Back to Journals » Journal of Pain Research » Volume 12
Desumoylation of aggrecan and collagen II facilitates degradation via aggrecanases in IL-1β-mediated osteoarthritis
Authors Zhu B, Cui G, Zhang Q, Cheng X, Tang S
Received 12 November 2018
Accepted for publication 1 April 2019
Published 12 July 2019 Volume 2019:12 Pages 2145—2153
DOI https://doi.org/10.2147/JPR.S194306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Bing Zhu,1 Guanxing Cui,1 Qifu Zhang,2 Xiankui Cheng,3 Shusen Tang1
1Department of Joint Surgery, The Affiliated Hospital of Weifang Medical University, Weifang, Shandong, People’s Republic of China; 2Department of Cancer Radiotherapy, The Affiliated Hospital of Weifang Medical University, Weifang, Shandong, People’s Republic of China; 3Department of Pathology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong, People’s Republic of China
Background: Aggrecan plays a crucial role in the ability of tissues to withstand compressive loads during the pathological progression of osteoarthritis (OA). Progressive loss of aggrecan from cartilage may result in exposure of the collagen matrix and can lead to its disintegration by metalloproteases. Although aggrecanases are expressed constitutively in human chondrocytes, the degradation of aggrecan is induced by proinflammatory cytokines; however, little is known about the underlying mechanisms.
Methods: Human primary chondrocytes from OA patients or healthy donors and human chondrogenic SW1353 cells were cultured and stimulated with IL-1β in vitro, the mRNA expressions and protein levels of MMP-13, ADAMTS-4, ADAMTS-5, SENP1, and SENP2 were determined using real time PCR and Western blot, respectively. The localizations of aggrecan and Col-II, as well as the SUMOylation modification of these proteins were analyzed using immunofluorescence and immunoprecipitation assays, respectively.
Results: Our results showed that a proinflammatory cytokine interleukin-1β induced the OA model and desumoylation of aggrecan and collagen type II because the small ubiquitin-like modifier 2/3 (SUMO2/3) was co-localized with aggrecan and collagen type II proteins and interacted physically with them. Mechanistic studies have shown that knockdown of SUMO2/3 expression can significantly enhance the rate of degradation of aggrecan and collagen type II at both the mRNA and protein levels in the OA model. In addition, SUMO-specific protease 2 (SENP2) plays important roles in the desumoylation of aggrecan, while knockdown of SENP2 can protect aggrecan and collagen type II. Clinical assays have shown that OA patients have higher SENP2 levels than healthy controls, and the SENP2 level correlates negatively with both aggrecan and collagen type II levels.
Conclusion: SENP2 desumoylates aggrecan and collagen type II proteins in the inflammation induced OA, and SENP2 expression correlates with OA progression.
Keywords: aggrecan, collagen type II, sumoylation, SENP2, osteoarthritis
Introduction
Osteoarthritis (OA) is a type of joint disease that features nonspecific synovial inflammation, articular cartilage degeneration, and subchondral bone abnormalities.1 It is the most common type of arthritis and affects millions of people worldwide. OA has a multifactorial etiology, which is the result of interactions between systemic and local factors. A better understanding of the mechanism underlying OA pathogenesis would enable the development of effective therapeutic methods.
An important manifestation of OA is the enhanced production of matrix-degrading enzymes matrix metalloproteinase-13 (MMP-13)2 and the reduced synthesis of aggrecan and collagen type II.3 As the major proteoglycan of articular cartilage, aggrecan enables tissue to withstand the compressive loads that confront them throughout life.4 This function is intimately related to the structure of the protein, particularly to its high level of substitution with sulfated glycosaminoglycan chains and its capacity to generate large molecular aggregates in conjunction with hyaluronan.5 Aggrecan is quite frequently modified with keratan sulfates and glycosaminoglycan chondroitin sulfates, which provide strong negative charges that enable water to be trapped in cartilage as opposed to repel when the cartilage is compressed, and thus provide cartilage with its shock absorption property. The primary histological symptom of cartilage damage in arthritis is loss of aggrecan, which results in the collagenous mesh being susceptible to mechanical abrasion and enzymatic assault.6 OA is the outcome of the progressive loss of aggrecan from cartilage, which results in both exposure of the collagen matrix and its disintegration by metalloproteases.7 Among the subgroup of aggrecanases, ADAMTS-4 and −5 act as pivotal roles in OA; therefore, they have emerged as therapeutic targets for arthritis. They are constitutively expressed in human chondrocytes, and their expression could also be induced by proinflammatory cytokines.8 It is not yet clear why the constitutively expressed aggrecanases do not lead to loss of aggrecan; we hypothesized that aggrecan might have some protective measure against the catalytic effects of aggrecanases.
Post-translational modification by the bonding of small ubiquitin-like modifier (SUMO) peptides impacts various cellular and subcellular functions, including cell survival and death, protein localization, protein stability, and transcriptional regulation.9 There are four mammalian SUMO genes: SUMO-1, SUMO-2, SUMO-3, and SUMO-4. SUMO-2 and SUMO-3 share 96% of their amino acid sequences, and so are referred to as SUMO-2/3, while SUMO-1 is approximately 50% similar to SUMO-2 and SUMO-3. In 2013, Frank et al proved that SUMO-1 has a close relationship with the pathological mechanisms of rheumatoid arthritis and SUMO-2/3 is differentially modulated by tumor necrosis factor-α (TNF-α) and selectively regulates TNF-α-mediated MMP expression through the nuclear factor-κB (NF-κB) pathway.10 Sumoylation is modulated by SUMO-specific proteases (SENPs). There are six types of SENPs in mammalian cells (SENP1-3 and SENP5-7). Among them, SENP1 and SENP2 are closely connected and are involved in both SUMO maturation and deconjugation.11 Wang et al showed that SENP2 is a desumoylation protease that has a significant role in the modulation of transforming growth factor-β signaling.12 Based on these previous studies, we decided to investigate the relationship between the highly homologous SUMO family members and the progressive loss of aggrecan during OA progression, and whether SENPs are involved in OA.
Although the precise pathological mechanism of OA is not fully understood, inflammatory responses are currently thought to be involved in the development and progression of OA.13 Secreted inflammatory factors such as proinflammatory cytokines are significant mediators of the disturbed metabolism in OA.14 The catabolic effects of IL-1β are mainly modulated through the activation of several signaling pathways such as that of p38 mitogen-activated protein kinase and c-Jun N-terminal kinase, and the most significant regulation is the activation of the NF-κB signaling pathway.15 The NF-κB signaling pathway can not only regulate the expression of multiple inflammatory genes but also participate in the expression or activation of matrix-degrading enzymes in OA.15,16 However, it is unclear whether IL-1β-activated NF-κB regulates sumoylation in OA.
In this study, we explored the interaction between SUMO family members and aggrecan protein. In addition, the specific sites of sumoylation on aggrecan were predicted using GPS-SUMO webserver (http://sumosp.biocuckoo.org). The results depicted a SUMO2 conjugation–deconjugation loop of aggrecan and further assigned a modulatory function of SENP2 in the SUMO signaling pathway in IL-1β-induced inflammation in primary human chondrocytes.
Materials and methods
Patient samples
Human cartilage samples were collected from 10 OA patients undergoing total knee replacement surgery and 10 traumatic amputees without OA or rheumatoid arthritis. Patients with OA were diagnosed according to the American College of Rheumatology criteria. Samples were collected from the Department of Joint Surgery, the Affiliated Hospital of Weifang Medical University, and Department of Pathology, Shandong Provincial Hospital Affiliated to Shandong University. Written informed consent was obtained from all patients, and this was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the Affiliated Hospital of Weifang Medical University.
Cell culture
Tissues from 10 traumatic amputees without OA or rheumatoid arthritis were used for further cellular analysis. Briefly, cartilage tissues were cut into small pieces, then sequentially digested in 0.15% trypsin for 2 hrs at 37°C and incubated overnight in 3 mg/mL collagenase type I at 37°C. After centrifugation, the cells were suspended in DMEM/Ham’s F-12 (Gibco, Grand Island, NY, USA) containing 10% fetal calf serum (Gibco), 2 mM L-glutamine, and 1% penicillin/streptomycin (Gibco). Cells were seeded at a density of 2×104 cells/cm2. Culture medium was changed every 3–4 days. Confluent primary chondrocytes were treated with 10 ng/mL recombinant human IL-1β (PeproTech, Rocky Hill, NJ, USA) for 12 hrs.
Human chondrogenic SW1353 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in Leibovitz’s L-15 Medium (Gibco) containing 10% FBS (Gibco) and 1% penicillin/streptomycin (Gibco) and used for a luciferase assay. Cells were incubated at 37°C in a humidified atmosphere of 100% air, and the medium was replaced every 2 or 3 days. After 24 hrs of serum starvation, cells were treated with 10 ng/ml IL-1β for 12 hrs and harvested for the subsequent experiments.
RNA extraction and quantitative reverse transcription polymerase chain reaction analysis
Total RNA was extracted from each cartilage sample and cell cultures using TRIzol (Invitrogen, Carlsbad, CA, USA) as per the manufacturer’s protocol. RNA quantity and quality were measured using a NanoDrop ND-1000 spectrophotometer. Total RNA was reverse transcribed to cDNA using Super-ScriptTM III Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions. qRT-PCR was performed using 2×SYBR Green PCR Master Mix (Arraystar, Rockville, MD, USA) on an Applied BiosystemsViiATM 7 Real-time PCR System. The qRT-PCR reaction conditions were as follows: denaturation at 95°C for 10 mins, followed by 40 cycles at 95°C for 10 s and 60°C for 60 s. The results were normalized to U6 or GAPDH expression to obtain △Ct values. Fold changes in expression were calculated using the 2−△△Ct method. All experiments were performed in triplicate.
Gene expression of ACANI (aggrecan), COL2A1 (collagen type II), MMP-13, ADAMTS-4, and ADAMTS-5 was detected and GAPDH was employed as an internal control. The following primers were used: MMP-13, 5ʹ-TGATGACATCAAGAAGGTGGTGAAG-3ʹ (forward), 5ʹ-TCCTTGGAGGCCATGTGGGCCAT-3ʹ (reverse); ACANI (aggrecan) 5ʹ-TGAGCGGCAGCACTTTGAC-3ʹ (forward), 5ʹ-TGAGTACAGGAGGCTTGAGG-3ʹ (reverse); COL2A1 (collagen type II) 5ʹ-GAACTGGTGGAGCAGCAAGA-3ʹ (forward), 5ʹ-AGCAGGCGTAGGAAGGTCAT-3ʹ (reverse);17 ADAMTS-4 5ʹ-GAGGAGGAGATCGTGTTTCCA-3ʹ (forward), 5ʹ-CCAGCTCTAGTAGCAGCGTC-3ʹ (reverse); ADAMTS-5 5ʹ-GAACATCGACCAACTCTACTCCG-3ʹ (forward), 5ʹ-CAATGCCCACCGAACCATCT-3ʹ (reverse); SENP1 5ʹ-AGTGAACCACAACTCCGTATTC-3ʹ (forward), 5ʹ-AAAAGATCGGTCCAAATGTCCTT-3ʹ (reverse); SENP2 5ʹ-GGCTGGTTAGGATTCTCGGC-3ʹ (forward), 5ʹ-GGCAGCATTGTAGAGACTGTTTT-3ʹ (reverse); GAPDH 5ʹ-CTGGGCTACACTGAGCACC-3ʹ (forward), 5ʹ-AAGTGGTCGTTGAGGGCAATG-3ʹ (reverse).
Western blotting
Cellular protein from cells or tissue prepared using buffer containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) was separated by SDS-PAGE following standard protocols (Amersham BioSciences, Piscataway, NJ, USA). The antibodies used for Western blotting were as follows: aggrecan (ab3778; Abcam, Cambridge, UK), collagen type II (ab3778; Abcam), MMP-13 (sc-515,284; Santa Cruz Biotechnology, Dallas, TX, USA), ADAMTS-4 (MAB4307; R&D Systems, Minneapolis, MN, USA), ADAMTS-5 (MAB2198; R&D Systems), and GAPDH (sc-166,574; Santa Cruz). An enhanced chemiluminescence system (Amersham BioSciences) was used for detection. Blots were quantified using the ImageJ densitometry program (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence assay
The expression levels of SUMO2/3, aggrecan, and collagen type II in primary human chondrocytes were analyzed by immunofluorescence microscopy. After washing three times with PBS, cells were fixed with 4% formaldehyde for 10 mins at room temperature, followed by blocking with 2.5% BSA for 1 hr at room temperature. Cells were subsequently incubated with anti-SUMO 2/3 (1:500; ab3742; Abcam), anti-aggrecan antibody (1:500; ab3778; Abcam), and anti-collagen type II antibody (1:500; ab3778; Abcam) overnight at 4°C in DPBS containing 2.5% BSA and 0.1% Tween 20. Samples were then washed three times with Dulbecco's Phosphate-Buffered Saline (DPBS), followed by probing with Fluorescein isothiocyanate (FITC) or Tetramethylrhodamine (TRITC) conjugate secondary antibody (1:1000; Thermo Fisher Scientific, Waltham, MA, USA) at room temperature for 1 hr. Thereafter, cells were counterstained with DAPI and analyzed using a fluorescence microscope.
Luciferase reporter assay
SW1353 cells were transfected with wild-type or mutant reporter plasmids (Stratagene, San Diego, CA, USA) and pRL-TK control reporter vector (Promega, Madison, WI, USA) using Lipofectamine 2000 (Invitrogen). The pSENP2-Luc plasmid contains a firefly luciferase reporter gene that was derived from the promoter or SENP2. The pRL-TK plasmid (Promega), which expresses Renilla luciferase, was used for the normalization of transfection efficiency. After 12 hrs of stimulation with IL-1β, the cells were harvested and their luciferase activities were measured using a dual luciferase reporter assay system (Promega). All experiments were performed in triplicate.
Statistical analysis
SPSS 13.0 software (SPSS Inc., Chicago, IL, USA) was applied for the statistical analysis. Data are shown as the mean ± SE of the mean. A parametric (Student's t-test) or nonparametric (Mann–Whitney) test was performed according to data distribution and the number of groups. Correlation between the expression levels of SENP-2 and ACAN or COL2A1 was analyzed using the Pearson correlation method in GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA), and values from ELISA data were compared by paired Student′s t-test. *p<0.05 and **p<0.01 were regarded as statistically significant unless otherwise stated.
Results
Aggrecan and collagen type II were downregulated by sumoylation modification in IL-1β-treated primary chondrocytes
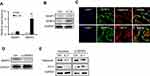
The degradation of the cartilage matrix could be induced by IL-1β and was mediated by several catabolic enzymes such as MMPs and aggrecanases. Therefore, we explored the effects of IL-1β on the expression of aggrecan, collagen type II, aggrecanases, ADAMTS-4, and ADAMTS-5. Based on previous studies, the expression of MMP-13 was selected as the biomarker, which has recently been proven to be strongly upregulated in IL-1β-induced cartilage matrix degradation.18 As expected, IL-1β treatment significantly increased the MMP13 protein levels and decreased the collagen type II and aggrecan levels (Figure 1A). Moreover, the expression of ADAMTS-4 was significantly increased, while that of ADAMTS-5 barely changed (Figure 1B). Through real-time PCR, we confirmed that the mRNAs of ADAMTS-4 and MMP-13 were all significantly upregulated (Figure 1C), while the mRNAs of aggrecan and collagen type II were not altered (Figure 1D). Therefore, we presume that the aggrecan and collagen type II proteins were modified for degradation.
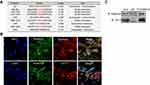
Recent research showed that sumoylation plays a significant role in protein degradation. We first predicted the possible sumoylation modification amino acid residues of aggrecan protein using the GPS-SUMO webserver, and the results showed that multiple lysine residues and peptides could be sumoylated potentially (Figure 2A). Immunofluorescence and immunoprecipitation assays were employed to explore whether sumoylation occurs during cartilage matrix degradation. We found that SUMO2/3 is located with aggrecan at the subcellular level (Figure 2B) and interacts with aggrecan. A similar association was also observed between SUMO2/3 and collagen type II (Figure 2C). These results showed that the sumoylation of collagen type II and aggrecan occur in normal primary chondrocytes.
SENP2 participates in the desumoylation of aggrecan and collagen type II
We investigated the expression levels of SENP1 and SENP2 in primary chondrocytes. The results indicated that SENP2 was significantly upregulated at both the mRNA and protein levels after treatment with IL-1β, whereas SENP1 expression barely changed (Figure 3A, B). The immunofluorescence assay revealed that SENP2 protein also co-localized with both aggrecan and CO-II proteins (Figure 3C). These data implied that SENP2-mediated desumoylation might be involved in the regulation of aggrecans and collagen type II protein sumoylation.
We performed an in vitro small interfering RNA (siRNA) assay to directly analyze whether SENP2 can catalyze the desumoylation of aggrecan and collagen type II proteins. We found that when chondrocytes were treated with SENP2 siRNA, the protein expression levels of the chondrocytes were significantly downregulated compared with that of a scrambled nucleotide (Figure 3D). When cells were transfected with SENP2 siRNA and treated with IL-1β, the decreased expression of aggrecan and collagen type II was reversed even to normal levels (Figure 3E), which indicated that the degradation of the cartilage matrix induced by IL-1β was attenuated.
Il-1β-induced NF-κB activation promoted transcriptional SENP2 expression
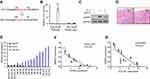
Because previous studies have shown that IL-1β could activate NF-κB signaling, we next explored whether NF-κB is involved in the regulation of SENP2 expression. First, bioinformatics analysis showed that one NF-κB-binding site is situated at the −64 to −55 regions on the SENP2 promoter. To confirm whether this binding site is involved in IL-1β-induced cartilage matrix degradation, the luciferase reporter plasmid, which contained both the wild and SENP2 mutant types, was constructed (Figure 4A). The luciferase reporter plasmid was transfected to SW1353 cells, a human chondrogenic cell line, and then treated with IL-1β. We found that luciferase activity was significantly enhanced in the wild-type group, while it barely changed in the mutant group (Figure 4B). To further confirm the function of NF-κB, we treated normal primary chondrocytes with the specific NF-κB inhibitor BAY11-7082. The expression of SENP2 in the normal primary chondrocytes was significantly downregulated when the cells were stimulated with IL-1β (Figure 4C). In conclusion, these results indicated that IL-1β promotes SENP2 expression through NF-κB.
Finally, an in vivo test was employed to further investigate whether desumoylation of aggrecan and collagen type II was present in the patients’ samples. The tissue samples stained with H&E to confirm OA among the patients (Figure 4D). The mRNA levels of ACAN, COAL2A1, and SENP2 were examined using real-time PCR (qPCR). We found that the expression of SENP2 was relatively high compared with healthy controls in 12 of the OA tissue samples, but the expression of both aggrecan and collagen type II was low (Figure 4E). When we analyzed the correlations between SENP2 and ACAN or COAL2A1, we found negative correlations between the groups (Figure 4F, G).
Discussion
Cartilage is unique due to its limited ability to regenerate and its avascular nature. It is composed of mature chondrocytes within a highly specialized matrix comprised of glycosaminoglycans and enables frictionless motion in the joints.19 Aggrecan is a high-molecular-weight proteoglycan that has a pivotal role in the structure of cartilage and functions of the joints. In addition, it enables cartilage and intervertebral discs to resist compressive loads. Aggrecan degradation in articular cartilage is an important event in early-stage OA.20 The major structure of the extracellular matrix is composed of aggrecan and collagen type II, which can preserve the normal physiological functions of cartilage. Loss of aggrecan and collagen type II contributes to accelerate the progression of OA. Thus, inhibition of the degradation of aggrecan and collagen type II may represent a novel therapeutic treatment for OA.21 The process that leads to clinical OA is considered to be triggered by different forms of trauma that result in inflammation and subsequently the release of enzymes and inflammatory mediators, which subsequently degrade the matrix, into the articular space.22 The specific pathway that leads to aggrecan degradation is not clear, although we believe that post-transcriptional modification is involved in this process.
As members of a subfamily of the ubiquitin-like proteins, SUMOs perform functions for the posttranslational modification of proteins, which represents actions involved in a variety of cellular and subcellular processes, including cell survival and death,10,23 protein localization,24 transcriptional regulation,25 and protein stability. Previous research has shown that the universal increase of SUMO-1 and SUMO-2/3 promotes the capacity of neurocytes to tolerate ischemic stress.26 Analogous increases of SUMO molecules and protective influences have been confirmed in salivary epithelial cells27 and cardiac tissues28 exposed to hypoxia.
Sumoylation participates in the progression of OA from several different angles. Wang et al found that sumoylation pathways are not only expressed in intervertebral disc cells but are also localized in nuclei. Both nucleus pulposus and annulus fibrosus cells are viable under hypoxia and upmodulate the expression of SENP1.29 Frank et al showed that the knockdown of SUMO-2/3 remarkably increases expression levels of MMP-3 and MMP-13 induced by IL-1β and TNF-α and is accompanied by increased NF-κB activity. The induction of MMP-3 and MMP-13 is inhibited by blocking the NF-κB pathway.10 Im et al reported that, besides the phosphorylation of Elk-1, dynamic posttranslational modification of Elk-1 by SUMO acts as an important mechanism for the modulation of MMP-13 gene expression. Furthermore, bFGF activates Elk-1 primarily through the ERK pathway and the increased phosphorylation of Elk-1 is accompanied by decreased conjugation of SUMO to Elk-1.30 Thus, we hypothesize that sumoylation might occur during the degradation of aggrecan and collagen type II.
Hundreds of studies have shown that aggrecan and collagen type II are degraded during the progression of OA; however, the detailed molecular mechanisms have not been clarified. For example, Bao et al evaluated the expression levels of chondrocyte-specific proteins (aggrecan and collagen type II) by qRT-PCR and Western blotting. In addition, the key protein levels of Hedgehog pathways and Wnt/β-catenin were also detected. The data showed that Hedgehog and Wnt/β-catenin pathways participate in the inflammatory effects of IL-18 in vitro and in the degradation of aggrecan and collagen type II.31 Zheng et al discovered that silibinin significantly inhibits IL-1β-induced degradation of collagen type II and aggrecan in human OA chondrocytes via experiments in mouse OA models. They found that treatment with silibinin can prevent the destruction of cartilage and the thickening of subchondral bone while it also relieves synovitis in vivo. Moreover, silibinin blocks IL-1β-induced NF-κB activation and the phosphorylation of PI3K/AKT.21 In this study, we revealed that IL-1β treatment remarkably decreased the expression of collagen type II and aggrecan at both the mRNA and protein levels. A bioinformatics prediction was employed to look for the potential sumoylation sites on aggrecan and collagen type II proteins, and we found that there are several sumoylation sites. Through co-immunoprecipitation and immunofluorescence assays, we found that the sumoylation of aggrecan and collagen type II occurred in normal primary chondrocytes; however, the protective function of sumoylation was weakened during the OA process. Thus, the roles of sumoylation/desumoylation pathways must be investigated during the progression of OA.
Wang et al showed that hypoxia in nucleus pulposus cells transiently upregulated the expression of SUMO-1, SUMO-2/3, SAE2, and UBC9, whereas SUMO-1 was elevated, and that SUMO-2/3, SAE1, SAE2, and UBC9 were attenuated by low oxygen in annulus fibrosus cells. Although downmodulation of SENP1 reduces the transcriptional activity of hypoxia-inducible factor-1α, the viability of intervertebral disc cells is not significantly lower under hypoxia conditions.29 Studies on SENP mutant mice revealed that SENP1 acts to desumoylate primarily SUMO1-conjugated proteins, while SENP2 preferentially de-conjugates SUMO2/3-conjugated proteins. This in vivo information is crucial when targeting SENPs for drug discovery, since the compounds identified will eventually be tested in animals.32 In our study, we found that SENP2-mediated desumoylation was involved in regulating the sumoylation of collagen type II and aggrecan proteins during OA progression. In addition, we found that knockdown of SENP2 attenuates the degradation of collagen type II and aggrecan in an IL-1β-mediated OA model. Moreover, we found that SENP2 expression correlated negatively with aggrecan and collagen type II expression in vivo and that desumoylation of collagen type II and aggrecan occurred in vivo probably via SENP2 regulation; thus, our research provides new insights into the mechanism of OA.
Acknowledgment
This study was supported by the Shandong Provincial Health and Family Planning Commission Project #2017WS776.
Disclosure
All authors declare no conflicts of interest in this work.
References
1. Zhang PL, Liu J, Xu L, Sun Y, Sun XC. Synovial fluid macrophage migration inhibitory factor levels correlate with severity of self-reported pain in knee osteoarthritis patients. Med Sci Monit. 2016;22:2182–2186.
2. Takahashi A, de Andres MC, Hashimoto K, et al. DNA methylation of the RUNX2 P1 promoter mediates MMP13 transcription in chondrocytes. Sci Rep. 2017;7(1):7771. doi:10.1038/s41598-017-08418-8
3. Huang K, Wu LD. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J Int Med Res. 2008;36(6):1149–1160. doi:10.1177/147323000803600601
4. Mort JS, Geng Y, Fisher WD, Roughley PJ. Aggrecan heterogeneity in articular cartilage from patients with osteoarthritis. BMC Musculoskelet Disord. 2016;17:89. doi:10.1186/s12891-016-1134-4
5. Roughley PJ, Mort JS. The role of aggrecan in normal and osteoarthritic cartilage. J Exp Orthop. 2014;1(1):8. doi:10.1186/s40634-014-0008-7
6. Ismail HM, Miotla-Zarebska J, Troeberg L, et al. Brief report: JNK-2 controls aggrecan degradation in murine articular cartilage and the development of experimental osteoarthritis. Arthritis Rheumatol. 2016;68(5):1165–1171. doi:10.1002/art.39547
7. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi:10.1002/art.34453
8. Ismail HM, Yamamoto K, Vincent TL, Nagase H, Troeberg L, Saklatvala J. Interleukin-1 acts via the JNK-2 signaling pathway to induce aggrecan degradation by human chondrocytes. Arthritis Rheumatol. 2015;67(7):1826–1836. doi:10.1002/art.39099
9. Wiechmann S, Gartner A, Kniss A, et al. Site-specific inhibition of the SUMO-conjugating enzyme Ubc9 selectively impairs SUMO chain formation. J Biol Chem. 2017. doi:10.1074/jbc.M117.794255
10. Frank S, Peters MA, Wehmeyer C, et al. Regulation of matrixmetalloproteinase-3 and matrixmetalloproteinase-13 by SUMO-2/3 through the transcription factor NF-kappaB. Ann Rheum Dis. 2013;72(11):1874–1881. doi:10.1136/annrheumdis-2012-202080
11. Jung HS, Kang YM, Park HS, et al. Senp2 expression was induced by chronic glucose stimulation in INS1 cells, and it was required for the associated induction of Ccnd1 and Mafa. Islets. 2016;8(6):207–216. doi:10.1080/19382014.2016.1235677
12. Wang R, Liu F, Zhao Y, et al. Reversible regulation of ORC2 SUMOylation by PIAS4 and SENP2. Oncotarget. 2017;8(41):70142–70155. doi:10.18632/oncotarget.19594
13. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16–21. doi:10.1016/j.joca.2012.11.012
14. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi:10.1038/nrrheum.2010.196
15. Wang Z, Huang J, Zhou S, et al. Anemonin attenuates osteoarthritis progression through inhibiting the activation of IL-1beta/NF-kappaB pathway. J Cell Mol Med. 2017; 21(12):3231–3243.
16. Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14(9):839–848. doi:10.1016/j.joca.2006.04.008
17. He J, Zhang J, Wang D. Down-regulation of microRNA-216b inhibits IL-1beta-induced chondrocyte injury by up-regulation of Smad3. Biosci Rep. 2017;37:2. doi:10.1042/BSR20160588
18. Morrovati F, Karimian Fathi N, Soleimani Rad J, Montaseri A. Mummy Prevents IL-1beta-induced inflammatory responses and cartilage matrix degradation via inhibition of NF-B subunits gene expression in pellet culture system. Adv Pharm Bull. 2018;8(2):283–289. doi:10.15171/apb.2018.033
19. Wang Z, Tran MC, Bhatia NJ, et al. Del1 knockout mice developed more severe osteoarthritis associated with increased susceptibility of chondrocytes to apoptosis. PLoS One. 2016;11(8):e0160684. doi:10.1371/journal.pone.0160684
20. Wu TJ, Fong YC, Lin CY, Huang YL, Tang CH. Glucose enhances aggrecan expression in chondrocytes via the PKCalpha/p38-miR141-3p signaling pathway. J Cell Physiol. 2018. doi:10.1002/jcp.26451
21. Zheng W, Feng Z, Lou Y, et al. Silibinin protects against osteoarthritis through inhibiting the inflammatory response and cartilage matrix degradation in vitro and in vivo. Oncotarget. 2017;8(59):99649–99665. doi:10.18632/oncotarget.20587
22. Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–809. doi:10.1002/jor.21359
23. Meinecke I, Cinski A, Baier A, et al. Modification of nuclear PML protein by SUMO-1 regulates Fas-induced apoptosis in rheumatoid arthritis synovial fibroblasts. Proc Natl Acad Sci U S A. 2007;104(12):5073–5078. doi:10.1073/pnas.0608773104
24. Li X, Luo Y, Yu L, et al. SENP1 mediates TNF-induced desumoylation and cytoplasmic translocation of HIPK1 to enhance ASK1-dependent apoptosis. Cell Death Differ. 2008;15(4):739–750. doi:10.1038/sj.cdd.4402303
25. Gomez-Del Arco P, Koipally J, Georgopoulos K. Ikaros SUMOylation: switching out of repression. Mol Cell Biol. 2005;25(7):2688–2697. doi:10.1128/MCB.25.7.2688-2697.2005
26. Datwyler AL, Lattig-Tunnemann G, Yang W, et al. SUMO2/3 conjugation is an endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2011;31(11):2152–2159. doi:10.1038/jcbfm.2011.112
27. Nguyen HV, Chen JL, Zhong J, et al. SUMOylation attenuates sensitivity toward hypoxia- or desferroxamine-induced injury by modulating adaptive responses in salivary epithelial cells. Am J Pathol. 2006;168(5):1452–1463. doi:10.2353/ajpath.2006.050782
28. Shao R, Zhang FP, Tian F, et al. Increase of SUMO-1 expression in response to hypoxia: direct interaction with HIF-1alpha in adult mouse brain and heart in vivo. FEBS Lett. 2004;569(1–3):293–300. doi:10.1016/j.febslet.2004.05.079
29. Wang F, Cai F, Shi R, Wei JN, Wu XT. Hypoxia regulates sumoylation pathways in intervertebral disc cells: implications for hypoxic adaptations. Osteoarthritis Cartilage. 2016;24(6):1113–1124. doi:10.1016/j.joca.2016.01.134
30. Im HJ, Sharrocks AD, Lin X, et al. Basic fibroblast growth factor induces matrix metalloproteinase-13 via ERK MAP kinase-altered phosphorylation and sumoylation of Elk-1 in human adult articular chondrocytes. Open Access Rheumatol. 2009;1:151–161.
31. Bao J, Ma C, Ran J, Xiong Y, Yan S, Wu L. Wnt/beta-catenin and Hedgehog pathways are involved in the inflammatory effect of Interleukin 18 on rat chondrocytes. Oncotarget. 2017;8(66):109962–109972. doi:10.18632/oncotarget.20584
32. Yang W, Sheng H, Wang H. Targeting the SUMO pathway for neuroprotection in brain ischaemia. Stroke Vasc Neurol. 2016;1(3):101–107. doi:10.1136/svn-2016-000031
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.