Back to Journals » Infection and Drug Resistance » Volume 16
Demographics and Clinical Outcomes of Culture-Positive versus Culture-Negative Pyogenic Liver Abscess in an Asian Population
Authors Liu Y , Liu J, Fu L, Jiang C , Peng S
Received 10 November 2022
Accepted for publication 12 January 2023
Published 16 February 2023 Volume 2023:16 Pages 903—911
DOI https://doi.org/10.2147/IDR.S395428
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yao Liu,1– 3 Jinqing Liu,1– 3 Lei Fu,1– 3 Chuan Jiang,1– 3 Shifang Peng1– 3
1Department of Infectious Diseases, Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 2Key Laboratory of Viral Hepatitis of Hunan Province, Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 3National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, People’s Republic of China
Correspondence: Shifang Peng, Department of Infectious Diseases, Xiangya Hospital, Central South University, Changsha, People’s Republic of China, Email [email protected]
Objective: Despite its high case-fatality risk, pyogenic liver abscess (PLA) lacks clear management guidelines in patients with negative microbial cultures. Our aim was to evaluate differences in clinical characteristics between patients with culture-negative liver abscess (CNLA) and those with culture-positive liver abscess (CPLA), and identify differences in the main causative pathogen.
Methods: In this study, we retrospectively collected medical records of PLA patients admitted to a teaching hospital from January 2010 to December 2019.
Results: In total, 324 PLA patients were enrolled in this study. Of these, 202 (62.3%) cases were confirmed cultural positive, including 109 patients (54%) and 20 (9.9%) patients infected with Klebsiella pneumoniae (K. pneumoniae) and Escherichia coli (E.coli), respectively. Patients in the CPLA group were older (p=0.029) and had higher prevalence of abscesses larger than 5 cm in diameter (p = 0.003), gas-forming rate (p = 0.016), and percutaneous drainage (p < 0.001) compared with CNLA group. Patients with CPLA had significantly longer hospitalizations than those with CNLA (p = 0.010). Nevertheless, there was no significant difference in in-hospital mortality between the two groups (p = 0.415). Compared with patients with E. coli, those with K. pneumoniae had higher incidence of diabetes mellitus (p = 0.041), solitary abscess (p < 0.001), localization in the right hepatic lobe (p = 0.033), abscess size larger than 5 cm (p < 0.001) and percutaneous drainage (p = 0.002), but mortality was not significantly different (p = 1.000).
Conclusion: No significant difference in in-hospital mortality was found between patients with CNLA and those with CPLA group. However, clinical characteristics and management were different between the main causative pathogens, including K. pneumoniae and E. coli.
Keywords: PLA, culture negative, culture positive, Klebsiella pneumoniae, Escherichia coli
Introduction
Pyogenic liver abscess (PLA) is a potentially life-threatening disease characterized by suppurating infections of the liver parenchyma with increasing incidence in many countries, including Korea, the United States, and China.1–3 Although specific medical interventions have contributed to significantly fewer deaths over the years, PLA mortality rate still remains relatively high, especially in Asia, where it is highest globally.4 The microbiology of PLA varies greatly, with Escherichia coli (E.coli), Klebsiella pneumoniae (K. pneumoniae), and Streptococcus spp. considered the most common causative organisms.5 In addition, multiple studies have shown that K. pneumoniae is the predominant pathogenic bacteria associated with PLA in Asia.6–8 Of the commonly used PLA treatment methods including antibiotic therapy, drainage, and surgery, antimicrobial treatment is the most important and first-line treatment. Pathogen identification and antimicrobial susceptibility data are essential for developing effective treatment regimens for PLA. Despite tremendous advances in microbiological diagnosis in recent years, the causative pathogen remains clearly undefined in many cases of liver abscess.9,10 Moreover, current research has focused more on culture-positive liver abscess (CPLA) patients than on culture-negative liver abscess (CNLA) patients. Consequently, it remains unclear whether the optimal treatment methods for CPLA can be used with CNLA patients, especially antimicrobial therapy. The purpose of the present study was to evaluate differences in clinical characteristics, treatment and prognosis between CPLA and CNLA groups, and compare the profile of two of the most commonly associated bacterial pathogens.
Materials and Methods
Study Design
To assess the microbiological and clinical characteristics of PLA, all hospitalized patients diagnosed with PLA from January 1, 2010 to December 31, 2019 in a tertiary teaching hospital were included in a retrospective study. The study was approved by the Institutional Ethics Committee of Xiangya Hospital, Central South University (Approval No. 202005055) and the conduct of the study was in accordance to the Declaration of Helsinki. Retrospective data of included patients were collected from the electronic medical record system. No additional samples were collected from the patients. Therefore, the ethics committee did not require written informed consent from study subjects.
Study Patients
Our study included patients who met the following criteria: the presence of typical clinical symptoms of PLA, including fever and abdominal pain; ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) evidence of liver abscess; laboratory findings showed elevated indicators of bacterial infection; and with blood culture or pus culture examinations. Patients were excluded based on the following criteria: age <18 years, missing or incomplete clinical data, fungal liver abscess, amoebic liver abscess, and both blood culture and pus culture results were lacking. Neither the blood culture nor pus culture results showed no microbial growth was defined as CNLA, and if any is positive, the patient was classified as CPLA. Polymicrobial infection was characterized by the presence of two or more microorganisms in blood or pus specimens.
Data Collection
Microbiological and medical data of all patients hospitalized due to PLA were retrieved from the electronic medical record system. The following information was collected and recorded: demographic data (age and gender), comorbidities, clinical features (symptoms and signs), laboratory (blood routine examination and liver function) and imaging findings (Doppler ultrasound, CT, and MRI), microbiological findings (blood culture and pus culture), treatments, and hospitalization days and outcomes.
Microbiological Characterization
All blood samples and pus samples were sent to the central laboratory of Xiangya Hospital for processing. Microbial identification and antimicrobial susceptibility testing were performed using the VITEK2 automated system (BioMérieux, France). After the blood culture bottle was positive, smear immediately, and transfer to Colombian blood plate and chocolate plate (BioMérieux, France), and at the same time inoculate a set of anaerobic bags (BioMérieux, France). After a single pure bacterium was cultivated, the corresponding turbidity was collected for the identification of the isolated bacterium. Drug susceptibility testing results were interpreted based upon the manual of Clinical and Laboratory Standards Institute.
Treatment Protocol
The treatment protocol for PLA in our institution was similar to the published literatures with some modifications.11 All patients with suspected PLA were firstly underwent ultrasonography, and CT or MRI were applied to confirm the results or examine complications. At least one set of blood culture were routinely performed in patients with PLA before administration of empiric broad-spectrum antibiotic agents. All patients in this study initially received intravenous empirical antimicrobial therapy. The empirical treatment options were third-generation cephalosporin or β-lactam/β-lactamase inhibitor combined with metronidazole according to the antimicrobial resistance surveillance data in our hospital. And we would adjust antibiotic therapy based on the cultures results and clinical, biochemical and radiologic assessments.
Patients who meet any of the following criteria will undergo percutaneous aspiration/drainage: (1) size of PLA > 5cm, (2) PLA < 5 cm while showed poor response to antibiotics treatment. Surgical drainage was performed in patients complicated with hepatobiliary calculi or tumors.
Statistical Analysis
All statistical analyses were conducted using SPSS version 18.0. Continuous data were presented as mean ± standard deviation (SD) and categorical variables as percentages. Chi-square (χ2 test) or Fisher’s exact test was used as categorical variables were compared, whereas continuous variables were compared using Student’s t-test or Mann–Whitney U-test. All analyses were considered statistically significant at p < 0.05.
Results
Demographic Characteristics of the Study Participants
A total of 528 PLA patients hospitalized from 2010 to 2019 were identified in the hospital’s system. After excluding ineligible patients, ultimately 324 were included in this retrospective study (Figure 1). Among the patients, 51.2% were male, with a mean age of 54.8 ± 12.5 years. K. pneumoniae and E. coli were the most frequent pathogens detected in 109 (54%) and 20 (9.9%) patients, respectively.
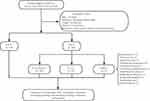 |
Figure 1 Workflow of the study. |
Clinical Characteristics of PLA Patients with CPLA versus CNLA
Demographic characteristics and clinical features of the patients with CPLA and CNLA are shown in Table 1. The mean age was 56.0 ± 11.8 years for the CPLA group, which was higher than 52.9 ± 13.5 years for the CNLA group (p = 0.029). Nevertheless, both groups did not differ significantly in terms of sex (p = 0.899). On the contrary, compared with the E. coli group, males were significantly higher in K. pneumoniae group (p = 0.001).
 |
Table 1 A Comparison of the Clinical Characteristics of PLA Patients |
Comorbidities
Diabetes mellitus was the most common comorbidity, which showed a higher incidence in the K. pneumoniae group than in the E. coli group (59.6% vs. 35%, p = 0.041). Patients with K. pneumoniae had more fatty liver but the difference was not significant (19.3% vs. 5%, p = 0.216). Patients with E. coli had a higher incidence of liver cirrhosis (20% vs. 1.8%, p = 0.003), hepatobiliary calculi (65% vs. 23.9%, p < 0.001) and history of hepatobiliary surgery (50% vs. 11%, p < 0.001) than those with K. pneumoniae. Nevertheless, comorbidities did not differ significantly between the CPLA and CNLA groups (Table 1).
Clinical Features
The most common symptom was fever (n = 282, 87%), which occurred in 89.3% and 85.6% of patients the CNLA group and CPLA group, respectively. There was an increased incidence of cough among CNLA patients when compared to CPLA patients (19.7% vs. 10.9%, p = 0.028). In both K. pneumoniae and E. coli groups, fever was the most common symptom, at 89% and 80%, respectively. This was followed by chills (n = 85, 65.9%), abdominal pain (n = 62, 48.1%), nausea or vomiting (n = 30, 23.3%), and coughs (n = 17, 13.2%) in both groups. Abdominal pain was more prevalent among patients with E. coli than those with K. pneumoniae (75% vs. 43.1%, p = 0.009) but did not differ significantly between the CNLA group and the CPLA group (Table 1).
Laboratory and Imaging Findings
No significant difference was found in white blood cell count (p = 0.165), neutrophil percentage (p = 0.069), hemoglobin (HB) (p = 0.615) and total bilirubin (p = 0.120) between the CPLA and CNLA groups. As compared to patients in the CNLA group, the CPLA group showed significantly higher levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (p < 0.001), but significantly lower albumin levels (p = 0.013). Patients in K. pneumoniae group and E. coli group had similar laboratory values for white blood cell count (p = 0.198), percentage of neutrophils (p = 0.874), albumin (p = 0.689), AST (p = 0.319), and total bilirubin (p = 0.164). However, ALT and HB levels were significantly higher in the K pneumoniae group (p = 0.006 and p = 0.008, respectively) (Table 2).
 |
Table 2 Comparison of Laboratory Findings in PLA Patients |
Imaging results showed that patients in the CPLA group were more likely to suffer from gas formation (p = 0.016) than those in the CNPA group. Besides, no significant difference was observed in the count of abscesses and intrahepatic location of abscesses between the two groups. Additionally, abscesses larger than 5 cm in diameter were more prevalent in patients with CPLA than those with CNLA (p = 0.003). Single abscess was five times as common as multiple abscesses in patients with K. pneumoniae, and a higher proportion of patients with K. pneumoniae had single abscess than those with E. coli (83.5% vs. 45%, p < 0.001). Compared with patients with E. coli, patients with K pneumoniae had a higher prevalence of abscesses in the right lobe (77.1% vs. 50%,) but less abscesses in left lobe and both lobes (11.9% vs. 20%, 9.2% vs. 30%, respectively) (p = 0.033). Patients with K. pneumoniae group more frequently had abscesses greater than 5 cm in diameter than those with E. coli group (p < 0.001) (Table 3).
 |
Table 3 Comparison of Imaging Findings for PLA Patients |
Microbiological Findings and Treatments
Blood and pus cultures were taken from 184 and 231 patients, respectively. Of these, blood sample was positive in only 38 cases whereas pus sample was positive in 175 cases. Among patients with CPLA, a higher number of PLA patients were infected with K. pneumoniae than E. coli (54% vs. 9.9%). Among K. pneumoniae cultures, only 19 cases (17.4%) showed multidrug resistance, whereas the remaining K. pneumoniae were sensitive to all antibiotics except ampicillin. By contrast, 13 (65%) of E.coli cultures exhibited multidrug resistance.
Most commonly used antibiotics including carbapenems, third-generation cephalosporin, β-lactam/β-lactamase inhibitor and metronidazole. No significant difference in antimicrobial therapy regimen was observed between the CNLA group and CPLA group. However, a significantly higher percentage of patients with CPLA received percutaneous drainage (n = 140, 69.3%) than those with CNLA (n = 57, 46.7%) (p < 0.001). Likewise, among patients with K. pneumoniae, percutaneous drainage was more prevalent than among those with E. coli (Table 4). Similar to previous study,12 there were more males in the group of percutaneous drainage combined antibiotics group than the antibiotics alone group. Meanwhile, PLA patients treated with percutaneous drainage had larger abscesses and lower total bilirubin levels than those treated with antibiotics alone. Then, the multivariate analysis showed that males (OR 2.009, P = 0.013), large abscesses (OR 1.032, P < 0.001) and low total bilirubin levels (OR 0.992, P = 0.045) were significantly correlated with percutaneous drainage (Supplementary Table 1).
 |
Table 4 Comparison of Treatment and Prognosis in PLA Patients |
Outcomes
Five cases died from septic shock or multiple organ failure. Overall in-hospital mortality was 1.9% (n = 6) and there was no significant difference in mortality between the CPLA group and CNLA group. However, hospital stay for CPLA patients was significantly longer compared with the CNLA group (Table 4).
Mortality rate in patients infected by K. pneumoniae was higher than in those infected by E.coli, but this difference was not significant (p = 1.000). Besides, no significant difference was detected in hospitalization days between patients with K. pneumoniae infections and those with E.coli infections (Table 4).
Discussion
The microbiologic etiology of PLA varies by geographic location. Streptococcus milleri was the most common pathogenic bacterium of PLAs in Europe,13 Canada14 and North America.15 However, several clinical studies have identified K. pneumoniae as the most common pathogen in mainland China.2,16–18 In the present study, among the pathogens responsible for PLA, K. pneumoniae is the most common, followed by E.coli, which is consistent with findings of previous studies from mainland China.
In our study, the main comorbidities for PLA patients were diabetes mellitus, hypertension, and history of hepatobiliary surgery, which is consistent with other studies.2,17 Patients with PLA were most commonly infected with K. pneumoniae, which showed a higher prevalence in diabetic patients.2,16,19–21 Consistent with these findings, we found that K. pneumoniae was the most common pathogen with a higher predominance in patients with diabetes mellitus compared with E.coli. Diabetes mellitus can promote bacterial growth because of impaired neutrophil activity and phagocytic function, as well as poorly controlled glucose levels.22 Therefore, good glycaemia control is crucial for effective management of patients with PLA caused by K. pneumoniae. Furthermore, patients with E.coli group had a higher prevalence of history of hepatobiliary surgery compared with those with K. pneumoniae. Since PLA patients with E.coli were more likely to have history of hepatobiliary surgery, it is reasonable to conclude that biliary tract infection is a risk factor for E.coli infection in PLA patients.2,20
A gas-forming abscess is a rare clinical symptom of liver abscess that is generally associated with high mortality rate. Lee et al23 and Yang et al24 suggested that the mortality of gas-forming abscess could be as high as 32% and 30%, respectively. In our study, patients with CPLA had higher gas-forming rates than those with CNLA. It is important to note, however, that CPLA and CNLA groups had no significant differences in prognosis. This finding was consistent with the propensity score analysis of Chan KS et al11 who showed that presence of gas may not have impact on outcomes. Patients with K. pneumoniae had a higher prevalence of single abscess predominantly located in the right lobe with a diameter of 5–10 cm, which was different from a previous study.2 This localization may be because the right liver lobe is the largest and is more inclined to receive the most blood flow from the portal vein and hepatic artery.9 In addition, bloodstream infections in patients with K. pneumoniae were the most common origin of infection.2
Treatment of liver abscesses is usually initiated before the pathogen is identified. Current antimicrobial guidelines generally recommend empiric therapy targeting gram-negative bacilli, gram-positive cocci, and anaerobes, with antibiotic choice based on host factors and local microbial prevalence. In previous studies,7 third-generation cephalosporins were the most commonly used antimicrobial agents. However, this finding was unsupported by our study, as the most frequently used antibiotics in the hospital were carbapenems. The reason for this antibiotic choice was that common pathogens, including E.coli, exhibited significant multidrug resistance. In addition, some inpatients in our hospital were transferred from other hospitals and may have used various other broad-spectrum antibiotics. Taking into account the proportion of patients treated percutaneously, the number of patients with K. pneumoniae–infected PLA was significantly higher than that of E.coli–infected PLA patients. The fact that the right lobe liver is the largest liver lobe, and relatively easy located and aspirated to avoid damage to major vessels and bile ducts account for this difference.20
As a life-threatening, intra-abdominal infection, PLA can result in high mortality (6% to 14%).25–28 In these studies, several factors were associated with PLA mortality, including age, sex, comorbidities, and prompt treatment. However, in our study, the overall hospital mortality was lower than that reported in previous studies, which might be related to the choice and practice time of the treatment strategies including the empiric antibiotics carbapenems,28 percutaneous drainage,26 and surgical drainage.28 In addition, it may also be related to the fact that the patient received early antibiotic treatment in other hospitals before being transferred to our hospital.
In the current study, we compared the demographics information, underlying diseases, clinical characteristics and management strategies between the CNLA and CPLA patients. Similar to findings of Shelat VG et al,29 we found that the age and ALT and AST levels were significantly different while the prognosis was comparable between CNLA and CPLA patients. However, the CPLA patients in the study of Shelat VG et al were only K. pneumoniae PLA patients, while in our study the CPLA were PLA patients with diverse pathogens including K. pneumoniae, E. coli and the like. Moreover, we have comprehensively compared with the properties of abscess including the location and degrees of sizes in our study. Despite the comparable in-hospital mortality between patients with CNLA and CPLA, we found length of stay was longer in CPLA compared to CNLA, which may be associated with age is older in CPLA group. The study of Chan KS et al30 suggested that age may be an independent predictor of hospitalisation stay in PLA while showed no impact on morbidity and mortality. In the current study, the proportion of CNLA patients was relatively high, this finding might partly attribute to the low positive rate of blood culture for the followed reasons. On the one hand, some of the patients were treated with antibiotics before hospitalization leading to low number of viable bacteria in abscess and blood cultures. On the other hand, the traditional culture-based methods were not suitable for fastidious pathogens. Therefore, when clinicians encounter CNLA patients, multiple blood cultures were recommended. And pus cultures were needed to improve the positive rate of bacterial cultures. Meanwhile, new technologies such as next-generation sequencing could be complemented with conventional culture method to identify more unique pathogens. In addition, no significant difference was found between CPLA and CNLA group in the type of use of the antibiotics. We speculated that similar antibiotic choices were used for CNLA and CPLA groups. In other words, since K. pneumoniae was the most common pathogen of PLA, we proposed that when clinicians encounter patients with CNLA, K. pneumoniae should be considered as the potential pathogen and choose the treatment strategy by referring to the K. pneumoniae PLA.
This study has some limitations that need to be considered. First, this was a retrospective study conducted at a single center and the results may not be generalizable to other populations. Second, some patients may have received antibiotics in other hospitals before completing blood and pus cultures in our hospital, which affects the positive rate of pus and blood cultures and the choice of antibiotics.
Conclusions
Our study revealed that K. pneumoniae and E.coli were the predominant pathogens of PLA in patients with differential demographics and underlying diseases and showed distinct clinical characteristics and management strategies. Despite the longer duration of hospital stay in CPLA group than CNLA group, the in-hospital mortality was comparable. Interestingly, our findings suggested that the choice of in CNLA group was similar to that of K. pneumoniae group.
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CNLA, culture-negative liver abscess; CT, computed tomography; CPLA, culture-positive liver abscess; K. pneumoniae, Klebsiella pneumoniae; E.coli, Escherichia coli; MRI, magnetic resonance imaging; PLA, pyogenic liver abscess.
Data Sharing Statement
The original data in this study can be obtained from the corresponding authors.
Acknowledgments
The authors thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81974080 and No. 82170640), the Independent Exploration and Innovation Project for Graduate students of Central South University (NO.2022ZZTS0853).
Disclosure
The authors declare no competing interests.
References
1. Meddings L, Myers RP, Hubbard J, et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol. 2010;105(1):117–124. doi:10.1038/ajg.2009.614
2. Yin D, Ji C, Zhang S, et al. Clinical characteristics and management of 1572 patients with pyogenic liver abscess: a 12-year retrospective study. Liver Int. 2021;41(4):810–818. doi:10.1111/liv.14760
3. Jun DW, Moon JY, Baeg SH, et al. 지역병원에서의 화농성 간농양의 임상적 고찰. [A clinical study of pyogenic liver abscess at two different local hospitals]. Korean J Hepatol. 2005;11(3):250–260. Korean.
4. Chen YC, Lin CH, Chang SN, Shi ZY. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the National Health Insurance Research Database of Taiwan, 2000–2011. J Microbiol Immunol Infect. 2016;49(5):646–653. doi:10.1016/j.jmii.2014.08.028
5. Jha AK, Das A, Chowdhury F, Biswas MR, Prasad SK, Chattopadhyay S. Clinicopathological study and management of liver abscess in a tertiary care center. J Nat Sci Biol Med. 2015;6(1):71–75. doi:10.4103/0976-9668.149091
6. Zhu X, Wang S, Jacob R, Fan Z, Zhang F, Ji G. A 10-year retrospective analysis of clinical profiles, laboratory characteristics and management of pyogenic liver abscesses in a Chinese hospital. Gut Liver. 2011;5(2):221–227. doi:10.5009/gnl.2011.5.2.221
7. Li W, Chen H, Wu S, Peng J. A comparison of pyogenic liver abscess in patients with or without diabetes: a retrospective study of 246 cases. BMC Gastroenterol. 2018;18(1):144. doi:10.1186/s12876-018-0875-y
8. Lo JZ, Leow JJ, Ng PL, et al. Predictors of therapy failure in a series of 741 adult pyogenic liver abscesses. J Hepatobiliary Pancreat Sci. 2015;22(2):156–165. doi:10.1002/jhbp.174
9. Foo NP, Chen KT, Lin HJ, Guo HR. Characteristics of pyogenic liver abscess patients with and without diabetes mellitus. Am J Gastroenterol. 2010;105(2):328–335. doi:10.1038/ajg.2009.586
10. Grosse K, Ohm D, Wurstle S, et al. Clinical characteristics and outcome of patients with enterococcal liver abscess. Sci Rep. 2021;11(1):22265. doi:10.1038/s41598-021-01620-9
11. Chan KS, Thng CB, Chan YH, Shelat VG. Outcomes of gas-forming pyogenic liver abscess are comparable to non-gas-forming pyogenic liver abscess in the era of multi-modal care: a propensity score matched study. Surg Infect. 2020;21(10):884–890. doi:10.1089/sur.2019.278
12. Liu Y, Li Z, Liu A, et al. Early percutaneous catheter drainage in protecting against prolonged fever among patients with pyogenic liver abscess: a retrospective cohort study. Ann Med. 2022;54(1):2269–2277. doi:10.1080/07853890.2022.2110612
13. Mohsen AH, Green ST, Read RC, McKendrick MW. Liver abscess in adults: ten years experience in a UK centre. QJM. 2002;95(12):797–802. doi:10.1093/qjmed/95.12.797
14. Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol H. 2004;2(11):1032–1038. doi:10.1016/S1542-3565(04)00459-8
15. Losie JA, Lam JC, Gregson DB, Parkins MD. Epidemiology and risk factors for pyogenic liver abscess in the Calgary Health Zone revisited: a population-based study. BMC Infect Dis. 2021;21(1):939. doi:10.1186/s12879-021-06649-9
16. Qian Y, Wong CC, Lai S, et al. A retrospective study of pyogenic liver abscess focusing on Klebsiella pneumoniae as a primary pathogen in China from 1994 to 2015. Sci Rep. 2016;6:38587. doi:10.1038/srep38587
17. Kong H, Yu F, Zhang W, Li X. Clinical and microbiological characteristics of pyogenic liver abscess in a tertiary hospital in East China. Medicine. 2017;96(37):e8050. doi:10.1097/MD.0000000000008050
18. Tian LT, Yao K, Zhang XY, et al. Liver abscesses in adult patients with and without diabetes mellitus: an analysis of the clinical characteristics, features of the causative pathogens, outcomes and predictors of fatality: a report based on a large population, retrospective study in China. Clin Microbiol Infect. 2012;18(9):E314–E330. doi:10.1111/j.1469-0691.2012.03912.x
19. Yoo JJ, Lee TK, Kyoung DS, Park MA, Kim SG, Kim YS. A population-based study of pyogenic liver abscess in Korea: incidence, mortality and temporal trends during 2007–2017. Liver Int. 2021;41(11):2747–2758. doi:10.1111/liv.15034
20. Chen SC, Wu WY, Yeh CH, et al. Comparison of Escherichia coli and Klebsiella pneumoniae liver abscesses. Am J Med Sci. 2007;334(2):97–105. doi:10.1097/MAJ.0b013e31812f59c7
21. Chan KS, Chia CTW, Shelat VG. Demographics, radiological findings, and clinical outcomes of Klebsiella pneumonia vs. non-Klebsiella pneumoniae pyogenic liver abscess: a systematic review and meta-analysis with trial sequential analysis. Pathogens. 2022;11(9):976. doi:10.3390/pathogens11090976
22. Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab. 2006;91(8):3084–3087. doi:10.1210/jc.2005-2749
23. Lee HL, Lee HC, Guo HR, Ko WC, Chen KW. Clinical significance and mechanism of gas formation of pyogenic liver abscess due to Klebsiella pneumoniae. J Clin Microbiol. 2004;42(6):2783–2785. doi:10.1128/JCM.42.6.2783-2785.2004
24. Yang CC, Chen CY, Lin XZ, Chang TT, Shin JS, Lin CY. Pyogenic liver abscess in Taiwan: emphasis on gas-forming liver abscess in diabetics. Am J Gastroenterol. 1993;88(11):1911–1915.
25. Yu SC, Ho SS, Lau WY, et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology. 2004;39(4):932–938. doi:10.1002/hep.20133
26. Alvarez Perez JA, Gonzalez JJ, Baldonedo RF, et al. Clinical course, treatment, and multivariate analysis of risk factors for pyogenic liver abscess. Am J Surg. 2001;181(2):177–186. doi:10.1016/s0002-9610(00)00564-x
27. Lok KH, Li KF, Li KK, Szeto ML. Pyogenic liver abscess: clinical profile, microbiological characteristics, and management in a Hong Kong hospital. J Microbiol Immunol Infect. 2008;41(6):483–490.
28. Wong WM, Wong BC, Hui CK, et al. Pyogenic liver abscess: retrospective analysis of 80 cases over a 10-year period. J Gastroenterol Hepatol. 2002;17(9):1001–1007. doi:10.1046/j.1440-1746.2002.02787.x
29. Shelat VG, Wang Q, Chia CL, Wang Z, Low JK, Woon WW. Patients with culture negative pyogenic liver abscess have the same outcomes compared to those with Klebsiella pneumoniae pyogenic liver abscess. Hepatobiliary Pancreat Dis Int. 2016;15(5):504–511. doi:10.1016/s1499-3872(16)60127-3
30. Chan KS, Junnarkar SP, Low JK, Huey CWT, Shelat VG. Aging is Associated with Prolonged Hospitalisation Stay in Pyogenic Liver Abscess-A 1:1 Propensity Score Matched Study in Elderly Versus Non-Elderly Patients. Malays J Med Sci. 2022;29(5):59–73. doi:10.21315/mjms2022.29.5.7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
