Back to Journals » Infection and Drug Resistance » Volume 16
Delphi Survey on the Current and Future Korean Guidelines for Isoniazid-Monoresistant Tuberculosis
Authors Seo W , Kim HW, Lee EG , An TJ , Kim S , Jeong YJ , Lee SH , Park Y, Mok J, Oh JY , Ko Y , Kim SH, Kwon SJ, Jung SS, Kim JW, Kim JS , Min J
Received 24 May 2023
Accepted for publication 1 August 2023
Published 11 August 2023 Volume 2023:16 Pages 5233—5242
DOI https://doi.org/10.2147/IDR.S420830
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Wan Seo,1 Hyung Woo Kim,2 Eung Gu Lee,3 Tai Joon An,4 Seunghoon Kim,5 Yun-Jeong Jeong,6 Sang Haak Lee,7 Yeonhee Park,8 Jeongha Mok,9 Jee Youn Oh,10 Yousang Ko,11 Sun-Hyung Kim,12 Sun Jung Kwon,13 Sung Soo Jung,14 Jin Woo Kim,1 Ju Sang Kim,2 Jinsoo Min15
1Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 2Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 4Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 5Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, St. Vincent’s Hospital, College of Medici ne, The Catholic University of Korea, Seoul, Republic of Korea; 6Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, Republic of Korea; 7Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Internal Medicine, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 8Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Daejeon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 9Department of Internal Medicine, Pusan National University Hospital, Busan, Republic of Korea; 10Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Republic of Korea; 11Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Hallym University Kangdong Sacred Heart Hospital, Seoul, Republic of Korea; 12Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Republic of Korea; 13Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Konyang University Hospital, Daejeon, Republic of Korea; 14Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Chungnam National University Hospital, Daejeon, Republic of Korea; 15Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
Correspondence: Jinsoo Min, Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul, 06591, Republic of Korea, Tel +82-2-2258-6785, Email [email protected]
Purpose: Isoniazid-monoresistant tuberculosis (Hr-TB) has emerged as a global challenge, necessitating detailed guidelines for its diagnosis and treatment. We aim to consolidate the Korean guidelines for Hr-TB management by gathering expert opinions and reaching a consensus.
Patients and Methods: A conventional Delphi method involving two rounds of surveys was conducted with 96 experts selected based on their clinical and research experience and involvement in nationwide tuberculosis studies and development of the Korean guidelines on tuberculosis. The survey consisted of three sections of questionnaires on diagnosis, treatment, and general opinions on Hr-TB.
Results: Among the 96 experts, 72 (75%) participated in the two rounds of the survey. A majority of experts (96%) strongly agreed on the necessity of molecular drug susceptibility testing (DST) for isoniazid and rifampin resistance in all tuberculosis patients and emphasized the importance of interpreting mutation types (inhA or katG) and additional molecular DST for fluoroquinolones for confirmed isoniazid-resistant cases. Over 95.8% of experts recommended treating Hr-TB with a combination of rifampin, ethambutol, pyrazinamide, and levofloxacin for six months, without exceeding 12 months unless necessary. They also acknowledged the drawbacks of long-term pyrazinamide use due to its side effects and agreed on shortening its duration by extending the duration of the rest of the treatment with a modified combination of choice.
Conclusion: This Delphi survey enabled Korean tuberculosis experts to reach a consensus on diagnosing and treating Hr-TB. These findings will be valuable for developing the upcoming revised Korean guidelines for Hr-TB management.
Keywords: tuberculosis, isoniazid-resistance, delphi technique
Introduction
Isoniazid (INH, H) is one of the key agents to treat tuberculosis (TB) today. After the first discovery of INH as a novel treatment for TB, it immediately became one of the “triple therapy”, a combination of streptomycin, para-aminosalicylic acid, and isoniazid.1 Even after replacing streptomycin and para-aminosalicylic acid with rifampicin (RIF, R), INH continues to be a first-line therapy. However, in the past decade, INH-monoresistant TB (Hr-TB) strains have emerged as a global challenge. The worldwide prevalence of Hr-TB among newly diagnosed TB patients is 7.4%, increasing to 11.4% among previously treated TB patients.2 In 2019, the World Health Organization (WHO) consolidated guidelines for drug-resistant TB treatment, recommending a regimen of RIF, ethambutol (EMB, E), pyrazinamide (PZA, Z), and levofloxacin (LFX) for patients with Hr-TB.3
The Korean Academy of Tuberculosis and Respiratory Disease (KATRD) adopted the WHO’s recommendation, releasing the fourth edition of the Korean Guidelines for Tuberculosis in 2020.4 The latest guidelines replace the previous recommendation of REZ for 6–9 months with a new chemotherapy regimen of RIF-EMB-PZA-LFX for 6 months in patients with Hr-TB. However, the current Hr-TB chapter of the guidelines only briefly introduces the consolidated WHO guidelines and their treatment recommendations. Considering the high incidence of Hr-TB among new TB patients and the prevalence of TB in the Republic of Korea, physicians need more detailed guidelines that include information on diagnosing INH resistance and when to switch to the new chemotherapy regimen. Therefore, this study aims to consolidate guidelines for Hr-TB management by gathering expert opinions and reaching a consensus on detecting Hr-TB, utilizing available diagnostic tools, and determining the optimal treatment strategy.
Materials and Methods
Study Design
We conducted a conventional Delphi method, a technique well introduced, and widely used in medical field,5,6 with two rounds of survey. The survey consisted of three sections with questions to be answered by experts anonymously: the first section for diagnosing Hr-TB, the second section for the treatment of Hr-TB, and the third section for general opinions on Hr-TB (Appendix 1). Regarding each question or statement, experts could choose from one of the followings: strongly agree, agree, neutral, disagree, and strongly disagree. Additionally, experts could leave comments or suggestions at the end of the survey. The first round of the survey was constructed using the Google Forms and sent to the experts via electronic mail. After two weeks, their replies were collected and analyzed. The result of the first round was sent back to the experts and the second round of survey with the same questionnaire was sent soon after. Again, their replies were collected after two weeks for final analysis (Figure 1).
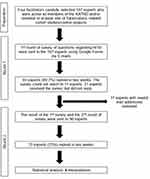 |
Figure 1 Flow chart of the Delphi method. Abbreviations: KATRD, Korean Academy of Tuberculosis and Respiratory Diseases; HrTB, Isoniazid-monoresistant tuberculosis. |
Facilitator Members and Experts
The questionnaire for the survey was developed and proposed by four facilitator members (Wan Seo, Hyung Woo Kim, Ju Sang Kim, and Jinsoo Min), all of whom are members of the KATRD with extensive clinical and academic experience in TB, as acknowledged in several publications.7–9 Ju Sang Kim is an advisory board member of the Korea Disease Control and Prevention and Jinsoo Min is a member of the Korean Multi-drug-resistant TB (MDR-TB) Consortium and the National TB Expert Review Committee. The questions in the survey primarily pertained to recent updates and changes recommended by the WHO.3,10 The facilitators reviewed the topics discussed in the WHO guidelines and created the questionnaire while considering the current situation in Korea. The 107 experts participating in the Delphi survey were thoughtfully selected by the four facilitators based on factors such as their clinical and research experience in TB and involvement in the Korean Guidelines for TB and/or other nationwide TB study groups.
The number of participating experts exceeded the usual range of 10–20, typical for consensus methods such as the nominal group technique (or the expert panel) and the Delphi technique.6 However, to achieve a robust consensus through a diverse range of expertise, we found it essential to involve a large number of experts. This approach was informed by the fact that the HrTB guidelines in Korea potentially influence not only a limited group of experts in university-affiliated hospitals, but also physicians in primary hospitals and many other professionals in the field. As results, the selected experts were KATRD members, working at tertiary referral hospitals, public hospitals, or government. To mitigate potential selection bias, we ensured the inclusion of more than one expert from each hospital or organization. Nineteen experts participated in nationwide TB cohort studies, and three experts were involved in a nationwide public-private mix TB control project in Korea. Eleven experts actively contributed to the development of the fourth edition of the Korean Guidelines for TB in 2020.
Results Interpretation and Proposing Recommendations
Overall, in terms of the median values for each question, the trend of responses of the second round remained to be the same as that of the first round with little refinement and no further round of survey was necessary (Appendix 1 and Table 1). The agreement rate was defined as the percentage of responses with either “strongly agree” or “agree”, whereas the disagreement rate was defined as the percentage of responses with either “disagree” or “strongly disagree”. For instance, if 100 experts responded to a question with the distribution 40/30/15/10/5 (Strongly agree/Agree/Neutral/Disagree/Strongly disagree), then the agreement rate would be 70% ((40+30)/100) and the disagreement rate would be 15% ((10+5)/100). If the agreement rate was greater than or equal to 70%, the statement in the question was decided to be recommended. Likewise, if the disagreement rate was greater than or equal to 70%, the statement in the question was decided to be recommended against. The rest of the cases were considered as neutral statements and were discarded in our final recommendation proposals.
 |
Table 1 The Second Round of the Delphi Survey on the Current and Future Guidelines for Diagnosis of Isoniazid-Monoresistant Tuberculosis |
Ethical Statement
This study was approved by the Institutional Review Board of the Catholic University of Korea (IRB No. UC22QIDI0134). Informed consent was obtained from the study participants before starting the survey.
Results
The first round of the survey was sent to 107 experts, and 96 experts received the survey emails. Eleven experts with invalid email addresses had to be removed without any further management in order to maintain the survey’s anonymity. Of the remaining 96 experts who received both emails from the first and second rounds of the survey, 72 (75%) responded and participated in the surveys. The responses for each question were counted and collected for statistical analysis by one of the four facilitators, who were only exposed to the email addresses (Figure 1).
In the first section for diagnosing Hr-TB, the median values for all six questions lay at either “strongly agree” or “agree”. Nearly 96% of the experts strongly agreed that conducting molecular drug susceptibility testing (DST) for INH and RIF resistance is essential for all TB patients, even in situations where the Xpert MTB/RIF assay has already been done (agreement rate of 90.3%). Most of the experts pointed out the usefulness of interpreting the type of mutations (inhA or katG) and additional rapid DST for fluoroquinolones (FQs) for those confirmed INH resistant (agreement rate of 74.6% and 91.5%, respectively). Nevertheless, 98.6% of the experts still preferred conventional culture-based phenotypic DST over the molecular DST and 73.6% prioritized the phenotypic DST result when there was a discrepancy between the results of phenotypic and molecular DSTs (Table 1).
In the second section for treatment of Hr-TB, the experts chose “strongly agree” or “agree” for most of the questions with agreement rate higher than 70%. This included changing the treatment regimens once INH resistance is confirmed in either phenotypic or molecular DSTs, treating Hr-TB with a combination of RIF-EMB-PZA-LFX for six months and not exceeding over 12 months unless it is necessary, and treating Hr-TB as MDR-TB when RIF has to be discontinued for any reason. Most experts were also well aware of the downside of the long-term use of PZA due to its side effects and agree on shortening its duration to two months by extending the duration of the rest of the regimen (agreement rate of 75%). In special situations where low-concentration resistance to INH or the inhA gene mutation was detected, some experts agreed on considering high-dose INH administration (agreement rate of 45.8%) (Table 2).
 |
Table 2 The Final Delphi Survey on the Current and Future Guidelines for Treatment of Isoniazid-Monoresistant Tuberculosis |
In the third section for general opinions on the current Korean guidelines for Hr-TB, 59.7% agreed that the current TB treatment guidelines (4th edition, 2020) require improvements in the section on Hr-TB, and 94.4% wished that the consensus reached through this Delphi survey would be reflected in the future revision of the Korean TB guidelines (Table 3).
 |
Table 3 The Final Delphi Survey on General Opinions on the Current Korean Guidelines for Isoniazid-Monoresistant Tuberculosis |
Discussion
Diagnosis of Hr-TB
Emerging from our survey results, we recommend conducting molecular DST using the initial culture isolates for all TB patients, regardless of the Xpert MTB/RIF assay (Table 4). This recommendation aligns with the WHO’s advice and is also in the context of the increasing prevalence of Hr-TB. A recent multicenter retrospective cohort study using culture-based phenotypic DST results from 4417 culture-confirmed TB patients who visited one of eight tertiary referral hospitals in Korea between 2015 and 2018 revealed that 7.2% (316/4417) of patients had Hr-TB, a higher proportion than those with RIF-resistant TB (RR-TB) (1.2%, 53/4417) or MDR-TB (4.1%, 179/4417).11 In a previous Korean nationwide study on anti-TB drug resistance in 2004, the prevalence of Hr-TB was 5.05% (133/2636) among new patients and 6.83% (19/278) among previously treated patients.12 The trend indicates a decline in MDR/RR-TB prevalence, while the proportion of Hr-TB has gradually increased.11,12 This highlights the concern that neglecting non-MDR Hr-TB may lead to a resurgence in the incidence of MDR-TB.13,14 Therefore, rapid detection of both RIF and INH-resistance using molecular methods will promote earlier diagnosis of Hr-TB and prompt switching to appropriate anti-TB regimens, ultimately reducing the burden of potential MDR-TB.
 |
Table 4 Summary of Newly Proposed Recommendations Based on the Delphi Survey on Isoniazid-Resistant Tuberculosis |
The experts who participated in our survey largely agreed on the usefulness of interpreting the type of mutation (inhA or katG) once molecular DST confirms the presence of INH resistance genetic mutations (Table 4. Recommendation 1.2). Regarding molecular DST for INH resistance, the WHO endorsed its use in 2008 based on evidence provided by a meta-analysis evaluating the diagnostic accuracy of commercially available line probe assays (LPAs): the INNO-LiPA Rif.TB assay (Innogenetics, Ghent, Belgium) and the GenoType MTBDRplus (version 1) (Hain Life-Science, Nehren, Germany).15 Those commercial LPAs, along with the AdvanSure MDR-TB GenoBlot Assay (AdvanSure; LG Life Science, South Korea), have been widely used in Korea and are familiar to primary care physicians. A previous study examining 206 genotypic INH-resistant strains of TB patients in Korea using the GenoType MTBDRplus assay showed 68.0% of the population with katG mutation and 35.0% with inhA mutation. The katG mutation exhibited high-level INH resistance in 94.8% of cases, while over half of the cases (51.5%) with inhA mutation showed low-level resistance.16
Our study identified a preference among experts for phenotypic DST over molecular DST when discrepancies between the results of the two tests occur when diagnosing Hr-TB (Table 4. Recommendation 1.4, 1.5). This can be explained by the notably lower sensitivity of the commercial LPAs for detecting INH resistance compared to RIF resistance. One main reason for this lower sensitivity is that these LPAs target only katG and inhA, while there are other genes associated with INH resistance, such as ahpC, furA, iniA, kasA, iniB, and iniC, exist.17 Therefore, early assumption on how one is resistant to INH based only on the molecular DST can lead to misdiagnosis and an inappropriate choice of treatment regimen.
The majority of experts (91.5%) agreed on conducting molecular DST for FQs in TB patients with confirmed INH resistance genetic mutations (Table 1). A recent multicenter retrospective cohort study in South Korea reported 1.2% (45/3720) resistance to any FQs in newly diagnosed TB patients and 1.3% (4/316) resistance in Hr-TB patients.11 Although the rate is relatively small compared to that of MDR-TB (20.1%, 36/179), because FQs are a key drug in the latest treatment of choice for Hr-TB, referring a sample for molecular DST for FQ is a prudent step to take before selecting a regimen.10 Currently, Korea has two commercial reference laboratories that offer gene sequencing for gyrA and gyrB resistance at no cost with financial support of the Korea Disease Control and Prevention Agency. The Korean Institute of Tuberculosis under the Korean National Tuberculosis Association also provides gene sequencing for gyrA and gyrB resistance for all Hr-TB cases confirmed either by phenotypic or molecular DSTs.
Treatment of Hr-TB
Our survey reaffirmed that most experts adhere to these guidelines, with a 95.8% agreement rate (69/72) on a 6-month course of RIF-EMB-PZA-LFX for Hr-TB. The latest Korean TB guidelines (the fourth edition of the Korean Guidelines for TB in 2020) adopted the WHO guidelines, recommending a 6-month course of RIF-EMB-PZA-LFX from the point of diagnosis of INH monoresistance. We also examined the other regimens and treatment durations suggested by global academies and organizations, including the WHO, Centers for Disease Control and Prevention, Infectious Diseases Society of America, and the Canadian Tuberculosis Standards. All guidelines published after the 2018 WHO guidelines introduced and recommended new regimens for Hr-TB, incorporating later generation FQs, preferably LFX (Table 5).
 |
Table 5 Regimens and Treatment Durations of Isoniazid-Resistant Tuberculosis Recommended by Academies and Organizations Worldwide |
In terms of when to initiate the switch in regimens for Hr-TB, our survey recorded an 84.7% agreement rate among experts when confirmed by molecular DST and a 97.2% agreement rate when confirmed by phenotypic DST (Table 2, Q1a and Q1b). A small percentage of expert (6.9% or 5/72) even disagreed on switching the regimen based solely on rapid DST. The statement in the WHO guidelines lacked specificity regarding the diagnosis of INH monoresistance before switching regimens, only mentioning “with confirmed rifampicin-susceptible and isoniazid-resistant”, without specifying whether phenotypic DST or molecular DST should be used to confirm INH monoresistance.3 Considering these results, our recommendation could not favor one DST over the other (Table 4, Recommendation 2.1). However, we anticipate that as molecular DST continues to improve in terms of sensitivity and specificity, differences in opinion will narrow.
Our survey highlighted the importance of LFX in treatment for Hr-TB. Of the experts, 66.7% did not agree on omitting LFX even in low-severity Hr-TB cases. A systemic review conducted with 19 cohort studies and 33 trials with 3744 patients with Hr-TB in 2017 showed that treatment with the previous WHO standard regimen for new patients resulted in treatment failure, relapse, and acquired multidrug resistance in 11%, 10%, and 8% of cases, respectively.18 Evidence from several cohort studies and randomized trials demonstrated significantly greater treatment success (adjusted odds ratio, 2.8; 95% confidence interval, 1.1–7.3) with addition of FQ to 6 months or more of REZ with or without INH ((H)REZ).19 Nevertheless, a vast majority of experts (93.1%) agreed on occasionally modifying the combination of anti-TB drugs, considering factors such as initial bacillary load, extent of lesions, culture conversion at two months, and potential pyrazinamide side effects (Table 2).
Reducing the duration of PZA to two months and extending the rest of the treatment duration could be an effective alternative strategy, with a 75.0% agreement rate among experts in our Delphi survey (Table 2). Long-term pyrazinamide use, in particular, raises concerns among physicians when treating Hr-TB with a 6-month course of RIF-EMB-PZA-LFX, due to its major side effects of hepatotoxicity, arthralgias, and prolonged inhibition of uric acid excretion. Patients with fulminant or sub-fulminant liver failure appeared to have a higher risk of death when treated with PZA.20 A recent retrospective cohort study of 626 notified HR-TB cases in London demonstrated that if the duration of (H)REZ treatment is long enough, a short PZA duration could be effective.21 Another retrospective multicenter cohort study of 318 notified HR-TB case in Korea revealed that shortening duration of PZA administration with additional FQ could be a safe alternative for patients with potential hepatotoxicity related PZA.22 In certain circumstances, particularly when PZA-related side effects are a concern, this alternative short-course PZA regimen may be a suitable option. (Table 4. Recommendation 2.3).
Conclusion
In conclusion, our Delphi survey, which was participated in by 96 experts, reached consensus on most of the major statements for diagnosing and treating Hr-TB. We strongly recommend conducting molecular DST using the initial culture isolates for all TB patients, regardless of the Xpert MTB/RIF assay. We advocate for the prompt switching of regimens once Hr-TB is suspected, but favor the conventional confirmation method with phenotypic DST. Once confirmed, we strongly recommend treating Hr-TB with a 6-month course of RIF-EMB-PZA-LFX. We anticipate for a few occasions when an alternative treatment strategy will be required due to concerns about long-term pyrazinamide side effects.
The strengths of our study include a high response rate (75%, with 72 out of 96 experts participating) and the utilization of the Delphi method to reach a consensus among experts, which is crucial for informing future guideline revisions on Hr-TB. However, this study also has limitations due to its focus on Korean TB experts, which may not adequately represent the perspectives of primary care physicians who are not specialized in TB and/or respiratory medicine yet are involved in diagnosing and treating TB patients. Conducting another survey involving non-TB expert physicians in the primary care field will be highly valuable for the revision of future TB guidelines.
Funding
This work was supported by the Research Program funded by the Korea National Institute of Health [grant number 2022E200100]. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Iseman MD. Tuberculosis therapy: past, present and future. Eur Respir J Suppl. 2002;20(Supplement 36):87s–94s. doi:10.1183/09031936.02.00309102
2. Dean AS, Zignol M, Cabibbe AM, et al. Prevalence and genetic profiles of isoniazid resistance in tuberculosis patients: a multicountry analysis of cross-sectional data. PLoS Med. 2020;17(1):e1003008. doi:10.1371/journal.pmed.1003008
3. World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment; 2019:120. Available from: https://www.who.int/publications/i/item/9789241550529.
4. The Korean Academy of Tuberculosis and Respiratory Disease (KATRD). Korean guidelines for tuberculosis (4th edition); 2020. Available from: https://www.lungkorea.org/bbs/index.html?code=guide&category=&gubun=&page=1&number=10303&mode=view&keyfield=&key=.
5. Choi H, Lee H, Ra SW, et al. Developing a diagnostic bundle for bronchiectasis in South Korea: a Modified Delphi Consensus Study. Tuberc Respir Dis. 2022;85(1):56–66. doi:10.4046/trd.2021.0136
6. Jones J, Hunter D. Qualitative Research: consensus methods for medical and health services research. BMJ. 1995;311(7001):376–380. doi:10.1136/bmj.311.7001.376
7. Kim HW, Min J, Choi JY, et al. Prevalence of latent tuberculosis infection among participants of the national LTBI screening program in South Korea - A problem of low coverage rate with current LTBI strategy. Front Public Health. 2022;10:1066269. doi:10.3389/fpubh.2022.1066269
8. Kim HW, Park S, Min J, et al. Hidden loss to follow-up among tuberculosis patients managed by public-private mix institutions in South Korea. Sci Rep. 2022;12(1):12362. doi:10.1038/s41598-022-16441-7
9. Kim JS, Kim YH, Lee SH, et al. Early bactericidal activity of delpazolid (LCB01-0371) in patients with pulmonary tuberculosis. Antimicrob Agents Chemother. 2022;66(2):e0168421. doi:10.1128/aac.01684-21
10. World Health Organization. WHO operational handbook on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection, 2021 update; 2021:162. Available from: https://www.who.int/publications/i/item/9789240030589.
11. Lee EG, Min J, Kang JY, et al. Age-stratified anti-tuberculosis drug resistance profiles in South Korea: a multicenter retrospective study. BMC Infect Dis. 2020;20(1):446. doi:10.1186/s12879-020-05157-6
12. Bai GH, Park YK, Choi YW, et al. Trend of anti-tuberculosis drug resistance in Korea, 1994–2004. Int J Tuberc Lung Dis. 2007;11(5):571–576.
13. Jenkins HE, Zignol M, Cohen T, Cattamanchi A. Quantifying the burden and trends of isoniazid resistant tuberculosis, 1994–2009. PLoS One. 2011;6(7):e22927. doi:10.1371/journal.pone.0022927
14. Stagg HR, Lipman MC, McHugh TD, Jenkins HE. Isoniazid-resistant tuberculosis: a cause for concern? Int J Tuberc Lung Dis. 2017;21(2):129–139. doi:10.5588/ijtld.16.0716
15. Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J. 2008;32(5):1165–1174. doi:10.1183/09031936.00061808
16. Seifert M, Catanzaro D, Catanzaro A, Rodwell TC, Mokrousov I. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: a systematic review. PLoS One. 2015;10(3):e0119628. doi:10.1371/journal.pone.0119628
17. Lee JH, Jo KW, Shim TS. Correlation between GenoType MTBDRplus assay and phenotypic susceptibility test for prothionamide in patients with genotypic isoniazid resistance. Tuberc Respir Dis. 2019;82(2):143–150. doi:10.4046/trd.2018.0027
18. Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(2):223–234. doi:10.1016/S1473-3099(16)30407-8
19. Fregonese F, Ahuja SD, Akkerman OW, et al. Comparison of different treatments for isoniazid-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med. 2018;6(4):265–275. doi:10.1016/S2213-2600(18)30078-X
20. Durand F, Bernuau J, Pessayre D, et al. Deleterious influence of pyrazinamide on the outcome of patients with fulminant or subfulminant liver failure during antituberculous treatment including isoniazid. Hepatology. 1995;21(4):929–932. doi:10.1002/hep.1840210407
21. Stagg HR, Bothamley GH, Davidson JA, et al. Fluoroquinolones and isoniazid-resistant tuberculosis: implications for the 2018 WHO guidance. Eur Respir J. 2019;54(4):1900982. doi:10.1183/13993003.00982-2019
22. Min J, Kim HW, Kang JY, et al. Comparison of different regimens with or without fluoroquinolone in isoniazid-resistant tuberculosis: a multicenter cohort study. PLoS One. 2022;17(8):e0273263. doi:10.1371/journal.pone.0273263
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
