’s effect. More likely, Ahmed et al,40 in Jordan and Muma et al41 in Zambia both found that abortion was associated with brucellosis seroprevalence. A possible reason is that abortion is often associated with Brucella infections.42
Conclusion and Recommendations
The results of a survey showed that most surveyed small holders dairy farmers did not know the cause of bovine brucellosis and were unaware of its symptoms. Smallholder farmers have limited knowledge of bovine brucellosis and have engaged in risky practices. The study results also revealed that the introduction of naive cattle to their farm without further investigation was common. A low level of individual animal-level seroprevalence of bovine brucellosis was found in the study area. Only one of the four risk factors associated with the disease in univariable logistic regression has become significantly associated with it in multivariable models. Multivariable logistic regression analysis revealed that herd size was a statistically significant risk factor. A small herd has a higher seropositivity to the disease, followed by a medium herd. Therefore, creating awareness about the disease is of paramount importance to mitigate its growing trend even if the prevalence was low in the current study. Testing new stock before introduction to farms and implementing a test and slaughter program before the disease spreads and becomes more prevalent are recommended steps. Moreover, the smallholder dairy farmers maintain the hygienic condition of the farm standard level and if not reduce the stock, which makes the dairy animals risk for the occurrence of bovine brucellosis.
Data Sharing Statement
On the author’s request, all information is available.
Ethical Approval and Consent to Participate
The study was carried out in accordance with animal welfare guidelines, and ethical approval was obtained from Arba Minch University's Ethical Review Committee (Certificate Ref. No.: AMU/AREC/8/2015). Also, the informed verbal consent was obtained from cattle owners to include their animals in the study. However, informed written consent was waived by the ethics committee as the majority of livestock owners could not read or write.
Acknowledgments
Smallholder farmers who took the time to respond to the survey and field veterinarians who actively participated during the study were acknowledged by the authors.
Author Contributions
All the stated authors have made a significant contribution to conception, study design, execution, and acquisition of data, analysis and interpretation. Again, all the authors took part in drafting, revising, or critically reviewing the article together with giving final approval of the version to be submitted to the agreed journal. Moreover, they all agreed to be accountable for all aspects of the work.
Funding
Arba Minch University provided funding for this research project.
Disclosure
The authors confirm that they have no competing interests.
References
1. CSA. Central Statistical Agency. Report on livestock and livestock characteristics. Stat Bulettin. 2018;II:587.
2. Bekele A, Molla B, Asfaw Y, Yigezu I. Bovine brucellosis I ranches and farms in south Eastern Ethiopia. Bull Anim Hlth Prod Afr. 2000;48:13–17.
3. McDermott JJ, Arimi SM. Brucellosis in Sub Saharan Africa: epidemiology, control and impact. Vet Microbiol. 2002;90:111–134. doi:10.1016/S0378-1135(02)00249-3
4. Megersa M, Feyissa A, Wondimu A, Jibat T. Herd composition and characteristics of dairy production in Bishoftu Town, Ethiopia. J Agric Extension Rural Dev. 2011;3(6):113–117.
5. Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Veterinary medicine. In: A Text Book of Disease of Cattle, Sheep, Pigs, Goats and Horses.
6. Acha N, Szyfres B. Zoonoses and communicable diseases common to man and animals: bacteriosis and mycosis. In: Scientific and Technical Publication No. 580. Washington DC: Pan American Health Organization, American Sanitary Bureau, Regional Office of the World Health Organization; 2001:40–62.
7. FAO. Food and Agricultural Organization of the United Nations. Guidelines for coordinated human and animal brucellosis surveillance. FAO Anim Prod Hlth. 2003;45:87.
8. Makita KE, Fevre M, Waiswa C, et al. Human brucellosis in urban and per-urban areas of Kampala, Uganda. Animal biodiversity and emerging diseases. Ann NY Acad Sci. 2008;1149:309–311. doi:10.1196/annals.1428.015
9. Radostits E, Gay CC, Hinchcliff KW, Constable PD. Disease caused by Brucella spp in veterinary medicine. In: Text Book of the Disease of Cattle, Sheep, Pigs, Goats and Horses.
10. Berhe G, Belihu K, Asfaw Y. Seroepidemiological investigation of bovine brucellosis in the extensive cattle production system of Tigray Region of Ethiopia. Int J Appl Res Vet Med. 2007;5:65–71.
11. Abeje S. Sero-Epidemiological Study of Bovine Brucellosis in and Around Bahir Dar [DVM Thesis]. Addis Ababa University; 1994.
12. Yilkal AB, Zessin KH, Azage T. A cross sectional study of bovine brucellosis and test performance in intra and peri urban production systems in and around Addis Ababa, Ethiopia. Bull Anim Hlth Prod Afr. 1998;46:217–224.
13. Fikadu K. An Epidemiological Survey of Bovine Brucellosis in the Eastern Amhara Regional State. Kombolcha Ethiopia: Kombolcha regional veterinary laboratory; 1999.
14. Alem W, Solomon G. A retrospective sero-epidemiology study of bovine brucellosis in different production systems in Ethiopia.
15. Tolosa TR, Belihu K, Belihu K. Seroprevalence study of bovine brucellosis in extensive management system in selected sites of Jimma zone, western Ethiopia. Bull Anim Health Prod Afri. 2008;56:25–37. doi:10.4314/bahpa.v56i1.32823
16. Haileselassie M, Shewit K, Moses K. Serological survey of bovine brucellosis in barka and arado breeds (Bos indicus) of Western Tigray, Ethiopia. Pak Vet J. 2010;94:28–35.
17. Megersa B, Biffa D, Niguse F, Rufael T, Asmare K, Skjerve E. Cattle brucellosis in traditional livestock husbandry practice in Southern and Eastern Ethiopia, and its zoonotic implication. Acta Vet Scand. 2011;53:24. doi:10.1186/1751-0147-53-24
18. Chencha District Livestock and Fishery Resource Office (CDLFRO). Report from Chencha District Livestock and Fishery Resource Office, Gamo Zone, SNNPR (Unpublished report). 2020.
19. Bonke District Livestock and Fishery Resource Office (BDLFRO). Report from Arba Minch Zuria District Livestock and Fishery Resource Office, Gamo Zone, SNNPR (Unpublished report). 2020.
20. Thrusfield MV. Epidemiology.
21. OIE. World Organization for Animal Health. Bovine brucellosis. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE Terrestrial Manual; 2012.
22. OIE. World Organization for Animal Health. Ovine and caprine brucellosis. In: Proceedings of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE Terrestrial Manual; 2009.
23. Lindahl E, Sattorov N, Boqvist S, Magnusson UA. Study of knowledge, attitudes and practices relating to brucellosis among small-scale dairy farmers in an urban and Peri- urban area of Tajikistan. PLoS One. 2015;10(2):e0117318. doi:10.1371/journal.pone.0117318
24. Kang’ethe EK, Ekuttan CE, Kimani VN, Kiragu MW. Investigations into the prevalence of bovine brucellosis and the risk factors that predispose humans to infection among urban dairy and non-dairy farming households in Dagoretti Division, Nairobi, Kenya. East Afr Med J. 2007;84:96–100.
25. Kansiime C, Mugisha A, Makumbi F, Mugisha S, Rwego IB. Knowledge and perceptions of brucellosis in the pastoral communities adjacent to Lake Mburo National Park, Uganda. BMC Public Health. 2014;14:242. doi:10.1186/1471-2458-14-242
26. Holt HR, Eltholth MM, Hegazy YM, El-Tras WF, Tayel AA, Guitian J. Brucella spp. infection in large ruminants in an endemic area of Egypt: cross-sectional study investigating seroprevalence, risk factors and livestock owner's knowledge, attitudes and practices (KAPs). BMC Public Health. 2011;11:341. doi:10.1186/1471-2458-11-341
27. Edao BM, Hailegebreal G, Berg S, et al. Brucellosis in the Addis Ababa dairy cattle; the myths and realities. BMC Vet Res. 2018;14:396. doi:10.1186/s12917-018-1709-4
28. Asmare K, Sibhat B, Molla B, et al. The status of bovine brucellosis in Ethiopia with special emphasis on exotic and cross bred cattle in dairy and breeding farms. Acta Trop. 2013;126:186–192. doi:10.1016/j.actatropica.2013.02.015
29. Geresu MA, Ameni G, Kassa T, Tuli G, Arenas A, Gezahegne MK. Seropositivity and risk factors for Brucella in dairy cows in Asella and Bishoftu towns, Oromia regional state, Ethiopia. Afr J Microbiol Res. 2016;10(7):203–213. doi:10.5897/AJMR2015.7707
30. Degefu H, Mohamud M, Hailemelekot M, Yohannes M. Sero prevalence of bovine brucellosis in agro pastoral areas of Jijiga zone of Somali National Regional State. Ethiop Vet J. 2011;15(1):37–47. doi:10.4314/evj.v15i1.67683
31. Asmare K, Asfaw Y, Gelaye E, Ayelet G. Brucellosis in extensive management system of zebu cattle in Sidama zone, southern Ethiopia. Afri J Agric Res. 2010;5:257–263.
32. Jergefa T, Kelay B, Bekana M, Teshale S, Gustafson H, Kindahl H. Epidemiological study of bovine brucellosis in three agroecological areas of central Oromiya, Ethiopia. Sci Tech Rev. 2009;28:933–943. doi:10.20506/rst.28.3.1939
33. Belihu K. Analysis of Dairy Cattle Breeding Practices in Selected Areas of Ethiopia [Ph.D. thesis]. Berlin Germany: Humboldt University; 2002.
34. Elemo KK, Geresu MA. Bovine brucellosis: seroprevalence and its associated risk factors in cattle from smallholder farms in Agarfa and Berbere districts of Bale Zone, South Eastern Ethiopia. J Anim Plant Sci. 2018;28(2):1–13.
35. Kebede T, Ejeta G, Ameni G. Seroprevalence of bovine brucellosis in smallholder farms in central Ethiopia (Wuchale-Jida district). Revue Méd Vét. 2008;159:3–9.
36. Alehegn E, Tesfaye S, Chane M. Seroprevalence of bovine brucellosis and its risk factors in cattle in and around Gondar Town, North West Gondar, Ethiopia. J Dairy Vet Anim Res. 2016;4(4):166.
37. Desalegn F, Berhe T, Gangwar SK. Seroprevalence study of bovine brucellosis in Assella government dairy farm of Oromia Regional State, Ethiopia. IJSN. 2011;2(3):692–697.
38. Hailemelekot M, Kassa T, Tefera M, Belihu K, Asfaw Y, Ali A. Seroprevalence of brucellosis in cattle and occupationally related humans in selected sites of Ethiopia. Ethiop Vet J. 2007;11:85–100.
39. Mussie H, Hailemelekot M. Seroprevalence Study of Brucellosis in Cattle and Human in Bahirdar Milkshed [Masters Thesis]; Debre Zeit, Ethiopia: FVM, AAU; 2005.
40. Ahmad MA, Adbelsalam QT, Mustafa MA, Mohammed MA. Seroprevalence and risk factors for bovine brucellosis in Jordan. J Vet Sci. 2009;10:61–65. doi:10.4142/jvs.2009.10.1.61
41. Muma JB, Samui KL, Siamudaala VM, et al. Prevalence of antibodies to Brucella spp. and individual risk factors of infection in traditional cattle, goats, sheep reared in livestock-wildlife interface areas of Zambia. Trop Anim Health Prod. 2006;38:195–206. doi:10.1007/s11250-006-4320-9
42. Schelling E, Diguimbaye C, Daoud S, et al. Brucellosis and Q fever seroprevalence of nomadic pastoralists and their livestock in Chad. Prev Vet Med. 2003;61:279–293. doi:10.1016/j.prevetmed.2003.08.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.