Back to Journals » Drug Design, Development and Therapy » Volume 16
Design, Synthesis, and Spectroscopic Studies of Some New α-Aminophosphonate Analogues Derived from 4-Hydroxybenzaldehyde with Special Reference to Anticancer Activity
Authors Ali OM , Alotaibi MT, Zaki YH , Amer HH
Received 12 January 2022
Accepted for publication 15 June 2022
Published 5 August 2022 Volume 2022:16 Pages 2589—2599
DOI https://doi.org/10.2147/DDDT.S357998
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Tin Wui Wong
Omar M Ali,1 Mohammed T Alotaibi,1 Yasser H Zaki,2 Hamada H Amer3
1Department of Chemistry, University College of Turabah, Taif University, Taif, 21944, Saudi Arabia; 2Department of Chemistry, Faculty of Science, Beni-Suef University, Beni-Suef, 62514, Egypt; 3Department of Animal Medicine and Infectious Diseases, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt
Correspondence: Yasser H Zaki; Omar M Ali, Email [email protected]; [email protected]
Introduction: As biological activity components, α-aminophosphonates and their moieties play important roles in medicinal chemistry. Alpha-phosphonic acids are significant α-amino acid counterparts. Due to its strong biological activity, this class of molecule has recently been discovered to have numerous medical applications.
Results and Discussion: A new class of α-aminophosphonates and arylidene derivatives was synthesized. Various spectroscopic and elemental analyses were used to confirm the prepared products. The produced materials were tested as anticancer against breast carcinoma cells and normal human cells (PBMC). Besides the analysis results, it was found that ( 7b, 4c, 5k, 6, 5a, 7c, 5f, 5b, and 5g) against MCF-7 line cells. As a reference anticancer drug, 5-fluorouracil was used. The anticancer activities showed that the compounds 7b, 4c, containing α-aminophosphonate and Schiff base groups, respectively, showed high inhibition activity against the MCF-7 cell line, with 94.32% and 92.45% inhibition compared to the inhibition by 5-FU with 96.02% inhibition. The results showed that the compounds 5k, 7b, 6, and 5a, respectively, had very low activity against normal human cells PBMC, with 12.77%, 13%, 13.13%, and 17.88% inhibition compared to the inhibition by 5-FU with 12.50% inhibition. The binding energy for non-bonding interactions between the ligand (studied compounds) and receptor, thymidylate synthase, was determined using molecular docking (pdb code: 1AN5).
Conclusion: α-aminophosphonate derivatives, arylidines, and disphosphonate derivatives derived from 4-hydroxybenzaldehyde were synthesized, purified, elucidated by spectroscopic analysis, and finally tested against carcinoma breast cancer to give high to moderate to low activity.
Keywords: synthesis, phosphonates, arylidene derivatives, 4-hydroxybenzaldehyde, anticancer activity
Graphical Abstract:
Introduction
α-Aminophosphonates and their moieties have important roles in medicinal chemistry as biological activity components.1 Alpha-phosphonic acids are important analogues of the α-amino acids. It has been found in recent years that this type of compound has many medical applications due to its high biological activity.2 α-Aminophosphonates are similar amino acids in their structure and are characterized by their high cell permeability.3 Recently, the interest in phosphonate synthesis through the three-component reaction with a natural iodine-coated catalyst has been observed and has already been tested and yielded results as an anti-HIV.4 Heterocyclic compounds that contain a pentagonal ring are called oxadiazole. When many of the α-aminophosphonates derived from them were synthesized, they were found to have very high activity as anti-liver cancer.5,6
On the other hand, it was found that α-aminophosphonates also have an anti-cancer effect, and they have an effect as antimicrobials in general and as anti-bacterial positive and gram-negative in particular.7–12 Schiff’s bases are considered one of the compounds known in the field of medicinal chemistry for their strong effects as new drugs, and it was found that when prepared with heterocyclic aldehydes, they showed high anti-fungal and bacterial activity.13–16
Molecular docking describes the proper orientation of any substance that binds to a specific protein and is critical in predicting the structure of a complex formed by two or more molecules.17 Because of its applications in medicine, the protein ligand interaction is the most interesting and entertaining case.18 A ligand is a tiny molecule that interacts with a protein’s binding site.19 Molecular docking is important because it is useful for learning about drug receptor interactions and is widely used to learn how a small molecule binds. Its activity filters drugs to their protein targets and leads to the prediction of small molecule affinity.20 We describe here the synthesis of α-aminophosphonates derivatives, which were evaluated against carcinoma breast cancer and normal human PBMC cells, as a continuation of our prior work in the synthesis of biologically active heterocycles.21–27 New alpha-aminophosphonate derivatives have been designed and manufactured. Hence, the chemical composition of the synthesized compounds were elucidated by spectroscopy, such as 13C NMR, elemental analysis, infrared (IR) and 1HNMR. After confirming the chemical composition of these compounds and their purification, they were tested against breast cancer cells (MCF-7), which results showed that the synthesized compounds showed moderate to high activity compared to 5-fluorouracil.28 It was found that breast cancer is the leading cause of death among women all over the world. In a very recent study, 60 female rats were divided into 6 groups. Negative control. The novel α-aminophosphonates and arylidene derivatives of 3-acetyl-1-aminoquinolin-2(1H)-one were synthesized and tested against infected breast cancer in rats. Histopathological examination showed a significant proliferation of tumor cells in the DMBA group. Treatment with alpha-aminophosphonate mainly reduced tumor mass. Bcl2 expression increased in DMBA-administered mice and then decreased in the treated groups, mostly with α-aminophosphonates. The level of CA15-3 was significantly decreased in the DMBA groups treated with α-aminophosphonates and arylidine derivatives of 3-acetyl-1-aminoquinolin-2(1H)-one. Gene expression of GST-P, PCNA, PDK and PIK3CA was decreased in the DMBA group treated with α-aminophosphonates.29 The binding energy for non-bonding interactions between the ligand (studied compounds) and the receptor, thymidylate synthase, was determined using a molecular docking study (pdb code: 1AN5).
Results and Discussion
Chemistry
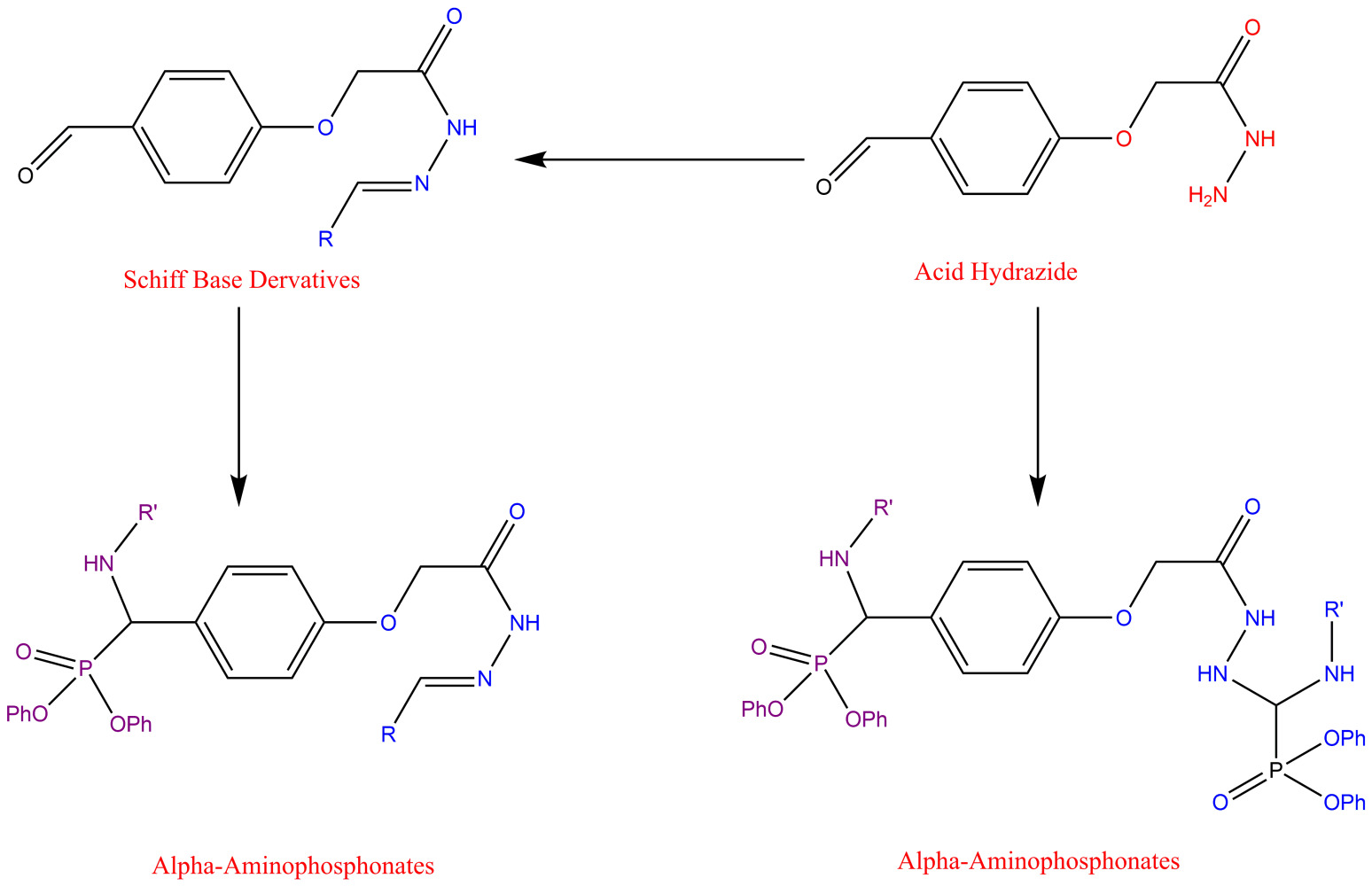
P-Hydroxybenzaldhyde (1) was permitted for the reaction with ethylchloroacetate and K2CO3 in acetone to provide ethyl 2-(4-formylphenoxy)acetate (2) in an 87% yield. The addition of hydrazine hydrate to a dissolved ethyl ester 2 in absolute ethanol at a boiling temperature in the presence of a condenser resulted in the administration of hydrazide 3, which when reacting with some aromatic aldehydes and a trace amount of AcOH yielded the arylidene derivatives 4a-d in 87–90% yields (Scheme 1 in the Supplementary Materials).
At room temperature, the reaction of 4a-d with the appropriate amines (1-naphthyl amine, 2-nitro aniline, or p–toluidine) in acetonitrile with the addition of triphenylphosphite and in the absence of perchloric acid as a catalytic agent gave the corresponding phosphonates 5a-l in 75–92% yields (Scheme 2 in the Supplementary Materials). The elucidation of 4a-d by 1H NMR spectra appears to be the disappearance of the NH2 group and the appearance of peaks in a broad peak around 9.32 for (NH) and 11.88 for (CHO group).
The elucidation of phosphonates 5a-l by 1H NMR spectra appear to be the disappearance of CHO groups and the appearance of peaks around 3.75 for (NH), a singlet peak around 6.00 for the (CH) group, a multiplet around 7.98 to 8.99 for CH-aromatic, and a broad peak around 9.22 for (NH group) and 8.71 for (CH) group. 13C NMR spectra appear to have peaks around 69.42 for (-CH-P-of phosphonates), the peaks of (CH-aromatics) appear around 109.54 to 157.24, a peak around 145.32 for (CH=N) and a peak around 171.12 for (CONH). The reaction of 3 with HCOOH at a boiling temperature in the presence of a condenser afforded diformyl-compound 6 in a 90% yield (Scheme 3 in the Supplementary Materials). In the presence of perchloric acid, the treatment of 6 in acetonitrile with the appropriate amines (1-naphthylamine, 2-nitroaniline, or p–toluidine) and the addition of triphenylphosphite yield the corresponding phosphonates 7a-c in 85–88% yields.
The elucidation of phosphonates 7a-c by 1H NMR spectra appear to be the disappearance of CHO groups and the appearance of peaks around 2.40 and 4.00 for (NH), a singlet peak around 6.00 for the (CH) group, a multiplet around 7.18 to 8.22 for CH-aromatic, 8.71 for the (CH) group, and a broad peak around 9.22 for the (NH group); 13C NMR spectra appears to have peaks around 69.07 and 85.78 for (-CH-P-of phosphonates), the peaks of (CH-aromatics) appear around 114.22 to 156.55 for (CH=N) and 165.83 for (CONH).
Anticancer Activity
The newly developed and synthesized compounds were examined for their ability to fight breast cancer. MCF-7 was inhibited by Schiff bases and α-aminophosphonate analogues generated from 4-Hydroxybenzaldehyde (7b, 4c, 5k, 6, 5a, 7c, 5f, 5b, and 5g). As a control, the medication 5-fluorouracil (5-FU) was used. According to the findings, compounds 7b and 4c, having α-aminophosphonate and Schiff base groups, respectively, displayed extremely strong inhibitory activity against the MCF-7 cell line, with 94.32% and 92.45% inhibition, respectively. Following that, 5k, 6 and 5a demonstrated strong action against breast cancer cells, with 83.14%, 82.65%, and 80.55% inhibition, respectively. Following that, 3, 7c, and 5f displayed moderate activity with 77.73%, 70.65%, and 67.34% inhibition, respectively. Finally, 5b and 5g demonstrated minimal activity, with 57.67% and 50.50% inhibition, respectively. In comparison to the suppression by 5-FU, which had a 96.02% inhibition (Table 1).
 |
Table 1 Inhibition Activity of the Synthesized Compounds Against MCF-7 |
On the other hand, the same compounds were tested against normal human cells (PBMC). According to the findings, the compounds 5k, 7b, 6, and 5a showed extremely poor action against normal human cells PBMC, with 12.77%, 13%, 13.13%, and 17.88% inhibition, respectively. Following that, 7c, 4c, and 3 showed minimal activity with 24.84%, 25.35%, and 29.55% inhibition, respectively. Compounds 5f, 5b, and 5g, on the other hand, displayed strong action against normal human cell PBMC, with inhibition rates of 32.85%, 39.22%, and 40.13%, respectively. In comparison to the suppression by 5-FU, which has a 12.50% inhibition (Tables 2).
 |
Table 2 Inhibition Activity Against Normal Human Cells PBMC |
Molecular Docking Simulation
For each synthesized compound, the docking simulation process was completed, and the best conformation was chosen as the compound with the highest negative binding energy value. Figures S1 and S2 in the Supplementary Materials illustrate the 3D structures, and Figures S3 and S4 in the Supplementary Materials illustrate the 2D structures of the ligand–receptor structures of all compounds studied. Table 3 displays the estimated binding energies and the interacting residues produced by docking for all compounds studied. All of the compounds studied formed stable complexes with receptors that had a high binding energy. Compound 7b had the best docking energy (highest binding energy) according to our findings, with a binding affinity of 10.31 kcal/mol, followed by compound 4c (−10.22 kcal/mol) (Table 3). This is consistent with the biological evidence acquired. As a result, the compounds studied, particularly compounds 7b and 4c, have the potential to be used as anti-Brest cancer. The most interacting residues in the 7b compound active site, according to molecular docking, were LEU 144, VAL 263, SER 55, ASP 170, GLU 83, PHE 177, GLY 174, CYS 147, ILE 80, and TYR 210.
 |
Table 3 The Binding Energies and the Interacting Residues Produced by Docking for All Compounds Studied |
Compound 4c interacted with ASN 178, GLY 174, ASP 170, PHE 177, CYS 147, TRP 81, and ILE 80. Compound 5k was seen to interact with VAL 263, PHE 177, LEU 173, CYS 147, LEU 144, TRP 84, TRP 81, and LYS 49. Compound 6 showed interaction with ARG 127, TRP 84, TRP 81, and ARG 22, as well as compound 5a interacted with VAL 263, LYS 260, ASN 178, PHE 177, and ILE 80. And compound 3 with ASN 178, CYS 147, LEU 144, and GLU 59. While compound 7c interacted with VAL 263, LYS 260, HS 208, GLY 174, LEU 173, CYS 147, TRP 81, ILE 80, HS 52, and LYS 49. And compound 5f interacted with TRY 210, HS 208, ASN 178, PHE 177, LEU 173, ASP 170, CYS 147, LEU 144, TRP 84, and ILE 80. Compound 5b was shown to interact with VAL 263, PHE 177, LEU 173, ASP 170, CYS 147, TRP 84, TRP 81, ILE 80, and CYS 51. And compound 5g interacted with VAL 263, PHE 177, LEU 173, TRP 84, GLU 83, TRP 81, ILE 80, THR 79, VAL 78, SER 55, and HS 52 as shown in Table 3.
Materials and Methods
General Information
Melting points were measured using a Kofler mass machine and were not corrected. Nuclear Magnetic Resonance for proton spectra were mapped onto the Varian Gemini Nuclear Magnetic Resonance for proton spectrometer at 500 MHz. Interactions were followed up by TLC using a 60 °F 245 aluminum silica plate. Primary analyses were carried out at Cairo University’s Microanalysis Center, Faculty of Science.
Experimental Procedures
Synthesis ethyl 2-(4-formylphenoxy)acetate (2).28
In the reaction of p-hydroxybenzaldhyde 1 (10.6 g, 0.1 mol) dissolved in acetone (250 mL) with the slow addition of ethylchloroacetate (12.25 g, 0.1 mol) and in the presence of anhydrous K2CO3 (13.8 g, 0.1 mol), the mix was boiled under reflux for 12h. After ensuring that the reaction was complete, the filtrate was evaporated under pressure and recrystallized with ethanol to yield yellow oil with an 87% yield. Rf = 0.48 (3% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 1.19 (t, 3H, J = 8.1 Hz, CH3CH2), 4.10 (q, 2H, J = 8.1 Hz, CH3CH2), 4.65 (s, 2H, CH2), 6.98 (d, 2H, J = 5.5 Hz, H-2), 7.56 (d, 2H, J = 5.5 Hz, H-3), 10.49 (s, 1H, CHO). Anal. Calc. for C11H12O4: C, 63.45; H, 5.81; Found C, 63.60; H, 5.95.
Synthesis of 2-(4-formylphenoxy) acetohydrazide (3).28
Compound 2 (2.08 g, 0.01 mol) was dissolved in absolute ethanol (30 mL), and then hydrazine hydrate (1.5 g, 0.03 mol) was added, and the reaction was heated under reflux for 5h. After completing the reaction and separating the resulting compound, it was purified by recrystallizing ethanol to obtain a high purity white product with a yield of 90%., m.p. 203–205°C, Rf = 0.31 (3% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 4.32 (brs, 2H, NH2), 4.55 (s, 2H, CH2), 6.92 (d, 2H, J= 5.5 Hz, Ar-H), 7.49 (d, 2H, J= 5.5 Hz, Ar-H), 9.33 (brs, 1H, NH), 10.42 (s, 1H, CHO); Anal. Calc. for C9H10N2O3: C, 55.67; H, 5.19; N, 14.43. Found C, 55.83; H, 5.34; N, 14.57.
Synthesis of arylidene derivatives 4a-d.
In absolute ethanol, different aromatic aldehydes (5 mmol) are allowed to react with compound 3 (5 mmol). After that, a catalytic quantity of acetic acid (glacial) was added to the mixture and it was refluxed for 15h. The product was separated to provide 4a-d (87–90%) yields. (Figures S5 and S6 in the Supplementary Materials)
(E)-N’-benzylidene-2-(4-formylphenoxy)acetohydrazide (4a).
Yellow powder (87%), m.p. 267–269 °C. Rf = 0.80 (3% EtOAc in CHCl3), 1H NMR (DMSO-d6): δ = 4.45 (s, 2H, CH2), 7.12–8.00 (m, 9H, Ar-H), 8.55 (s, 1H, CH), 9.32 (brs, 1H, NH), 11.88 (s, H, CHO); Anal. Calc. for C16H14N2O3: C, 68.07; H, 5.00; N, 9.92. Found C, 68.19; H, 5.16; N, 9.81.
N’-(4-(dimethylamino) benzylidene)-2-(4-formylphenoxy)-acetohydrazide (4b).
White powder (88%), m.p. > 300 °C. Rf = 0.45 (3% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 3.10 (s, 6H, 2CH3), 4.45 (s, 2H, CH2), 7.20–8.05 (m, 8H, Ar-H), 8.45 (s, 1H, CH), 9.32 (brs, 1H, NH), 11.80 (s, 1H, CHO); Anal. Calc. for C18H19N3O3: C, 66.45; H, 5.89; N, 12.91. Found C, 66.57; H, 6.03; N, 13.06.
2-(4-formylphenoxy)-N’-(3, 4, 5-trimethoxybenzylidene) acetohydrazide (4c).
White powder (90%), m.p. = 212–214 °C. Rf = 0.50 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 3.92 (s, 9H, 3CH3), 4.65 (s, 2H, CH2), 7.20–7.95 (m, 6H, Ar-H), 8.22 (s, 1H, CH), 8.62 (brs, 1H, NH), 10.52 (s, 1H, CHO); Anal. Calc. for C19H20N2O6: C, 61.28; H, 5.41; N, 7.52. Found C, 61.43; H, 5.56; N, 7.37.
2-(4-formylphenoxy)-N’-(4-nitrobenzylidene) acetohydrazide (4d).
White powder (90%), m.p. = 250–252 °C. Rf = 0.65 (3% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 4.63 (s, 2H, CH2), 7.10–7.85 (m, 8H, Ar-H), 8.30 (s, 1H, CH), 8.52 (brs, 1H, NH), 10.95 (s, 1H, CHO); Anal. Calc. for C16H13N3O5: C, 58.72; H, 4.00; N, 12.84. Found: C, 58.88; H, 3.89; N, 12.98.
General procedure for the synthesis of α-aminophosphonates 5a- l.
In 20 mL MeCN, triphenylphosphite (5 mmol) was added to a mixture of Schiff bases 4 (a-d) (5 mmol) and different amines (5 mmol). 1 mL of perchloric acid was added dropwise, and the reaction mixture was stirred at room temperature for 20h. The solvent was evaporated under low pressure, and the gum was tutrated with diethyl ether and dried to give 5 (a- l) in 75–92% yields. (Figures S7–S18 in the Supplementary Materials).
Diphenyl ((4-(2-(2-benzylidenehydrazinyl)-2-oxoethoxy)phenyl)-(naphthalen-1-yl-amino)methyl)phosphonate (5a).
Yellow gum (75%), Rf = 0.60 (3% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 3.75 (brs, 1H, NH), 4.63 (s, 2H, CH2), 6.00 (s, 1H, CH), 6.98–7.99 (m, 26H, Ar-H), 8.00 (brs, 1H, NH), 8.45 (s, 1H, CH); 13C NMR (100 MHz, CDCl3): δ = 68.77 (CH2), 69.42 (CH-P-), 109.54, 114.34, 118.98, 120.41, 124.82, 125.05, 126.11, 127.65, 127.89, 128.32, 128.87, 129.22, 130.25, 131.11, 133.65, 134.37, 141.55, 147.22, 150.23, 157.24 (C-Aromatic), 145.32 (CH=N), 171.12 (CONH). Anal. Calc. for C38H32N3O5P: C, 71.13; H, 5.03; N, 6.55. Found C, 71.24; H, 5.15; N, 6.68.
Diphenyl ((4-(2-(2-benzylidenehydrazinyl)-2-oxoethoxy)phenyl)((2-nitrophenyl)-amino)methyl)phosphonate (5b).
Yellow gum (75%), Rf = 0.55 (3% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 3.75 (brs, 1H, NH), 4.60 (s, 2H, CH2), 6.00 (s, 1H, CH), 6.98–7.99 (m, 23H, Ar-H), 8.12 (brs, 1H, NH), 8.55 (s, 1H, CH); 13C NMR (100 MHz, CDCl3): δ = 69.00 (CH2), 69.42 (CH-P-), 114.65, 118.73, 120.39, 121.41, 125.94, 127.56, 128.76, 129.23, 130.35, 131.31, 131.77, 133.38, 135.27, 146.37, 150.43, 156. 94 (C-Aromatic), 144.72 (CH=N), 170.52 (CONH). Anal. Calc. for C34H29N4O7P: C, 64.15; H, 4.59; N, 8.80. Found: C, 64.27; H, 4.43; N, 8.66.
Diphenyl ((4-(2-(2-benzylidenehydrazinyl)-2-oxoethoxy)phenyl)(p-tolylamino)-methyl)phosphonate (5c).
Yellow gum (77%), Rf = 0.60 (6% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 2.20 (s, 3H, CH3), 3.75 (brs, 1H, NH), 4.60 (s, 2H, CH2), 6.00 (s, 1H, CH), 6.98–7.99 (m, 23H, Ar-H), 8.12 (brs, 1H, NH), 8.55 (s, 1H, CH); 13C NMR (100 MHz, CDCl3): δ = 20.98 (CH3), 69.08 (CH2), 69.50 (CH-P-), 113.45, 114.13, 120.29, 121.45, 127.36, 128.43, 128.76, 129.17, 129.61, 130.13, 131.45, 133.77, 144.62, 150.55, 156.78 (C-Aromatic), 145.30 (CH=N), 171.00 (CONH). Anal. Calc. for C35H32N3O5P: C, 69.41; H, 5.33; N, 6.94. Found: C, 69.55; H, 5.48; N, 7.06.
Diphenyl ((4-(2-(2-(4-(dimethylamino)benzylidene)hydrazinyl)-2-oxoethoxy)-phenyl)(naphthalen-1-ylamino)methyl)phosphonate (5d).
Brown gum (82%), Rf = 0.35 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 3.10 (s, 6H, 2CH3), 4.11 (brs, 1H, NH), 4.45 (s, 2H, CH2), 6.23 (s, 1H, CH), 6.82–8.12 (m, 25H, Ar-H), 8.00 (brs, 1H, NH), 8.32 (s, 1H, CH); 13C NMR (100 MHz, CDCl3): δ = 40.35 (-N(CH3)2), 68.76 (CH2), 69.53 (CH-P-), 108.46, 110.67, 113.95, 119.00, 120.22, 121.31, 123.00, 124.19, 125.36, 126.32, 127.11, 127.65, 128.16, 128.48, 128.77, 130.12, 134.66, 146.55, 150.50, 153.09, 154.60, 157. 51 (C-Aromatic), 144.53 (CH=N), 171.07 (CONH). Anal. Calc. for C40H37N4O5P: C, 70.16; H, 5.45; N, 8.18. Found C, 70.03; H, 5.58; N, 8.29.
Diphenyl ((4-(2-(2-(4-(dimethylamino)benzylidene)hydrazinyl)-2-oxoethoxy)phenyl)((2-nitrophenyl)amino)methyl)phosphonate (5e).
Brown gum (80%), Rf = 0.75 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 3.10 (s, 6H, 2CH3), 4.11 (brs, 1H, NH), 4.45 (s, 2H, CH2), 6.23 (s, 1H, CH), 6.82–8.12 (m, 22H, Ar-H), 8.00 (brs, 1H, NH), 8.32 (s, 1H, CH); 13C NMR (100 MHz, CDCl3): δ = 41.00 (-N(CH3)2), 69.11 (CH2), 70.04 (CH-P-), 111.48, 114.22, 114.76, 118.13, 120.15, 121.27, 123.42, 125.45, 127.34, 128.17, 128.55, 130.25, 131.44, 134.97, 146.72, 150.10, 153.17, 156.53 (C-Aromatic), 145.07 (CH=N), 172.11 (CONH). Anal. Calc. for C36H34N5O7P: C, 63.62; H, 5.04; N, 10.30. Found C, 63.75; H, 5.15; N, 10.43.
Diphenyl ((4-(2-(2-(4-(dimethylamino)benzylidene)hydrazinyl)-2-oxoethoxy)-phenyl)(p-tolylamino)methyl)phosphonate (5f).
Brown oil (80%), Rf = 0.57 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ =2.24 (s, 3H, CH3), 3.10 (s, 6H, 2CH3), 4.13 (brs, 1H, NH), 4.45 (s, 2H, CH2), 6.23 (s, 1H, CH), 6.82–8.10 (m, 22H, Ar-H), 8.12 (brs, 1H, NH), 8.22 (s, 1H, CH); 13C NMR (100 MHz, CDCl3): δ = 22.00 (CH3), 41.97 (-N(CH3)2), 68.76 (CH2), 69.53 (CH-P-), 112.06, 113.40, 114.46, 120.32, 121.45, 123.09, 127.17, 128.00, 128.33, 128.86, 129.34, 129.56, 131.00, 144.39, 150.24, 153.12, 156. 66 (C-Aromatic), 145.14 (CH=N), 170.23 (CONH). Anal. Calc. for C37H37N4O5P: C, 68.51; H, 5.75; N, 8.64. Found: C, 68.39; H, 5.88; N, 8.77.
Diphenyl ((naphthalen-1-ylamino)(4-(2-oxo-2-(2-(3,4,5-trimethoxy-benzylidene)-hydrazinyl)ethoxy)phenyl)methyl)phosphonate (5g).
Brown oil (85%), Rf = 0.63 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ =3.90 (s, 9H, 3CH3), 4.15 (brs, 1H, NH), 4.55 (s, 2H, CH2), 6.20 (s, 1H, CH), 6.72–8.10 (m, 23H, Ar-H), 8.12 (brs, 1H, NH), 8.24 (s, 1H, CH); 13C NMR (100 MHz, CDCl3): δ = 56.22 (2xOCH3), 61.12 (OCH3), 69.00 (CH2), 69.57 (CH-P-), 104.00, 110.02, 114.34, 119.13, 120.30, 121.39, 124.73, 125.09, 126.24, 127.45, 127.68, 128.07, 128.38, 128.99, 130.61, 134.65, 141.43, 147.57, 150.26, 153.00, 156.84 (C-Aromatic), 147.43 (CH=N), 171.77 (CONH). Anal. Calc. for C41H38N3O8P: C, 67.30; H, 5.23; N, 5.74. Found: C, 67.44; H, 5.11; N, 5.61.
Diphenyl (((2-nitrophenyl)amino)(4-(2-oxo-2-(2-(3,4,5-trimethoxybenzylidene)hydrazinyl)ethoxy)phenyl)methyl)phosphonate (5h).
Brown oil (85%), Rf = 0.55 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 3.90 (s, 9H, 3CH3), 4.13 (brs, 1H, NH), 4.45 (s, 2H, CH2), 6.23 (s, 1H, CH), 6.82–8.10 (m, 20H, Ar-H), 8.15 (brs, 1H, NH), 8.22 (s, 1H, CH); 13C NMR (100 MHz, CDCl3): δ = 55.46 (2xOCH3), 60.67(OCH3), 68.15 (CH2), 69.00 (CH-P-), 105.13, 114.04, 114.43, 118.63, 120.29, 121.45, 125.47, 127.36, 128.45, 128.78, 130.22, 131.50, 135.71, 141.25, 146.56, 150.43, 153.21, 156.47(C-Aromatic), 147.54 (CH=N), 172.07 (CONH). Anal. Calc. for C37H35N4O10P: C, 61.16; H, 4.85; N, 7.71. Found: C, 61.25; H, 4.72; N, 7.83.
Diphenyl ((4-(2-oxo-2-(2-(3,4,5-trimethoxybenzylidene)hydrazinyl)-ethoxy)phenyl)(p-tolylamino)methyl)phosphonate (5i).
Brown oil (80%), Rf = 0.60 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ =2.24 (s, 3H, CH3), 3.90 (s, 9H, 3CH3), 4.20 (brs, 1H, NH), 4.65 (s, 2H, CH2), 6.11 (s, 1H, CH), 7.11–7.42 (m, 20H, Ar-H), 8.10 (brs, 1H, NH), 8.45 (s, 1H, CH); 13C NMR (100 MHz, CDCl3): δ = 22.12 (CH3), 55.46 (2xOCH3), 60.67(OCH3), 69.12 (CH2), 69.32 (CH-P-), 104.43, 113.47, 114.87, 118.63, 120.33, 121.54, 127.48, 128.07, 128.33, 129.00, 129.56, 129.91, 130.66, 141.45, 144.05, 150.75, 153.61, 156.78 (C-Aromatic), 146.59 (CH=N), 171.63 (CONH). Anal. Calc. for C38H38N3O8P: C, 65.60; H, 5.51; N, 6.04. Found: C, 65.49; H, 5.39; N, 5.92.
Diphenyl ((naphthalen-1-ylamino)(4-(2-(2-(4-nitrobenzylidene)-hydrazinyl)-2-oxo-ethoxy)phenyl)methyl)phosphonate (5j).
Yellow gum (86%), Rf = 0.66 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ =3.98 (brs, 1H, NH), 4.55 (s, 2H, CH2), 6.00 (s, 1H, CH), 6.62–8.00 (m, 25H, Ar-H), 8.20 (s, 1H, CH), 8.42 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3): δ = 69.15 (CH2), 69.70 (CH-P-), 109.11, 114.14, 119.36, 120.29, 121.45, 124.11, 124.36, 124.67, 125.18, 126.43, 127.36, 128.32, 128.65, 130.22, 134.30, 139.55, 147.74, 150.65, 156.36 (C-Aromatic), 144.98 (CH=N), 170.83 (CONH). Anal. Calc. for C38H31N4O7P: C, 66.47; H, 4.55; N, 8.16. Found: C, 66.60; H, 4.66; N, 8.29.
Diphenyl ((4-(2-(2-(4-nitrobenzylidene)hydrazinyl)-2-oxoethoxy)-phenyl)((2-nitro-phenyl)amino)methyl)phosphonate (5k).
Yellow gum (88%), Rf = 0.42 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ =3.98 (brs, 1H, NH), 4.55 (s, 2H, CH2), 6.00 (s, 1H, CH), 6.62–8.00 (m, 22H, Ar-H), 8.20 (s, 1H, CH), 8.42 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3): δ = 68.67 (CH2), 69.13 (CH-P-), 114.12, 114.56, 118.56, 120.29, 121.45, 124.13, 124.46, 125.09, 127.91, 128.45, 130.22, 135.87, 139.39, 146.00, 150.67, 156.78 (C-Aromatic), 144.87 (CH=N), 171.00 (CONH). Anal. Calc. for C34H28N5O9P: C, 59.91; H, 4.14; N, 10.28. Found: C, 59.77; H, 4.25; N, 10.15.
Diphenyl ((4-(2-(2-(4-nitrobenzylidene)hydrazinyl)-2-oxoethoxy)-phenyl)(p-tolylamino) methyl)phosphonate (5l).
Yellow gum (92%), Rf = 0.70 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ =2.22 (s, 3H, CH3), 3.98 (brs, 1H, NH), 4.55 (s, 2H, CH2), 6.00 (s, 1H, CH), 6.62–8.00 (m, 22H, Ar-H), 8.20 (s, 1H, CH), 8.42 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3): δ = 21.75 (CH3), 69.17 (CH2), 69.63 (CH-P-), 113.10, 114.23, 120.15, 121.38, 124.40, 124.58, 127.65, 128.78, 129.45, 129.86, 130.00, 140.07, 145.51, 150.72, 156.63 (C-Aromatic), 145.60(CH=N), 171.43 (CONH). Anal. Calc. for C35H31N4O7P: C, 64.61; H, 4.80; N, 8.61. Found: C, 64.72; H, 4.68; N, 8.48.
Synthesis of N’-Formyl-2-(4-formylphenoxy) acetohydrazide (6).
For 14h, hydrazide 3 (10 mmol) and formic acid (30 mL) were refluxed. The excess of formic acid was evaporated under low pressure to give 6 in (90%) yields. (Figure S19 in the Supplementary Materials)
White crystals (90%), m.p. 158–160 °C. Rf = 0.38 (3% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 2.21 (brs, 1H, NH), 4.35 (s, 2H, CH2), 7.22 (d, 2H, j = 5.5 Hz), 7.92 (d, 2H, j= 5.5 Hz), 8.12 (brs, 1H, NH), 8.72 (s, 1H, CHO), 10.00 (s, 1H, CHO); Anal. Calc. for C10H10N2O4: C, 54.05; H, 4.54; N, 12.61. Found: C, 54.17; H, 4.49; N, 12.48.
General procedure for the synthesis of phosphonates 7a-c.
A mixture of the aromatic dialdehyde 6 (5 mmol), triphenylphosphite (5 mmol) and different amines (5 mmol) was dissolved in acetonitrile. A catalytic quantity of perchloric acid (0.5 mL) was added dropwise, and the reaction was stirred at room temperature for 24 h. The solvent was withdrawn under decreased pressure, and the residue of the gum was tutrated with diethyl ether and dried to provide 7a-c in 85–88% yields. (Figures S20–S25 in the Supplementary Materials)
Diphenyl((naphthalen-1-ylamino)(2-(2-(p-tolyloxy)acetyl)hydrazinyl)methyl)phosphonatediphenyl((naphthalen-1-ylamino)(phenyl)methyl)phosphonate (7a).
Yellow powder (85%) m.p. 152–154 °C. Rf = 0.56 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 2.40 (brs, 1H, NH), 4.00 (brs, 2H, 2NH), 4.45 (s, 2H, CH2), 6.00 (s, 1H, CH), 7.18–8.22 (m, 38H, Ar-H), 8.71 (s, 1H, CH), 9.22 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3): δ = 66.45 (CH2), 69.07 (CH-P-), 85.78 (CH-P-), 114.22, 114.34, 118.35, 120.23, 121.46, 124.44, 125.34, 127.56, 128.35, 128.76, 129.00, 129.19, 130.69, 133.43, 146.44, 150.10, 156.55 (C-Aromatic), 165.83 (CONH). Anal. Calc. for C54H46N4O8P2: C, 68.93; H, 4.93; N, 5.95. Found: C, 68.79; H, 5.04; N, 6.07.
Diphenyl((2-nitrophenyl)amino)(2-(2-(p-tolyloxy)acetyl)hydrazinyl)methyl)phosphonatediphenyl((2-nitrophenyl)amino)(phenyl)methyl)phosphonate (7b).
Yellow powder (87%) m.p. 142–144 °C. Rf = 0.37 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 2.40 (brs, 1H, NH), 4.00 (brs, 2H, 2NH), 4.45 (s, 2H, CH2), 6.00 (s, 1H, CH), 7.18–8.22 (m, 32H, Ar-H), 8.71 (s, 1H, CH), 9.22 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3): δ = 66.45 (CH2), 69.07 (CH-P-), 85.78 (CH-P-), 114.54, 114.78, 118.65, 120.54, 121.30, 125.13, 127.70, 128.51, 130.00, 131.60, 135.80, 146.73, 150.00, 156.90 (C-Aromatic), 166.72 (CONH). Anal. Calc. for C46H40N6O12P2: C, 59.36; H, 4.33; N, 9.03. Found: C, 59.49; H, 4.45; N, 8.88.
Diphenyl((4-methylphenyl)amino)(2-(2-(p-tolyloxy)acetyl)hydrazinyl)methyl)phosphonatediphenyl((4-methylphenyl)amino)(phenyl)methyl)phosphonate (7c).
Yellow powder (88%) m.p. 132–134 °C. Rf = 0.45 (5% EtOAc in CHCl3). 1H NMR (DMSO-d6): δ = 2.00 (s, 6H, 2CH3), 2.40 (brs, 1H, NH), 4.00 (brs, 2H, 2NH), 4.45 (s, 2H, CH2), 6.00 (s, 1H, CH), 7.18–8.22 (m, 32H, Ar-H), 8.71 (s, 1H, CH), 9.22 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3): δ = 21.56 (CH3), 65.78 (CH2), 68.15 (CH-P-), 86.00 (CH-P-), 113.14, 114.65, 120.54, 121.79, 127.65, 128.74, 129.40, 129.80, 130.44, 144.20, 150.23, 156.72 (C-Aromatic), 164.93 (CONH). Anal. Calc. for C48H46N4O8P2: C, 66.35; H, 5.34; N, 6.45. Found: C, 66.47; H, 5.45; N, 6.56.
Anticancer Activity
Cell Line Propagation
Cell propagation was started with Dulbecco’s modified Eagle Medium (DMEM), which contained 1% L-glutamine, 50 g/mL gentamicin, 10% heat inactivated fetal bovine serum, and HEPES buffer at the start of pretreatment. At 37 degrees Celsius, the cells were incubated. As a result, they were promoted twice in week.30 Viability assays were used to measure cytotoxicity, with the cells being prepared in a 96-well plate to accommodate a concentration of 1×104 cells per well in 100 L of growth media. After 24 hours, various medium concentrations were administered and then supplemented using a multi-channel pipette. 96-well plates were used to spread the monolayers.
Modified microtiter plate incubation was done for 48 hours at 37 degrees Celsius and 5% CO. Three wells were used to concentrate the test material. The control cells were then cultivated with and without the test confinement, as well as with and without the addition of dimethyl sulfoxide. Dimethyl sulfoxide has a maximum inactivation concentration of 0.1%. When the cell was cultured at 37 °C for 24 hours, different concentrations of samples were obtained. A colorimetric approach was used to calculate cell yield. Following incubation, a 30-minute period of 1% crystal violet solution was applied to each well’s remaining cell medium. The patches that remained were cleaned with distilled water.
The wells were filled with 30% glacial acetic acid, and then absorption measurements at 490 nm and a spectroscopic background correction were performed on the wells that did not have spots. Because there are not any substances that have been evaluated, Because the experiment was done in triplicate, samples were compared to cellular controls. The cytotoxicity efficacy was then determined. As a result, the optical density of the samples was determined using a microplate reader. The following equation was used to determine the number of viable cells as well as the percentage of cells that survived: [(ODt/ODc)] x 100%, where ODc is the average optical density of untreated cells and ODt is the average optical density of all treated wells in all tested samples. To determine the degree of cancer cell survival following therapy, histograms of live cells and medication concentrations were created.31 The IC estimate of healthy cells can be determined using graphical displays of the dose-response curve for all concentrations (GraphPad Prism Software; San Diego, CA, USA).32
Molecular Docking
Materials
AutoDockTools 1.5.6, PyRx and BIOVIA Discovery Studio programs were used in the molecular docking study. ChemDraw3D Ultra software was used to draw all the chemical structures of all studied compounds and the PDB (Protein Data Bank) site.
Ligand Preparation
In order to avoid repetition, ChemDraw3D Ultra was used to refine the structures of all the compounds studied. Using the open source babel program, the structures were then transformed into PDBQT format.
Protein Preparation
The PDB (Protein Data Bank) site was used to obtain the 3D crystal structure of thymidylate synthase (PDB ID: 1AN5). The BIOVIA Discovery Studio software was used to remove small molecules from the crystal structures of (1AN5).30,33
Molecular Docking Study
Polar hydrogens and Kollman charges were applied to the protein, and a PDBFQT format file was generated using the AutoDockTools 1.5.6 program. The protein was created using the protein preparation wizard in AutoDockTools 1.5.6. Polar hydrogens and Kollman charges were applied to the protein, and a PDBFQT format file was generated using AutoDockTools. (1AN5) was completely devoid of water molecules. The ligand torsions were calculated by first detecting the roots in AutoDockTools 1.5.6 and then setting the aromaticity parameters to 7.5.
The receptor was given a grid size of 60 Å × 60 Å × 60 Å, and the molecular docking operation was assigned to the Lamarckian genetic algorithm (LGA). After docking, the best pose was chosen based on binding energy, ligand–receptor interactions, and active site residues. The docked posture was simply compared to the cocrystallised structure, and the root mean square deviation (RMSD) was less than 1.0 Å. All torsions were allowed to rotate during docking. The traditional docking procedure for rigid and fluid ligand docking included ten separate runs per ligand, 2.5×106 energy measurements, a total of 27,000 iterations, a mutation rate of 0.02, a crossover rate of 0.80, and an elitism value of 1.
The likelihood of conducting a local search on a person in the population was 0.06 using a limit of 300 iterations per local search. Following docking, the ten solutions were classified as having RMS differences of less than 1.0. The clusters were sorted based on the cluster’s lowest energy representation. The effects of the docking process were visualized using the BIOVIA Discovery Studio program.
Conclusions
In this study, α-aminophosphonate derivatives, arylidines, and disphosphonate derivatives derived from 4-hydroxybenzaldehyde were synthesized, purified, elucidated by spectroscopic analysis, and finally tested against carcinoma breast cancer to give high to moderate to low activity. A molecular docking study was used to determine the binding energy for non-bonding interactions between the ligand (studied compounds) and receptor, thymidylate synthase (pdb code: 1AN5). All of the compounds studied formed stable complexes with receptors that had a high binding energy. Compound 7b had the best docking energy (highest binding energy) according to our findings, with a binding affinity of 10.31 kcal/mol, followed by compound 4c (−10.22 kcal/mol) (Table 3).
Data Sharing Statement
The figures/data presented in this study are available on request from the corresponding author.
Ethics
The cell lines were purchased commercially from the ATCC https://www.lgcstandards-atcc.org/search#q=RH30&sort=relevancy; no further approval is required.
Acknowledgments
We acknowledge Taif University for Researchers Supporting Project number (TURSP-2020/81) and Taif University, Taif, Saudi Arabia.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Rezaei Z, Khabnadideh S, Zomorodian K, et al. Design, synthesis, and antifungal activity of new α -aminophosphonates. Int J Med Chem. 2011;2011:1–11. doi:10.1155/2011/678101
2. Walęcka-Kurczyk A, Walczak K, Kuźnik A, Stecko S, Październiok-Holewa A. The synthesis of α-aminophosphonates via enantioselective organocatalytic reaction of 1-(N-Acylamino) alkylphosphonium salts with dimethyl phosphite. Molecules. 2020;25(2):405. doi:10.3390/molecules25020405
3. Azizi N, Rajabi F, Saidi MR. A mild and highly efficient protocol for the one-pot synthesis of primary α-amino phosphonates under solvent-free conditions. Tetrahedron Lett. 2004;45(50):9233–9236. doi:10.1016/j.tetlet.2004.10.092
4. Baddi L, Ouzebla D, El Mansouri A-E, Smietana M, Vasseur -J-J, Lazrek HB. Efficient one-pot, three-component procedure to prepare new α-aminophosphonate and phosphonic acid acyclic nucleosides. Nucleosides Nucleotides Nucleic Acids. 2020;40(1):43–67. doi:10.1080/15257770.2020.1826516
5. Amer HH, Ali OM, Salama AA, El-gendy MS, Houssin OK. Synthesis of some new 1, 3, 4-oxadiazole derivatives bearing sugars and α-aminophosphonate derived from 4-nitrophenol as anticancer agents. Natl J Physiol Pharmacy Pharmacol. 2018;8(9):1275–1286.
6. Liu M, Song W, Du X, et al. NQO1-selective activated prodrug of triptolide: synthesis and antihepatocellular carcinoma activity evaluation. ACS Med Chem Lett. 2018;9(12):1253–1257. doi:10.1021/acsmedchemlett.8b00404
7. Rasal SA, Dhavan PP, Jadhav BL, Shimpi NG. Synthesis of new α‐aminophosphonates using nanoscale nickel‐based metal–organic framework as a heterogeneous catalyst and their antibacterial activity. Appl Organomet Chem. 2020;34(2):e5317. doi:10.1002/aoc.5317
8. Joossens J, Ali OM, El-Sayed I, et al. Small, potent, and selective diaryl phosphonate inhibitors for urokinase-type plasminogen activator with in vivo antimetastatic properties. J Med Chem. 2007;50(26):6638–6646. doi:10.1021/jm700962j
9. Joossens J, Van der Veken P, Surpateanu G, et al. Diphenyl phosphonate inhibitors for the urokinase-type plasminogen activator: optimization of the P4 position. J Med Chem. 2006;49(19):5785–5793. doi:10.1021/jm060622g
10. Ali OM, Amer HH, Abdeen E, Khallaf OH. Preparation, spectroscopic, and biological characterizations of novel α-aminophosphonates bearing paracetamol. Natl J Physiol Pharmacy Pharmacol. 2018;8(8):1219–1225. doi:10.5455/njppp.2018.8.0517614052018
11. Srinivasulu D, Vijaya Bhaskara Reddy M, Rajasekhar D, Balaji M, Nagaraju C. Design, synthesis and antimicrobial activity of α-aminophosphonates of quinoline and their molecular docking studies against DNA gyrase A. Lett Drug Des Discov. 2013;10(10):967–976. doi:10.2174/15701808113109990035
12. Kenawy E-RS, Azaam MM, Saad-Allah KM. Synthesis and antimicrobial activity of α-aminophosphonates containing chitosan moiety. Arab J Chem. 2015;8(3):427–432. doi:10.1016/j.arabjc.2013.12.029
13. Mesbah M, Douadi T, Sahli F, Issaadi S, Boukazoula S, Chafaa S. Synthesis, characterization, spectroscopic studies and antimicrobial activity of three new Schiff bases derived from Heterocyclic moiety. J Mol Struct. 2018;1151:41–48. doi:10.1016/j.molstruc.2017.08.098
14. Ali OM, Amer HH, Mosaad AA, Abdel-Rahman A-H. Synthesis and antimicrobial activity of new phenytoin derivatives and their acyclic nucleoside analogs. Chem Heterocyclic Compounds. 2012;48(7):1043–1049. doi:10.1007/s10593-012-1097-9
15. Amer HH, El-Kousy SM, Salama WM, Sheleby AH. Synthesis and antimicrobial activity of new synthesized benzimidazole derivatives and their acyclic nucleoside analogues. Org Chem Curr Res. 2016;5:2–8. doi:10.4172/2161-0401.1000159
16. Ali OM, Amer HH, Nayel M, Abdel-Rahman AA. Synthesis and antimicrobial activity of new synthesized paracetamol derivatives and their acyclic nucleoside analogues. Int J Sci Res Publ. 2016;4:408–418.
17. Bhati S, Kaushik V, Singh J. In silico identification of piperazine linked thiohydantoin derivatives as novel androgen antagonist in prostate cancer treatment. Int J Pept Res Ther. 2019;25(3):845–860. doi:10.1007/s10989-018-9734-5
18. Sharma NK, Jha KK. Molecular docking: an overview. J Adv Sci Res. 2010;1(1):67–72.
19. Vijesh AM, Isloor AM, Telkar S, Arulmoli T, Fun H-K. Molecular docking studies of some new imidazole derivatives for antimicrobial properties. Arab J Chem. 2013;6(2):197–204. doi:10.1016/j.arabjc.2011.10.007
20. Morris GM, Goodsell DS, Halliday RS, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19(14):1639–1662. doi:10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B
21. Gomha SM, Zaki YH, Abdelhamid AO. Utility of 3-Acetyl-6-bromo-2H-chromen-2-one for the synthesis of new heterocycles as potential antiproliferative agents. Molecules. 2015;20(12):21826–21839. doi:10.3390/molecules201219803
22. Fouad SA, El-Gendey MS, Ahmed EM, Hessein SA, Ammar YA, Zaki YH. Convenient synthesis of some new thiophene, pyrazole, and thiazole derivatives bearing biologically active sulfonyl guanidine moiety. Polycycl Aromat Compd. 2021;1–19. doi:10.1080/10406638.2021.1988999
23. Hosny MA, Zaki YH, Mokbel WA, Abdelhamid AO. Synthesis, characterization, antimicrobial activity and anticancer of some new pyrazolo [1, 5-a] pyrimidines and pyrazolo [5, 1-c] 1, 2, 4-triazines. Med Chem (Los Angeles). 2020;16(6):750–760. doi:10.2174/1573406415666190620144404
24. Abdelhamid AO, El Sayed IE, Zaki YH, Hussein AM, Mangoud MM, Hosny MA. Utility of 5-(furan-2-yl)-3-(p-tolyl)-4, 5-dihydro-1 H-pyrazole-1-carbothioamide in the synthesis of heterocyclic compounds with antimicrobial activity. BMC Chem. 2019;13(1):1–18. doi:10.1186/s13065-019-0566-y
25. Zaki YH, Gomha SM, Mohamed AMG. Utility of 2-thioxo-pyrido [2, 3-d] pyrimidinone in synthesis of pyridopyrimido [2, 1-b][1, 3, 5]-thiadiazinones and pyridopyrimido [2, 1-b][1, 3] thiazinones as antimicrobial agents. Chem Cent J. 2017;11(1):1–10. doi:10.1186/s13065-017-0286-0
26. Zaki YH, Sayed AR, Elroby SA. Regioselectivity of 1, 3-dipolar cycloadditions and antimicrobial activity of isoxazoline, pyrrolo [3, 4-d] isoxazole-4, 6-diones, pyrazolo [3, 4-d] pyridazines and pyrazolo [1, 5-a] pyrimidines. Chem Cent J. 2016;10(1):1–13. doi:10.1186/s13065-016-0163-2
27. Abdelhamid AO, Abdelall EKA, Zaki YH. Reactions with hydrazonoyl halides 62: synthesis and antimicrobial evaluation of some new imidazo [1, 2‐a] pyrimidine, imidazo [1, 2‐a] pyridine, imdazo [1, 2‐b] pyrazole, and quinoxaline derivatives. J Heterocycl Chem. 2010;47(2):477–482.
28. Amer HH, Alotaibi SH, Trawneh AH, Metwaly AM, Eissa IH. Anticancer activity, spectroscopic and molecular docking of some new synthesized sugar hydrazones, arylidene and α-aminophosphonate derivatives. Arab J Chem. 2021;14(10):103348. doi:10.1016/j.arabjc.2021.103348
29. Nassan MA, Aldhahrani A, Amer HH, et al. Investigation of the anticancer effect of α-aminophosphonates and arylidine derivatives of 3-acetyl-1-aminoquinolin-2(1H)-one on the DMBA model of breast cancer in albino rats with in silico prediction of their thymidylate synthase inhibitory effect. Molecules. 2022;27(3):756. doi:10.3390/molecules27030756
30. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi:10.1016/0022-1759(83)90303-4
31. Gomha SM, Riyadh SM, Mahmmoud EA, Elaasser M. Synthesis and anticancer activities of thiazoles, 1, 3-thiazines, and thiazolidine using chitosan-grafted-poly (vinylpyridine) as basic catalyst. Heterocycles. 2015;91(6):1227–1243. doi:10.3987/COM-15-13210
32. Ahamed MR, Narren SF, Sadiq AS. Synthesis of 2-mercaptobenzimidazole and some of its derivatives. Al-Nahrain J Sci. 2013;16(2):77–83.
33. Dassault Systèmes Biovia. Biovia, Discovery Studio Modeling Environment. San Diego, CA, USA: Dassault Systèmes Biovia; 2016.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
