Back to Journals » Journal of Pain Research » Volume 17
Delayed-Onset Muscle Soreness Alters Mechanical Sensitivity, but Not Thermal Sensitivity or Pain Modulatory Function
Authors Peterson J, Chesbro G, Bemben MG , Larson RD, Pereira HM, Black CD
Received 28 November 2023
Accepted for publication 30 January 2024
Published 8 February 2024 Volume 2024:17 Pages 571—581
DOI https://doi.org/10.2147/JPR.S449787
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Jessica Peterson,1– 3 Grant Chesbro,1 Michael G Bemben,1 Rebecca D Larson,1 Hugo M Pereira,1 Christopher D Black1
1Department of Health and Exercise Science, University of Oklahoma, Norman, OK, USA; 2Department of Kinesiology, New Mexico State University, Las Cruces, NM, USA; 3North Florida Foundation of Research and Education, Malcom Randall Veterans Medical Center, Gainesville, FL, USA
Correspondence: Jessica Peterson, Department of Kinesiology MSC 3M, New Mexico State University, P.O. Box 30001, Las Cruces, NM, 88003-8001, USA, Tel +1 405 318-9494, Email [email protected]
Introduction: Many clinical musculoskeletal pain conditions are characterized by chronic inflammation that sensitizes nociceptors. An unresolved issue is whether inflammation affects all nociceptors in a similar manner. Exercise-induced muscle damage (EIMD) has been proposed as a model for simulating clinical inflammatory pain in healthy samples. We sought to test the effect of EIMD on various painful stimuli (pressure and thermal), central pain processing (via the nociceptive flexion reflex) and endogenous pain modulation via conditioned pain modulation and exercise-induced hypoalgesia.
Methods: Eighteen participants (9F, age: 24.6 ± 3.3) were recruited for repeated measures testing and each completed pain sensitivity testing prior to and 48 hours after an eccentric exercise protocol. The participants performed a minimum of 6 rounds of 10 eccentric knee extension exercises to induce muscle damage and localized inflammation in the right quadriceps. Force decrements, knee range-of-motion, and delayed onset muscle soreness (DOMS) were used to quantify EIMD.
Results: There was a significant main effect of time for pressure pain (%diff; − 58.9 ± 23.1; p = 0.02, ηp2 = 0.28) but no significant main effect was observed for limb (%diff; − 15.5 ± 23.9; p = 0.53, ηp2 = 0.02). In contrast, there was a significant interaction between time and limb (p < 0.001, ηp2 = 0.47) whereby participants had lower pressure pain sensitivity in the right leg only after the damage protocol (%diff; − 105.9 ± 29.2; p = 0.002).
Discussion: Individuals with chronic inflammatory pain usually have an increased sensitivity to pressure, thermal, and electrical stimuli, however, our sample, following muscle damage to induce acute inflammation only had sensitivity to mechanical pain. Exercise induced inflammation may reflect a peripheral sensitivity localized to the damaged muscle rather than a global sensitivity like those with chronic pain display.
Keywords: pain sensitivity, exercise induced muscle damage, acute inflammation, pain modulation
Introduction
Many clinical musculoskeletal pain conditions are characterized by chronic inflammation1,2 with pro-inflammatory cytokines activating or sensitizing nociceptors, termed central sensitization, leading to hyperalgesia or increased pain sensitivity.3–5 Understanding how, and the time-course by which inflammation affects distinct populations of nociceptors as well as how it affects central pain processing is important for developing targeted interventions. Examining this question in clinical pain populations is challenging. Patients may have a similar diagnosis, but experience heterogeneous symptoms.6 Additionally, assessments of pain sensitivity have been shown lead to a worsening of symptoms7 in certain patient population, further complicating testing in these populations. Therefore, an alternative approach is needed.
Exercise-induced muscle damage (EIMD) has been proposed as an ecologically valid model of clinical inflammatory pain.8,9 It induces localized inflammation concomitant with heightened pain sensitivity,7 strength deficits leading to impairment of activities of daily living,10 and increased self-care behaviors.11 Using the EIMD model, Taguchi et al,12 demonstrated that localized inflammation increased peripheral sensitivity to mechanical, but not thermal or biochemical stimuli. Multiple studies have confirmed this finding of selective sensitization to mechanical stimuli in animal models of EIMD.12–14 In humans, EIMD has also been shown to lead to increased mechanical sensitivity, but not sensitivity to injection of warm water or capsaicin.14 Together these findings suggest EIMD and the inflammation consequent to it, likely affects thermal sensitive C-fibers, polymodal C-fibers and pressure sensitive A-delta fibers in skeletal muscle differently.12,14,15
Chronic inflammatory pain also impairs endogenous pain inhibition when assessed via conditioned pain modulation (CPM) and exercise induced hypoalgesia (EIH).16,17 Both conditioned pain modulation (CPM) and exercise-induced hypoalgesia (EIH) have been consistently demonstrated in young, healthy, pain-free populations.18–20 However, CPM is reduced in inflammatory conditions such as fibromyalgia,21 osteoarthritis,22 pancreatitis,23 and COVID-19.24 Additionally, EIH is also impaired in chronic pain conditions including fibromyalgia,25 chronic whiplash disorder26 and Gulf War syndrome.27 Little is known about the time-course of progression from normal to impaired endogenous pain inhibition in those with chronic inflammation. A previous study found acute inflammation from EIMD did not impair EIH28 in healthy young adults—indicating a few days of localized inflammation was likely not sufficient to alter EIH. However, no data exist on CPM.
The nociceptive flexion reflex (NFR) represents a measure of spinal nociceptive excitability.29 It is assessed as the threshold nociceptive input required to elicit/trigger reflexive motor activation. NFR has been shown to occur at a lower threshold in those with chronic pain and may serve as a maker of central nervous system sensitization.29 To our knowledge it has not been assessed following EIMD. Combining localized assessment of pressure and thermal thresholds with NFR, CPM, and EIH could provide a more complete picture of the potential peripheral and central changes in pain sensitivity that accompany EIMD. As such, the purpose of this study was three-fold. We sought to determine the effects of acute, localized inflammation from EIMD on 1) sensitivity to painful pressure and thermal stimuli, 2) the NFR as a marker of spinal sensitization, and 3) endogenous pain inhibition assessed via CPM and EIH. It was hypothesized that localized inflammation from EIMD would heighten sensitivity to mechanical, thermal, and electrical stimuli in the damaged limb, but not in the undamaged limb, and that CPM and EIH would be reduced in the days following eccentric exercise.
Materials and Methods
Sample
Eighteen individuals participated [24.6 ± 3.3 years and 50% female (N=9/18)] (Table 1). This was sufficient to detect a moderate effect (Cohen’s d = 0.5 SD) using repeated measures 2 limb (damaged vs control) × 2 time point (pre vs post EIMD) completely within ANOVA assuming an alpha of 0.05, a power of 0.8, and a correlation between repeated measurements of 0.9.30 Participants self-reported being free of musculoskeletal injuries and chronic pain conditions, and other diseases known to affect sensory processing. Females were tested during the luteal phase of their menstrual cycle. Participants refrained from resistance training, consumption of non-steroidal anti-inflammatory drugs (NSAIDS) and other pain medications, and the use of therapeutic modalities over the duration of the study.
 |
Table 1 Participant Characteristics |
Experimental Procedure
The repeated measures procedures were approved by the University of Oklahoma Institutional Review Board (IRB number 9972) and complied with the Declaration of Helsinki. Participants completed three visits over the course of five days in the Sensory Muscle Function Laboratory located in the Department of Health and Exercise Science at the University of Oklahoma. During the first visit, consent was obtained, and participants were familiarized with the exercise and pain testing protocols, and completed a host of questionnaires covering physical activity readiness (PAR-Q), menstrual history, drug history, a current pain location questionnaire, physical activity (International Physical Activity Questionnaire; IPAQ),31 pain catastrophizing (PCS),32 pain attitudes (PAQ),33 Profile of Mood States (POMS)34 and a 100mm visual analog scale (VAS) to assess movement evoked muscle pain/soreness. Anthropometric data were collected using a stadiometer (Secamodel 242, Chino, CA) and electronic scale (Tanita Model WB-627A, Tokyo Japan). Baseline knee joint range-of-motion (ROM) was then assessed with a goniometer. Participants were familiarized with the assessment of pressure pain threshold (PPT), heat pain threshold (HPT), and the nociceptive flexion reflex (NFR) to improve their reliability.35 Finally, participants were familiarized with the maximal voluntary contraction (MVC) protocol that was used to assess knee extensor strength.
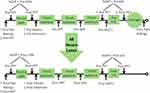
During visit two, participants completed a pain location questionnaire and rated their muscle pain/soreness. CPM was then assessed as was ROM. Next PPT, HPT, and NFR were assessed on both legs with a washout period of 15-min of quiet in order to minimize carryover. MVC was then determined and EIH was assessed. Participants then performed eccentric exercise to induce muscle damage. Following the EIMD protocol, participants’ MVC and soreness were reassessed. During visit 3, which occurred 48 ± 2 hours after the EIMD protocol, all procedures from visit 2 were repeated except for the eccentric exercise protocol (Figure 1).
Assessment of Pressure Pain Thresholds (PPT)
A handheld digital pressure algometer (Algomed, Medoc, Ramat Yishai, Israel) was used to assess PPT’s in the right and left vastus lateralis. A constant rate of pressure (30 kPa/second) was applied with the participant ending each trial by pressing a button to indicate when they deemed the applied pressure first “hurt.” The mean of three trials was calculated for each testing site and used as the criterion measure of PPT.35,36
Assessment of Heat Pain Thresholds (HPT)
A Medoc TSA-II (Ramat Yishai, Israel) was used to deliver heat stimuli to the vastus lateralis. A randomized staircase protocol was employed with a baseline thermode temperature of 32 °C and temperature increased and decreased at 5°C·s−1 intervals.37 Once the target temperature has been reached, it was applied for 5 seconds. After each stimulus, the participant indicated if the temperature was “painful” or “not painful”. Once a temperature was rated as “painful”, subsequent stimuli were presented in a randomized fashion in decreasing temperature increments until the threshold temperature was established.
Nociceptive Flexion Reflex (NFR)
Participants were placed in a prone position. The skin under the stimulation and EMG electrodes was abraded and wiped isopropyl alcohol. Pairs of circular (1.25inch) stimulating electrodes (PALS Platinum; Axelgaard; Fallbrook, CA, USA) were placed over the sural nerve inferior to the lateral malleolus. EMG electrodes were placed over the long head of the biceps femoris, 10cm superior to the popliteal fossa and a ground electrode was placed on the lateral epicondyle of the femur. A constant current stimulator (model DS7AH; Digitimer, Hertfordshire, England) controlled by a custom-written program in Biopac Acknowledge software was used to deliver square wave pulses (5 × 1msec pulses at 300 Hz). Stimulation began at 2mA, and was progressively increased in 3mA increments every 10sec to avoid temporal summation and progressive augmentation.38 This was done until the investigator observed the reflex within the EMG signal. Participant informed the investigator if the stimulus “hurt” after each application and to rate it on a 0–10 scale. If pain reached a 5/10 prior to the reflex being evoked, testing stopped. A BioNomadix dual channel wireless EMG system was used (Biopac, Goleta, CA, USA) at a sampling rate of 2000 Hz, amplified, and band-pass filtered (10 and 500 Hz). Recordings were collected and analyzed using Biopac AcKnowledge software (version 4.3). The amplitude of the reflex was recorded based upon the first appearance of the R3 segment of the reflex which has a latency of approximately 100–125ms.38
Conditioned Pain Modulation
PPT (previously described) was assessed on both legs as the test stimulus. Participants then submerged their foot into a cold-water bath as the conditioning stimulus that was maintained between 2°C and 4°C. After 60 seconds, participants removed their foot and PPT were reassessed. If participants withdrew their foot early, PPT was assessed immediately upon withdrawal. CPM was calculated as the percent change between PPT before immersion and PPT after immersion. Perceived pain during immersion was rated on a 10-point scale.
Exercise Induced Hypoalgesia and MVC
PPT (previously described) was assessed in both legs. Participants were then placed in an upright seated position on an isometric dynamometer (KinCom; Biopac, Goleta, CA, USA) with their dominant knee extended to 110 degrees (full leg extension being 180 degrees). The ankle of was secured against to an immobile lever arm. The force signal was digitalized using Biopac and displayed. Three MVC’s with three minutes rest between each attempt were performed by kicking against the lever arm as forcefully as possible for 3 seconds. The highest value was taken as their MVC. Participants were then instructed to hold 25% of their MVC until task failure (defined as force falling by 10% for 3 seconds). Visual feedback of target force and verbal encouragement were provided. PPTs were again measured immediately following exercise. EIH was calculated as the percent change in PPTs before and after exercise.
Muscle Soreness
A visual analog scale (VAS) was used to gather information regarding the participant’s ratings of perceived muscle soreness.39,40 A 100mm line with the words “no pain at all” and “worst imaginable pain” on opposite sides of the scale was used. Participants drew a line perpendicular to the printed line representing their perceived soreness during a body weight squat.
Knee Range of Motion
A goniometer was used to measure extended and flexed knee joint angles. Participants actively extended and flexed the knee joint while lying in prone position. Measurements were taken twice for each joint angle on left and right sides, and the mean value of the two measurements was used to calculate the total ROM of the joint.
EIMD Protocol
The participants were placed in an upright seated position on an isometric dynamometer (KinCom; Biopac, Goleta, CA, USA) with their dominant ankle secured to an immobile lever arm. They then performed six bouts of ten maximum voluntary eccentric knee extensions through a 100° ROM (170° to 70° of knee extension) at a speed of 20°·s−1. The dynamometer passively returned the knee to 170° after each eccentric action. One minute of rest was provided between sets. MVC was determined at completion of 6 sets. If MVC had declined by ≥20% then exercise ceased. If it had not, two additional sets of 10 eccentric contractions were performed.
Statistical Analysis
Statistical analyses were performed using SPSS 23 (IBM Armonk, New York, NY, USA). Descriptive characteristics are reported as means ± SD. Independent-measures t–tests were used to compare values for all descriptive variables age, height, weight, BMI, POMS scales, PCS, PAQ, and IPAQ domains between male and female participants. Normality was assessed using a Shapiro–Wilk test. Pre and 48-hr post EIMD MVC were compared using a paired sample t–test. ROM and all pain assessments (PPT, HPT, NFR, CPM, and EIH) were compared using a mixed model ANOVA with repeated factors for time (pre vs post EIMD) × and leg (damaged-leg vs control leg) and sex (male vs female) as a between factor. Pairwise comparisons of simple effects were performed using a Bonferroni correction for multiple comparisons. The significance level for all tests was set at p < 0.05.
Results
Participant Physical and Psychological Characteristics
Participant characteristics are shown in Table 1. Men were taller than women, but otherwise no differences were observed in physical activity, mood states, pain catastrophizing, or pain attitudes (P > 0.05).
Assessment of Muscle Damage
Immediately following the eccentric exercise protocol, MVC declined −32.0 ± 14.8% across all participants. Forty-eight hours later, MVC remained reduced (−17.7 ± 20.3%). Active knee ROM declined 9.1 ± 8.0° in the eccentrically exercised leg compared to the unexercised limb 1.2 ± 5.3 (0.9%; p < 0.001) and VAS assessed soreness increased from 2.9 ±3.5 to 24.4 ± 23.8 mm in the EIMD leg. These findings are indicative that EIMD occurred in the eccentrically exercised leg, as intended.
Pain Sensitivity Following EIMD
Pressure: The time × leg × sex interaction for PPT was not significant (P = 0.90), nor was the leg × sex (P = 0.19) and time × sex (P = 0.12) interaction, or the main effect for sex (P = 0.12) so data from men and women were collapsed and to increase sample size. There was a significant time × leg interaction (P = 0.002). Post-hoc testing using a Bonferroni correction demonstrated a higher PPT values at Pre in the damaged leg compared to the control leg (P = 0.02; Figure 2A). Additionally, a decline in PPT (−13%; P = 0.002) from pre to post EIMD in the damaged leg and no change (2.2%; P = 0.60) in the control leg.
Thermal: No changes were observed in the thermal pain threshold. The time × leg × sex interaction for HPT was not significant (P = 0.67; Figure 2B), nor was the time × leg (P = 0.25), leg × sex (P = 0.28) and time × sex (P = 0.90) interactions, nor the main effects for leg (P = 0.14), time (P = 0.42) or sex (P = 0.61).
Flexion Reflex: No changes were observed in the NFR. The time × leg × sex interaction for NFR was not significant (P = 0.30; Figure 2C), nor was the time × leg (P = 0.39), leg × sex (P = 0.42) and time × sex (P = 0.99) interactions, nor the main effects for leg (P = 0.71), time (P = 0.53) or sex (P = 0.056).
Pain Modulation Following EIMD
EIH: The time × leg × sex interaction for EIH was not significant (P = 0.28; Figure 3A), nor was the time × leg (P = 0.48), leg × sex (P = 0.86) and time × sex (P = 0.051) interactions, nor the main effects for time (P = 0.43) and sex (P = 0.57). A significant main effect for leg was found (P = 0.04) with the EIH response being consistently larger in the exercised leg (17.3% vs 25.8%).
CPM: The time × leg × sex interaction for CPM was not significant (P = 0.20; Figure 3B), nor was the leg x sex (P = 0.40) and time x sex (P = 0.14) interaction, or the main effect for sex (P = 0.97). There was a significant time x leg interaction (P = 0.016) so data from men and women were collapsed. Post-hoc testing using a Bonferroni correction demonstrated a difference between the control and damaged leg in the Pre damage condition (P = 0.002) with larger values observed in the control leg. This difference was no longer present in the Post damage condition (P = 0.72). EIMD had no effect on the magnitude of CPM in the damaged leg (Pre vs Post; P = 0.66), but in the control leg, CPM declined (P = 0.008) approximately 60%.
Discussion
Our primary findings were as follows: 1) EIMD resulted in mechanical, but not thermal hypersensitivity, 2) EIMD did not alter the NFR threshold, and 3) EIMD did not alter endogenous pain inhibition assessed by either CPM or EIH. A secondary finding was that there were no sex differences in the EIMD, NFR, EIH, or CMP response. This provided further evidence that localized inflammation from EIMD sensitizes thermal sensitive and polymodal C-fibers, as well as A-delta fiber differently in human models of pain. A lack of effect of EIMD on the NFR threshold as well as the EIH or CPM response provides evidence that acute, short-term inflammation is not sufficient to induce measurable central sensitization in the spinal cord and does not alter endogenous pain inhibition in a manner similar to that observed in chronic pain conditions.
It is accepted that mechanical hyperalgesia underlies the development of DOMS following EIMD. Inflammation consequent to EIMD leads to the activation and/or sensitization of a host of afferent nerves receptors such as transient receptor potential vanilloid (TPRV) 1 and 441,42 and acid sensing-ion channels (ASIC)42,43 which are implicated in mechanical hypersensitivity. Therefore, our findings of reduced PPTs and the presences of DOMS in the damaged leg was expected and agrees with observations from a range of previous studies.10–12,14,28,41–43 A potentially more interesting finding was that EIMD did not alter thermal (heat) pain thresholds in the damaged muscle. This has been observed in animal models of EIMD12,14 when heated Krebs solution was applied to muscle thin-fibers and in human when warm saline was injected into damaged muscle. C-fibers and their neurons expressing TRPV1 play a role in heat hyperalgesia,13 but EIMD does not appear sufficient to activate this pathway. Several studies12,14,15 suggest sensitization of polymodal C-fibers (fibers sensitive to both heat and mechanical stimuli), which express TRPV1, drives mechanical hypersensitivity following EIMD. A mechanistic answer to this question is beyond the scope of the current study. Therefore, further study is clearly needed to elucidate the divergent responses of these fibers to heat and mechanical stimuli following EIMD.
A novel finding of this study was that EIMD of the quadriceps did not alter the NFR. A recent meta-analysis44 found lower NFR threshold in a host of chronic pain conditions such as fibromyalgia, whiplash, headache, spinal pain, joint pain, and chronic widespread pain—providing clear evidence of central sensitization in those with chronic pain. Experiments have identified a host of factors that can modify the NFR threshold by increasing or decreasing tonic supraspinal inhibition.45,46 It was of interest to us to determine if short term (1–2 days), localized inflammation from EIMD might be able to induce spinal sensitization as chronic inflammation is a hallmark of many pain conditions. The lack of change in NFR in the present study provides the first human evidence that alterations in spinal excitability do not underly the development of DOMS.
Our findings that EIMD did not alter EIH agrees with the single previous study on this topic28 and to our knowledge our finding that EIMD did not alter CPM is the first of its kind. Endogenous pain modulation is often reduced in chronic inflammatory pain conditions such as fibromyalgia, knee osteoarthritis, and shoulder myalgia. EIH tends to be larger in the exercising limb (local) than at distal sites,47–49 as was found in the present study, suggesting a role for both peripheral and central factors. Our findings indicate localized inflammation in the exercising limb did not alter the peripheral or central components of EIH which agrees with a previous report from our lab.28 Similar to EIH, EIMD did not alter CPM in the damaged leg further indicating a lack of modification in the spinal and supraspinal components that contribute to CPM. CPM in the undamaged, control leg decreased after EIMD. This finding is difficult to interpret as prior to the induction of muscle damage, the magnitude of CPM was larger in the undamaged leg and the reduction following EIMD made CPM similar between legs (see Figure 3B). It is possible CPM was erroneously high in the “Pre” condition in the undamaged leg or it may have been a consequence of placing both feet in the ice bath. Given that it occurred in the undamaged leg, and that PPT, TPT, and NFR were not altered in that limb it seems unlikely that the reduced CPM was due to EIMD or DOMS in the contralateral limb. Some evidence using fMRI and EEG observed that the brain functional network had changed post EIMD, despite different locations of pain induced.50,51 Thus, the brain functional network may also change after EIMD in present study, however further investigation using these methods is warranted.
This study has several experimental considerations. First, there was a lack of diversity among participants (primarily Caucasian males and females) and this reduces generalizability of results to other populations. As mentioned above, we did not measure a true distal site (such as the trapezius muscle) in the CPM assessment which might have demonstrated different affect. Another consideration to the study is that the NFR was measured in the hamstring, but EIMD was induced in quadriceps. This was done in order to evoke a larger amount of acute inflammation given the quadriceps is a larger muscle than the hamstring and to allow for comparison to previous studies. However, our results might have differed if the stimulation site (sural nerve) or assessment site were in areas or muscles that had been directly damaged. Another limitation to this study is that there was a lack of a chronic pain group which may have helped to determine if pressure pain sensitivity, thermal sensitivity, conditioned pain modulation, and exercise induced hypoalgesia are any different from what would be expected in those without musculoskeletal injury or chronic pain conditions.
Conclusion
Our main finding was that pressure pain sensitivity, but not thermal pain sensitivity was reduced following eccentric exercise and EIMD, similar to a growing body of animal data. EIMD and its associated localized inflammation did result in central sensitization as no changes were observed in pain sensitivity, to pressure or heat, in the undamaged leg nor was the NFR threshold altered. Finally, EIMD did not alter CPM or EIH in the damaged leg. Taken together the findings of the present study suggest localized inflammation following eccentric exercise selectively sensitize mechanosensitive nociceptors, but do not alter central pain processing or endogenous pain modulation. Studies invoking a longer period of inflammation are necessary to explore the time-course of the transition to impaired central sensitization and modulatory function observed in many chronic pain population. Additionally, studies comparing experimentally induced muscle pain and those with chronic pain are warranted.
Acknowledgments
The authors would like to thank the participants for their time and effort throughout the project. An abstract on some of the findings presented in this paper was presented at the American College of Sport Medicine Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in Medicine and Science in Sport and Exercise. DOI: 10.1249/01.mss.0000878032.71202.7b
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Funding for this study was provided by the Department of Health and Exercise Science Helen Riddle Dissertation Award and the College of Arts and Sciences Robberson Research Award to Jessica Peterson.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Fernandes JC, Martel‐Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(12):237–246.
2. Ohtori S, Miyagi M, Inoue G. Sensory nerve ingrowth, cytokines, and instability of discogenic low back pain: a review. Spine Surg Related Res. 2018;2(1):11–17. doi:10.22603/ssrr.2016-0018
3. Dailey DL, Rakel BA, Vance CGT, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154(11):2554–2562. doi:10.1016/j.pain.2013.07.043
4. Zhang J-M, An J. Cytokines, inflammation and pain. Inter Anesthes Clinics. 2007;45(2):27. doi:10.1097/AIA.0b013e318034194e
5. Wegner A, Elsenbruch S, Maluck J, et al. Inflammation-induced hyperalgesia: effects of timing, dosage, and negative affect on somatic pain sensitivity in human experimental endotoxemia. Brain Behav Immun. 2014;41:46–54. doi:10.1016/j.bbi.2014.05.001
6. Vellucci R. Heterogeneity of chronic pain. Clin. Drug Invest. 2012;32(1):3–10. doi:10.2165/11630030-000000000-00000
7. Dannecker EA, Sluka KA. Pressure and activity-related allodynia in delayed-onset muscle pain. Clin J Pain. 2011;27(1):42–47. doi:10.1097/AJP.0b013e3181f04818
8. George SZ, Dover GC, Fillingim RB. Fear of pain influences outcomes after exercise-induced delayed onset muscle soreness at the shoulder. Clin J Pain. 2007;23(1):76–84. doi:10.1097/01.ajp.0000210949.19429.34
9. George SZ, Dover GC, Wallace MR, et al. Biopsychosocial influence on exercise-induced delayed onset muscle soreness at the shoulder: pain catastrophizing and catechol-o-methyltransferase (COMT) diplotype predict pain ratings. Clin J Pain. 2008;24(9):793–801. doi:10.1097/AJP.0b013e31817bcb65
10. Dannecker EA, Knoll V, Robinson ME. Sex differences in muscle pain: self-care behaviors and effects on daily activities. J Pain. 2008;9(3):200–209. doi:10.1016/j.jpain.2007.10.014
11. Dannecker EA, Gagnon CM, Jump RL, et al. Self-care behaviors for muscle pain. J Pain. 2004;5(9):521–527. doi:10.1016/j.jpain.2004.09.003
12. Taguchi T, Sato J, Mizumura K. Augmented mechanical response of muscle thin-fiber sensory receptors recorded from rat muscle–nerve preparations in vitro after eccentric contraction. J Neurophysiol. 2005;94(4):2822–2831. doi:10.1152/jn.00470.2005
13. Walder RY, Radhakrishnan R, Loo L, et al. TRPV1 is important for mechanical and heat sensitivity in uninjured animals and development of heat hypersensitivity after muscle inflammation. Pain. 2012;153(8):1664–1672. doi:10.1016/j.pain.2012.04.034
14. Queme F, Taguchi T, Mizumura K, et al. Muscular heat and mechanical pain sensitivity after lengthening contractions in humans and animals. J Pain. 2013;14(11):1425–1436. doi:10.1016/j.jpain.2013.07.010
15. Itoh K, Kawakita K. Effect of indomethacin on the development of eccentric exercise-induced localized sensitive region in the fascia of the rabbit. Jpn J Physiol. 2002;52(2):173–180. doi:10.2170/jjphysiol.52.173
16. Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthes. 2010;23(5):611–615. doi:10.1097/ACO.0b013e32833c348b
17. Trouvin A-P, Simunek A, Coste J, et al. Mechanisms of chronic pain in inflammatory rheumatism: the role of descending modulation. Pain. 2022;10:1097.
18. Naugle KM, Fillingim RB, Riley JL. A meta-analytic review of the hypoalgesic effects of exercise. J Pain. 2012;13(12):1139–1150. doi:10.1016/j.jpain.2012.09.006
19. Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101(1):155–165. doi:10.1016/S0304-3959(02)00324-X
20. Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care. 2015;9(2):131–137. doi:10.1097/SPC.0000000000000126
21. Potvin S, Larouche A, Normand E, et al. No relationship between the ins del polymorphism of the serotonin transporter promoter and pain perception in fibromyalgia patients and healthy controls. Eur J Pain. 2010;14(7):742–746. doi:10.1016/j.ejpain.2009.12.004
22. Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88(1):69–78. doi:10.1016/S0304-3959(00)00310-9
23. Olesen SS, Brock C, Krarup AL, et al. Descending inhibitory pain modulation is impaired in patients with chronic pancreatitis. Clin Gastro Hepatol. 2010;8(8):724–730. doi:10.1016/j.cgh.2010.03.005
24. Peterson JA, Bemben MG, Larson RD, et al. Symptomatic but not asymptomatic COVID-19 impairs conditioned pain modulation in young adults. J Pain. 2022;23(11):1923–1932. doi:10.1016/j.jpain.2022.06.010
25. Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118(1–2):176–184. doi:10.1016/j.pain.2005.08.007
26. Van Oosterwijck J, Nijs J, Meeus M, et al. Lack of endogenous pain inhibition during exercise in people with chronic whiplash associated disorders: an experimental study. J Pain. 2012;13(3):242–254. doi:10.1016/j.jpain.2011.11.006
27. Cook DB, Stegner AJ, Ellingson LD. Exercise alters pain sensitivity in gulf war veterans with chronic musculoskeletal pain. J Pain. 2010;11(8):764–772. doi:10.1016/j.jpain.2009.11.010
28. Black CD, Tynes BK, Gonglach AR, et al. Local and generalized endogenous pain modulation in healthy men: effects of exercise and exercise-induced muscle damage. Pain Med. 2016;17(12):2422–2433. doi:10.1093/pm/pnw077
29. Smith SM, Dworkin RH, Turk DC, et al. The potential role of sensory testing, skin biopsy, and functional brain imaging as biomarkers in chronic pain clinical trials: immpact considerations. J Pain. 2017;18(7):757–777. doi:10.1016/j.jpain.2017.02.429
30. Peterson JA, Schubert DJ, Campbell J, et al. Endogenous pain inhibitory function: endurance-trained athletes vs active controls. Pain Med. 2019;20(9):1822–1830. doi:10.1093/pm/pnz014
31. Hallal PC, Victora CG. Reliability and validity of the international physical activity questionnaire (IPAQ). Med Sci Sports Exerc. 2004;36(3):556. doi:10.1249/01.MSS.0000117161.66394.07
32. Sullivan M, Bishop S, Pivik J. The pain catastrophizing scale: development and validation. Psychological Assessment. 1995;7(4):524–532. doi:10.1037/1040-3590.7.4.524
33. Yong HH, Gibson SJ, de L. Horne DJ, et al. Development of a pain attitudes questionnaire to assess stoicism and cautiousness for possible age differences. J Gerontol B Psychol Sci Soc Sci. 2001;56(5):P279–84. doi:10.1093/geronb/56.5.P279
34. McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971:27.
35. Black CD, Pickowitz KE. Day-to-day reliability of pressure pain threshold and pain ratings in college-aged men. Int J Rehabil Res. 2015;38(3):213–218. doi:10.1097/MRR.0000000000000111
36. Lautenbacher S, Kunz M, Strate P, et al. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115(3):410–418. doi:10.1016/j.pain.2005.03.025
37. Gracely RH, Lota L, Walter DJ, et al. A multiple random staircase method of psychophysical pain assessment. Pain. 1988;32(1):55–63. doi:10.1016/0304-3959(88)90023-1
38. Skljarevski V, Ramadan N. The nociceptive flexion reflex in humans–review article. Pain. 2002;96(1–2):3–8. doi:10.1016/S0304-3959(02)00018-0
39. Bodian CA, Freedman G, Hossain Set al. The visual analog scale for pain: clinical significance in postoperative patients. J Am Soc Anesthes. 2001;95(6):1356–1361.
40. Langley G, Sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatol Inter. 1985;5(4):145–148. doi:10.1007/BF00541514
41. Ota H, Katanosaka K, Murase S, et al. TRPV1 and TRPV4 play pivotal roles in delayed onset muscle soreness. PLoS One. 2013;8(6):e65751. doi:10.1371/journal.pone.0065751
42. Fujii Y, Ozaki N, Taguchi T, et al. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140(2):292–304. doi:10.1016/j.pain.2008.08.013
43. Matsubara T, Hayashi K, Wakatsuki K, et al. Thin-fibre receptors expressing acid-sensing ion channel 3 contribute to muscular mechanical hypersensitivity after exercise. Eur J Pain. 2019;23(10):1801–1813. doi:10.1002/ejp.1454
44. Amiri M, Esmaili H, Hamad AH, et al. Nociceptive flexion reflex threshold in chronic pain patients: a needed update for the current evidence. Am J Phys Med Rehabil. 2021;100(8):750–759. doi:10.1097/PHM.0000000000001626
45. Miller JC, Boureau F, Albe-Fessard D. Supraspinal influences on nociceptive flexion reflex and pain sensation in man. Brain Res. 1979;179(1):61–68. doi:10.1016/0006-8993(79)90489-X
46. Grönroos M, Pertovaara A. Capsaicin-induced central facilitation of a nociceptive flexion reflex in humans. Neurosci Lett. 1993;159(1–2):215–218. doi:10.1016/0304-3940(93)90837-B
47. Kosek E, Lundberg L. Segmental and plurisegmental modulation of pressure pain thresholds during static muscle contractions in healthy individuals. Eur J Pain. 2003;7(3):251–258. doi:10.1016/S1090-3801(02)00124-6
48. Vaegter HB, Handberg G, Graven-Nielsen T. Hypoalgesia after exercise and the cold pressor test is reduced in chronic musculoskeletal pain patients with high pain sensitivity. Clin J Pain. 2016;32(1):58–69. doi:10.1097/AJP.0000000000000223
49. Peterson JA, Lohman C, Larson RD, et al. Body composition does not influence conditioned pain modulation and exercise-induced hyperalgesia in healthy males and females. Eur J Pain. 2022;26(8):1800–1810. doi:10.1002/ejp.2005
50. Boissoneault J, Penza CW, George SZ, Robinson ME, Bishop MD. Comparison of brain structure between pain-susceptible and asymptomatic individuals following experimental induction of low back pain. Spine J. 2020;20(2):292–299. doi:10.1016/j.spinee.2019.08.015
51. Bush NJ, Schneider V, Sevel L, Bishop MD, Boissoneault J. Associations of regional and network functional connectivity with exercise-induced low back pain. J Pain. 2021;22(12):1606–1616. doi:10.1016/j.jpain.2021.05.004
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.