Back to Journals » Journal of Pain Research » Volume 16
Cytotoxicity of Local Anesthetics on Bone, Joint, and Muscle Tissues: A Narrative Review of the Current Literature
Authors Zhang K, Li M, Yao W, Wan L
Received 19 November 2022
Accepted for publication 30 January 2023
Published 27 February 2023 Volume 2023:16 Pages 611—621
DOI https://doi.org/10.2147/JPR.S398329
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ellen M Soffin
Kaiwen Zhang, Meihong Li, Wenlong Yao, Li Wan
Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Li Wan, Department of Anaesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China, Email [email protected]
Background: Local anesthetics are commonly used in surgical procedures to control pain in patients. Whilst the cardiotoxicity and neurotoxicity of local anesthetics have received much attention, the cytotoxicity they exert against bone, joint, and muscle tissues has yet to be well recognized.
Objective: This review aimed to raise awareness regarding how local anesthetics may cause tissue damage and provide a deeper understanding of the mechanisms of local anesthetic-induced cytotoxicity. We summarized the latest progress on the cytotoxicity of local anesthetics and the underlying mechanisms and discussed potential strategies to reduce it.
Findings: We found that the toxic effects of local anesthetics on bone, joint, and muscle tissues were time- and concentration-dependent in vitro. Local anesthetics induced apoptosis, necrosis, and autophagy through specific cellular pathways. Altogether, this review indicates that toxicity of local anesthetics may be avoided by rationally selecting the appropriate anesthetic, limiting the total amount, and determining the lowest effective concentration and duration.
Keywords: local anesthetics, cytotoxicity, myotoxicity, chondrotoxicity, cell death mechanism
Introduction
Peripheral nerve blocks are an important component of multimodal postoperative pain management. They have been shown to decrease pain scores and opioid use in the immediate postoperative period.1 Although this method is becoming increasingly safe, using local anesthetics still carries potential risks. Previous studies have focused on the cytotoxicity of local anesthetics in the nervous and cardiovascular systems. With the application of fascial plane blocks, such as transverse abdominis block, erector spinae plane block, and periarticular infiltration, the cytotoxicity of local anesthetics to muscles, bones, and joints has attracted much attention. In vitro studies have found that local anesthetics can be toxic to myocytes,2–4 chondrocytes,5–8 tendon cells,9–11 intervertebral disc (IVD) cells,12–14 and mesenchymal stem cells(MSCs),15–17 leading to reduced cell metabolism and increased apoptosis, necrosis, and autophagic activity.
In this review, we would like to raise awareness regarding tissue damage caused by different local anesthetics and provide a deeper understanding of the mechanisms of local anesthetic-induced cytotoxicity. Although the cytotoxicity of local anesthetics is not common in clinical practice, to use the anesthetic more safely due to the variance in cytotoxicity among local anesthetics, the information provided here may help guide anesthesiologists in their medication selection.
Methods
A literature search was performed using PubMed, Embase, Cochrane Library, and Web of Science without any language restriction. The keywords “cytotoxicity”, “toxicity”, and “local anesthetics” were used to search. Studies from January 1, 2000, until October 28, 2022, and any referenced articles deemed significant were included. Randomized controlled trials, case reports, retrospective studies, meta‐analyses and systematic reviews were included. The articles unrelated to the bone, joint and muscle were excluded. Articles were assessed for relevance, and data were qualitatively analyzed.
Local Anesthetics Induced Cytotoxicity in a Concentration- and Time-Dependent Manner
Sixteen studies using four different local anesthetics were included in this review (Table 1). The data showed that different local anesthetics might lead to varying degrees of decreased cell death. Lidocaine and bupivacaine were found to be more cytotoxic than ropivacaine and mepivacaine.8,15 Studies showed significant cytotoxicity with high concentrations of bupivacaine (>0.175%) and lidocaine (>1%);3,12 the number of dead cells increased in a concentration- and time-dependent manner, whereby cell death obviously increased after exposure of >1 h.10 In contrast, ropivacaine was the least toxic at clinically used concentrations, with no significant dead cells after treatment with varying concentrations.6 These findings, however, were limited to in vitro situations. Whether local anesthetics induce cytotoxicity in a concentration- and time-dependent manner in vivo remains to be explored.
 |
Table 1 Studies on Local Anesthetic Cytotoxicity Affecting Bone, Joint, and Muscle Tissues |
Cytotoxicity of Local Anesthetics in Research Studies
Myotoxicity
Local anesthetics may induce myotoxicity; the higher the concentrations and the longer the duration of exposure to local anesthetics, the greater the damage to the muscles.2–4 Clinically relevant local anesthetics concerning myotoxicity included lidocaine, ropivacaine, and bupivacaine, in increasing order of toxicity. The addition of epinephrine to local anesthetics may also increase myotoxicity.18,19 The histological changes in muscle injury caused by different local anesthetics were similar, whereby a few minutes after local injection, the damage was the most obvious in the area close to the muscle fibers; a few hours after local injection, the sarcoplasmic reticulum dissolved and degenerated, and the myocytes showed oedema and necrosis. However, the basal layer, vascular system, neurons, and connective tissue structures remained intact. Characteristic fiber damage and eosinophils appeared in the adjacent areas.20 Nevertheless, muscle injury caused by local anesthetics is entirely reversible: regeneration is complete within three to four weeks.18
Although many animal studies have confirmed the myotoxicity of local anesthetics, few clinical reports exist.21 Studies have shown that approximately 0.53% and 0.11% of patients with ophthalmic blocks and adductor canal blocks experience myotoxicity symptoms.18 The actual rate of myotoxicity may be higher as local pain at the injection site often causes anesthesiologists to ignore damage to the muscle. Most myotoxicity is missed due to rapid and complete recovery.21 Additionally, the acute inflammatory response associated with surgery and acute-onset flaccid limb weakness may mask myotoxicity. Therefore, most cases of local anesthetic-induced myotoxicity are clinically overlooked.
Chondrotoxicity
In few clinical studies, applying intra-articular local anesthetics by pain pumps may contribute to joint and bone damage.22 Hansen et al23 found that 19 of 177 patients undergoing shoulder arthroscopic surgery received postoperative intra-articular analgesia, and 12 patients, all of which had pain pumps, developed post arthroscopic glenohumeral chondrolysis (PAGCL) 19 months after surgery. Other studies have shown that the risk of PAGCL is associated with flow rates since, as compared to low flow rates, it is prominently higher in patients with high.24 Moreover, for shoulder arthroscopy, using subacromial rather than intra-articular pain pumps prevents the development of PAGCL.23,25 Chondrolysis was also observed after arthroscopic surgery in the knee in a study in which 21 patients who received intra-articular bupivacaine with a low or high flow rate after knee surgery developed severe postoperative knee chondrolysis, causing pain in the knee during daily activities.26
Kreuz et al24 showed that exposure to local anesthetics for >1 hour significantly affected cultured chondrocytes. The half-lives of lidocaine, mepivacaine, ropivacaine, and bupivacaine are 1.6, 1.9, 1.9, and 3.5 hours, respectively, which may account for the higher chondrotoxicity of bupivacaine. Local anesthetics have concentration- and time-dependent chondrotoxic effects in vitro (Table 1).6–8 Although in vitro studies indicated potential chondrocyte toxicity for bupivacaine and lidocaine, it was not clear the true duration of contact from local anesthetics injection into a peripheral joint in vivo. However, given the variance in chondrotoxicity among local anesthetics, the studies we have included here could help guide anesthesiologists in their medication selection. As far as clinical selection is concerned, studies have shown that low concentrations of ropivacaine and mepivacaine are chosen over bupivacaine and lidocaine as they are less toxic for cartilage.6,24
Tendon Toxicity
Injections of local anesthetics around tendons are commonly used to treat pain. In a rotator cuff study, Nuelle et al9 assessed the effect of local anesthetics on tendons. They exposed supraspinatus tendon cell explants to lidocaine (0.5% and 1%) and bupivacaine (0.0625%, 0.125%, and 0.25%) and cultured them for seven days; exposure to 1% lidocaine (p < 0.001), but not to 0.125% or 0.0625% bupivacaine, significantly decreased cell viability.
In addition, Sung et al10 investigated the potential cytotoxic effects of bupivacaine, ropivacaine and lidocaine on cultured human rotator cuff tenofibroblasts and found that the survival rate of these cells decreased significantly with an increase in anesthetic concentration and exposure time. They further identified an increased generation of reactive oxygen species (ROS) and caspase activation as factors mediating tenofibroblast death and observed that 0.2% ropivacaine exerted the least toxic effects. These findings were corroborated by Zhang et al,11 suggesting that appropriate concentrations and exposure times should be chosen carefully when using local anesthetics to avoid associated side effects.
IVD Toxicity
Intra-IVD injection of local anesthetics has been used in diagnosing or treating discogenic back pain. Currently, bupivacaine is often administered intraoperatively and postoperatively, which is the most commonly used intervention to reduce pain by local, spinal, or epidural injection. Due to its mechanism of inhibiting sensitization of nerve endings and reducing inflammation in degenerative IVDs, in the treatment of back pain, the efficacy of bupivacaine has been demonstrated during clinical practice.12 However, specific adverse effects of bupivacaine have been reported, particularly concerning its cytotoxicity towards IVD cells in vitro.12 This was first observed by Quero et al,14 who found that the viability of these cells decreased when exposed to bupivacaine.
The higher the concentration of the local anesthetic, the worse the damage conferred to the IVD cells. Cai et al13 exposed cultured IVD cells to lidocaine, bupivacaine, and ropivacaine and found that the cytotoxicity of ropivacaine was lower than that of both lidocaine and bupivacaine.
MSCs Toxicity
It is common to use allogenic or autologous human MSCs for tissue repair by local injection or surgical implantation.15 In orthopedic cartilage repair surgery, intra-articular injection of human bone marrow MSCs can be performed preoperatively, intraoperatively, and postoperatively with local anesthetics.16,27 However, in vitro data, suggest that the use of local anesthetics at the same concentration clinically as that used in vitro can have cytotoxic effects on human MSCs.11,17 There are differences in cytotoxicity among different local anesthetics: ropivacaine is significantly less toxic than bupivacaine and lidocaine, whilst they have the same anesthetic efficacy.15,16,28–32
The safety of local anesthetics combined with bone marrow MSCs in intra-articular treatment has been widely studied. Dregalla et al15 studied the effect of local anesthetics on MSCs viability and investigated the mechanism by which local anesthetics induce the death of these cells. They exposed cultured and expanded MSCs from three donors to ropivacaine, lidocaine, bupivacaine, and mepivacaine. They demonstrated that 24 hours of exposure significantly affected the viability and adhesion of MSCs, and all local anesthetics except ropivacaine caused cell death even after brief exposure.15
Mechanisms of Local Anesthetic-Induced Cytotoxicity
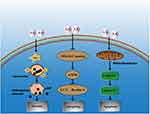
Cytotoxicity can develop within a few minutes of exposure to local anesthetics in vitro.17 The underlying mechanisms responsible for these adverse effects include apoptosis, necrosis, and autophagy (Table 2, Figure 1).
 |
Table 2 Mechanisms of Local Anesthetic-Induced Cytotoxicity |
Local Anesthetic-Induced Apoptosis
Fluorescence microscopic observations confirmed that the cytotoxicity observed in chondrocytes, IVD cells, and tenocytes was mainly caused by apoptosis after prolonged exposure to local anesthetics.6 Local anesthetics could induce cell apoptosis by inhibiting mitochondrial energy metabolism,33–39 which was based on a variety of mechanisms, such as mitochondrial uncoupling, reduction of the mitochondrial membrane potential, and reduction of the respiratory chain protein content.34 All these phenomena could be observed during long-term local anesthetic exposure (>24 hours).10,40 Grishko et al36 analysed the chondrotoxicity of different local anesthetics in human chondrocyte cultures and found 1% and 2% lidocaine as well as 0.5% bupivacaine induced mitochondrial DNA damage, decreased mitochondrial protein levels, and enhanced mitochondrial membrane permeability; subsequently, caspases were activated, leading to cell apoptosis. Further studies showed that local anesthetics could induce various cellular changes, including DNA breakage, chromatin concentration, and apoptotic body formation,15,24 all indicated apoptosis.
Local Anesthetic-Induced Necrosis
Whilst at low concentrations, local anesthetics could induce apoptosis in myocytes, higher concentrations predominantly cause necrosis in these cells.40 Correspondingly, studies have shown that bupivacaine and ropivacaine mainly cause necrosis at high concentrations,28 which was caused by lysosomal membrane permeabilization (LMP) mediated by ROS.41–43 Treatment of IVD cells with bupivacaine produces ROS in a dose-dependent manner,37 and ROS could quickly diffuse into lysosomes and interact with free iron. LMP was induced by lipid peroxidation of the lysosomal membrane through the Fenton reaction, which was followed by the release of the lysosomal content into the cytosol, subsequently leading to cell death.37 Transmission electron microscopy revealed nuclear alterations, swollen organelles, rupture of the plasma membrane, and cytolysis following exposure to local anesthetics, which confirmed local anesthetic-induced cell necrosis.44
Local Anesthetic-Activated Autophagy
Local anesthetics could activate autophagy by inhibiting the PI3K/Akt/mTOR signaling pathway. Autophagy was important in regulating cell metabolism and was often associated with apoptosis in the cellular stress response.45,46 The Beclin-1 expression level and LC3-II/I ratio were widely used as indicators of autophagy activation.47–49 Clinically relevant concentrations of Local anesthetics induced upregulation of autophagic activity by inhibiting PI3K/Akt/mTOR signalling.50–52 Activation of this pathway negatively regulated autophagy and inhibiting autophagy activation might be a protective mechanism against bupivacaine cytotoxicity.
Discussion
With the popularization of ultrasound visualization technology, peripheral nerve block technology has been widely used, and the related local anesthetic toxicity has aroused the concern of anesthesiologists. Any complications that must be detected in time. Timely detection and effective treatment can significantly improve the clinical prognosis. It is also the key for the peripheral nerve block technique to be widely promoted. Therefore, we should pay close attention to the cytotoxicity caused by local anesthetics, even though the related clinical reports are not much.
This review summarized the findings on the cytotoxicity of local anesthetics to different cells. We found that treatment with ropivacaine resulted in less cell death than bupivacaine. Furthermore, the toxicity effect of local anesthetics was time- and concentration-dependent. The above was based on in vitro studies reviewed here, and these effects remained to be established in clinical cohorts. In clinical practice, the anesthesiologist can determine the minimum effective concentration of the peripheral block, the optimal duration of the protocol for the peripheral block, and the precise delivery of the injection to the target site.53 The use of ultrasound-guided peripheral nerve block may help to reduce the dose of local anesthetics using ultrasound-guided peripheral nerve block to avoid potential risks.33
Pichiorri et al reported a clinical spinal cord infarction due to cocaine use.54 The mechanisms might include vasoconstriction and disruption of blood flow autoregulation in the nervous system.55 However, the effects of local anesthetics administered spinally were debatable. Although lidocaine was claimed to cause vasoconstriction at low doses and vasodilation at high concentrations in the peripheral circulation,56 the spinal administration of lidocaine has been observed to raise, maintain, or decrease spinal cord blood flow in diverse ways.55
Although some anesthetics are similarly used “off-label”, lidocaine is still not permitted for intravenous usage as an analgesic with potential risks. 2.5–3.5 mcg/mL of lidocaine is the therapeutic plasma level. In the central neurological and cardiovascular systems, plasma concentrations higher than 5 mcg/mL are thought to result in an increased sodium channel blockage and local anesthetic systemic toxicity (LAST).57 Seizures, myocardial depression, and severe cardiac arrhythmias are the symptoms which may result in cardiac arrest. Despite its extremely low incidence—less than 100 instances reported in the previous 30 years— LAST can develop after using any local anesthetic, while bupivacaine use is more frequently linked to cardiotoxicity.57
Wound infiltration with local anesthetics has recently been shown to minimize postoperative discomfort following various surgical procedures successfully. The possible toxicity of local anesthetics brought on by their high plasma concentrations is one of the most critical barriers to the widespread adoption of this technique.58 Systemic absorption may be increased by significant surgical incisions and soft-tissue dissection, usually during major orthopedic surgery.59 Given that toxicity varies among local anesthetics, to prevent the occurrence of toxic effects, it is necessary to raise awareness regarding how local anesthetics may cause tissue damage. We found that local anesthetics caused cell death through oxidative stress, mitochondrial and lysosomal dysfunction, and autophagy pathways. Local anesthetic cytotoxicity-induced tissue damage and metabolic changes should be kept to a minimum.12
The specific form of cell death caused by local anesthetics seems to be related to the duration of exposure, the concentration and the type of local anesthetics. Studies indicated that bupivacaine mainly caused necrosis, whereas lidocaine predominantly induced apoptosis.17,28 At low concentrations, local anesthetics induced apoptosis in myocytes, whereas higher concentrations mainly caused necrosis of these cells.28 Fluorescence microscopy confirmed that the short-term toxicity of local anesthetics on chondrocytes, IVD cells, and tenocytes was driven primarily by necrosis rather than apoptosis.11 However, after prolonged exposure to local anesthetics for more than 24 hours, the number of apoptotic cells significantly increased.6
In addition, the identification of the underlying signaling pathways, as well as the development of protective agents that could reduce local anesthetic-induced cytotoxicity, require further research. Potential protective agents include mitochondrial fission inhibitors,36 caspase activity modulators,12 scavengers of ROS,44 and autophagy inhibitors.50 Mitochondria should be among the primary targets of such protective agents and may contribute to protecting tissues exposed to local anesthetics.
Furthermore, the effects of adjuvants to local anesthetics on the tissue or cellular injury are mixed. It was thought that using epinephrine and local anesthetics simultaneously would increase myotoxicity.60,61 Epinephrine has been demonstrated to increase significantly the skeletal muscle necrosis caused by lidocaine, even at low dosages where there is minimal damage on its own.61 This potentiation was directly related to the drug’s capacity to postpone local anesthetic absorption from the injection site.
On the other hand, Moser et al62 found that the metabolic activity could be improved in human chondrocytes in vitro when local anesthetics and hyaluronic acid (HA) were administered simultaneously compared to local anesthetics alone. Ishida et al discovered that the surface marker CD44 on chondrocytes played a crucial role in anchoring the extracellular matrix components through binding HA. More significantly, the CD44-HA pathway stimulated various signals contributing to chondrocyte proliferation and matrix production.63 Therefore, for patients with symptomatic knee osteoarthritis, co-administration of local anesthetics with hyaluronic acid may offer an alternative, less chondrotoxic approach. However, protective agents can only reduce the toxicity of anesthetic drugs at the cellular level; whether they are effective in vivo or clinically remains to be explored.
LAs may have anti-neoplastic actions within cancer cells, according to the evidence gathered from many laboratory investigations.64–66 Along with their direct effects on cancer cells, LAs also have anti-inflammatory qualities that may be used to control the pro-cancer consequences of the stress response and maintain or improve immune cell function.67 Although in vitro studies can help establish biological plausibility, their findings are not always transferable to in vivo settings, and clinical results in humans have been contradictory.68 One retrospective study, for example, found that regional block during breast cancer surgery was associated with a lower risk of postoperative metastasis. Another study found that radical prostatectomy combined with epidural analgesia under general anesthesia significantly reduced recurrence.69,70 Further research, however, revealed no benefit for colon or prostate cancer when patients underwent surgery with the general and epidural anesthetic.71
Data from cell cultures or animal models may not apply to human tissue. For clinicians, this requires follow-up on these findings in clinical research studies to find if a threshold dose and exposure time for a toxic effect might exist. Cell viability and tissue integrity can be affected by various personal factors, including body weight, the severity of a preexisting disease, or the test site. Such lesions are still difficult to diagnose in daily practice. Other local phenomena like pain and inflammation negate semiological signals of toxicity induced by LAs. MRI imaging in conjunction with other ambiguous symptoms may help in diagnosis.
Limitations
This review focused on the effects of local anesthetics. Most studies were done in vitro; thus, the in vivo effects still needed to be fully understood. In vitro results might have shown higher cell toxicity than in vivo examinations would show due to direct cell incubation. In vitro, local anesthetics did not have to pass various barriers such as the cartilage matrix or the synovial membrane. The other weakness of this review is that some results interpreted from data obtained in animals might not be transferrable to human tissue. Varying methods and protocols made it challenging to conduct general comparisons, which is a common problem within literature reviews such as this.
Conclusion
Toxic effects of local anesthetics were shown to be time- and concentration-dependent and ropivacaine showed the lowest cytotoxicity in vitro. Local anesthetics decreased cell viability by interacting with molecules in cell pathways leading to cell death by autophagy, necrosis, or apoptosis. Based on these results, clinical anesthesiologists could reduce local anesthetics-induced toxicity by rationally selecting local anesthetics, using limited total amounts, and determining the lowest effective concentration and duration.
Data Sharing Statement
All data relevant to this study can be obtained from the corresponding authors upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by a grant from the Natural Science Foundation of Hubei Province (No. 2019CFB444 to LW) and a grant from the National Natural Science Foundation of P.R. China (No. 82171228 to WLY).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Duellman TJ, Gaffigan C, Milbrandt JC, et al. Multi-modal, pre-emptive analgesia decreases the length of hospital stay following total joint arthroplasty. Orthopedics. 2009;32(3):167.
2. Hofmann P, Metterlein T, Bollwein G, et al. The myotoxic effect of bupivacaine and ropivacaine on myotubes in primary mouse cell culture and an immortalized cell line. Anesth Analg. 2013;117(3):634–640. doi:10.1213/ANE.0b013e31829e4197
3. Metterlein T, Hoffmann P, Spath R, et al. In vitro myotoxic effects of bupivacaine on rhabdomyosarcoma cells, immortalized and primary muscle cells. Cancer Cell Int. 2015;15:75. doi:10.1186/s12935-015-0229-6
4. Ling X, Ma X, Kuang X, et al. Lidocaine inhibits myoblast cell migration and myogenic differentiation through activation of the notch pathway. Drug Des Devel Ther. 2021;15:927–936. doi:10.2147/dddt.S290002
5. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90(5):986–991. doi:10.2106/JBJS.G.01033
6. Jacob B, Zippelius T, Kloss N, et al. Local anesthetics’ toxicity toward human cultured chondrocytes: a comparative study between lidocaine, bupivacaine, and ropivacaine. Cartilage. 2019;10(3):364–369. doi:10.1177/1947603518758436
7. Park J, Sutradhar BC, Hong G, et al. Comparison of the cytotoxic effects of bupivacaine, lidocaine, and mepivacaine in equine articular chondrocytes. Vet Anaesth Analg. 2011;38(2):127–133. doi:10.1111/j.1467-2995.2010.00590.x
8. Adler DMT, Frellesen JF, Karlsen CV, et al. Evaluation of the in vitro effects of local anesthetics on equine chondrocytes and fibroblast-like synoviocytes. Am J Vet Res. 2021;82(6):478–486. doi:10.2460/ajvr.82.6.478
9. Nuelle CW, Cook CR, Stoker AM, et al. In vitro toxicity of local anesthetics and corticosteroids on supraspinatus tenocyte viability and metabolism. J Orthop Translat. 2017;8:20–24. doi:10.1016/j.jot.2016.08.002
10. Sung CM, Hah YS, Kim JS, et al. Cytotoxic effects of ropivacaine, bupivacaine, and lidocaine on rotator cuff tenofibroblasts. Am J Sports Med. 2014;42(12):2888–2896. doi:10.1177/0363546514550991
11. Zhang AZ, Ficklscherer A, Gulecyuz MF, et al. Cell toxicity in fibroblasts, tenocytes, and human mesenchymal stem cells-a comparison of necrosis and apoptosis-inducing ability in ropivacaine, bupivacaine, and triamcinolone. Arthroscopy. 2017;33(4):840–848. doi:10.1016/j.arthro.2016.10.026
12. Iwasaki K, Sudo H, Yamada K, et al. Cytotoxic effects of the radiocontrast agent iotrolan and anesthetic agents bupivacaine and lidocaine in three-dimensional cultures of human intervertebral disc nucleus pulposus cells: identification of the apoptotic pathways. PLoS One. 2014;9(3):e92442. doi:10.1371/journal.pone.0092442
13. Cai XY, Xiong LM, Yang SH, et al. Comparison of toxicity effects of ropivacaine, bupivacaine, and lidocaine on rabbit intervertebral disc cells in vitro. Spine J. 2014;14(3):483–490. doi:10.1016/j.spinee.2013.06.041
14. Quero L, Klawitter M, Nerlich AG, et al. Bupivacaine--the deadly friend of intervertebral disc cells? Spine J. 2011;11(1):46–53. doi:10.1016/j.spinee.2010.11.001
15. Dregalla RC, Lyons NF, Reischling PD, et al. Amide-type local anesthetics and human mesenchymal stem cells: clinical implications for stem cell therapy. Stem Cells Transl Med. 2014;3(3):365–374. doi:10.5966/sctm.2013-0058
16. Rahnama R, Wang M, Dang AC, et al. Cytotoxicity of local anesthetics on human mesenchymal stem cells. J Bone Joint Surg Am. 2013;95(2):132–137. doi:10.2106/JBJS.K.01291
17. Breu A, Scheidhammer I, Kujat R, et al. Local anesthetic cytotoxicity on human mesenchymal stem cells during chondrogenic differentiation. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):937–945. doi:10.1007/s00167-014-3312-y
18. Hussain N, McCartney CJL, Neal JM, et al. Local anesthetic-induced myotoxicity in regional anaesthesia: a systematic review and empirical analysis. Br J Anaesth. 2018;121(4):822–841. doi:10.1016/j.bja.2018.05.076
19. McFate JA, Soparkar CNS, Sami M, et al. Local anesthetic orbicularis myotoxicity: a possible unrecognized cause of post-blepharoplasty lagophthalmos. Eur J Plast Surg. 2014;37(4):201–204. doi:10.1007/s00238-013-0924-2
20. Zink W, Graf BM. Local anesthetic myotoxicity. Reg Anesth Pain Med. 2004;29(4):333–340. doi:10.1016/j.rapm.2004.02.008
21. Neal JM, Salinas FV, Choi DS. Local anesthetic-induced myotoxicity after continuous adductor canal block. Reg Anesth Pain Med. 2016;41(6):723–727. doi:10.1097/aap.0000000000000466
22. Matsen FA, Papadonikolakis A. Published evidence demonstrating the causation of glenohumeral chondrolysis by postoperative infusion of local anesthetic via a pain pump. J Bone Joint Surg. 2013;95-A. doi:10.2106/JBJS.L01104
23. Hansen BP, Beck CL, Beck EP, et al. Postarthroscopic glenohumeral chondrolysis. Am J Sports Med. 2007;35(10):1628–1634. doi:10.1177/0363546507304136
24. Kreuz PC, Steinwachs M, Angele P. Single-dose local anesthetics exhibit a type-, dose-, and time-dependent chondrotoxic effect on chondrocytes and cartilage: a systematic review of the current literature. Knee Surg Sports Traumatol Arthrosc. 2018;26(3):819–830. doi:10.1007/s00167-017-4470-5
25. Järvelä T, Järvelä S. Long-term effect of the use of a pain pump after arthroscopic subacromial decompression. Arthroscopy. 2008;24(12):1402–1406. doi:10.1016/j.arthro.2008.07.013
26. Noyes FR, Fleckenstein CM, Barber-Westin SD. The development of postoperative knee chondrolysis after intra-articular pain pump infusion of an anesthetic medication: a series of twenty-one cases. J Bone Joint Surg Am. 2012;94(16):1448–1457. doi:10.2106/JBJS.K.01333
27. Mohanty ST, Bellantuono I. Intra-femoral injection of human mesenchymal stem cells. Methods Mol Biol. 2013;976:131–141. doi:10.1007/978-1-62703-317-6_10
28. Wu T, Smith J, Nie H, et al. Cytotoxicity of local anesthetics in mesenchymal stem cells. Am J Phys Med Rehabil. 2018;97(1):50–55. doi:10.1097/PHM.0000000000000837
29. Breu A, Eckl S, Zink W, et al. Cytotoxicity of local anesthetics on human mesenchymal stem cells in vitro. Arthroscopy. 2013;29(10):1676–1684. doi:10.1016/j.arthro.2013.06.018
30. Guo X, Gong J, Yang G, et al. 罗哌卡因对大鼠骨髓间充质干细胞增殖和迁移能力的影响 [Effect of ropivacaine on proliferation and migration of rat bone marrow mesenchymal stem cells]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013;38(11):1152–1159. Chinese. doi:10.3969/j.issn.1672-7347.2013.11.012
31. Haasters F, Polzer H, Prall WC, et al. Bupivacaine, ropivacaine, and morphine: comparison of toxicity on human hamstring-derived stem/progenitor cells. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2138–2144. doi:10.1007/s00167-011-1564-3
32. Keck M, Zeyda M, Gollinger K, et al. Local anesthetics have a major impact on viability of preadipocytes and their differentiation into adipocytes. Plast Reconstr Surg. 2010;126(5):1500–1505. doi:10.1097/PRS.0b013e3181ef8beb
33. Cela O, Piccoli C, Scrima R, et al. Bupivacaine uncouples the mitochondrial oxidative phosphorylation, inhibits respiratory chain complexes I and III and enhances ROS production: results of a study on cell cultures. Mitochondrion. 2010;10(5):487–496. doi:10.1016/j.mito.2010.05.005
34. Grishko V, Xu M, Wilson G, et al. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010;92(3):609–618. doi:10.2106/JBJS.H.01847
35. Irwin W, Fontaine E, Agnolucci L, et al. Bupivacaine myotoxicity is mediated by mitochondria. J Biol Chem. 2002;277(14):12221–12227. doi:10.1074/jbc.M108938200
36. Grishko VI, Ho R, Wilson GL, et al. Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthritis Cartilage. 2009;17(1):107–113. doi:10.1016/j.joca.2008.05.009
37. Cai XY, Xia Y, Yang SH, et al. Ropivacaine- and bupivacaine-induced death of rabbit annulus fibrosus cells in vitro: involvement of the mitochondrial apoptotic pathway. Osteoarthritis Cartilage. 2015;23(10):1763–1775. doi:10.1016/j.joca.2015.05.013
38. Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. doi:10.1038/s41418-017-0012-4
39. Song Z, Fan TJ. Tetracaine induces apoptosis through a mitochondrion-dependent pathway in human corneal stromal cells in vitro. Cutan Ocul Toxicol. 2018;37(4):350–358. doi:10.1080/15569527.2018.1468342
40. Nouette-Gaulain K, Capdevila X, Rossignol R. Local anesthetic ‘in-situ’ toxicity during peripheral nerve blocks: update on mechanisms and prevention. Curr Opin Anaesthesiol. 2012;25(5):589–595. doi:10.1097/ACO.0b013e328357b9e2
41. Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27(50):6434–6451. doi:10.1038/onc.2008.310
42. Johansson AC, Appelqvist H, Nilsson C, et al. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis. 2010;15(5):527–540. doi:10.1007/s10495-009-0452-5
43. Repnik U, Hafner Cesen M, Turk B. Lysosomal membrane permeabilization in cell death: concepts and challenges. Mitochondrion. 2014;19:49–57. doi:10.1016/j.mito.2014.06.006
44. Cai X, Liu Y, Hu Y, et al. ROS-mediated lysosomal membrane permeabilization is involved in bupivacaine-induced death of rabbit intervertebral disc cells. Redox Biol. 2018;18:65–76. doi:10.1016/j.redox.2018.06.010
45. Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol. 2011;21(7):387–392. doi:10.1016/j.tcb.2011.03.007
46. Ito M, Yurube T, Kakutani K, et al. Selective interference of mTORC1/RAPTOR protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism with Akt and autophagy induction. Osteoarthritis Cartilage. 2017;25(12):2134–2146. doi:10.1016/j.joca.2017.08.019
47. Chaabane W, User SD, El-Gazzah M, et al. Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Arch Immunol Ther Exp. 2013;61(1):43–58. doi:10.1007/s00005-012-0205-y
48. Jain MV, Paczulla AM, Klonisch T, et al. Interconnections between apoptotic, autophagic and necrotic pathways: implications for cancer therapy development. J Cell Mol Med. 2013;17(1):12–29. doi:10.1111/jcmm.12001
49. Matsunaga K, Saitoh T, Tabata K, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11(4):385–396. doi:10.1038/ncb1846
50. Yang G, Li Z, Mei H, et al. Bupivacaine at clinically relevant concentrations induces toxicity in human intervertebral disc cells via the induction of autophagy in vitro. Mol Med Rep. 2019;20(1):837–843. doi:10.3892/mmr.2019.10279
51. Fan YL, Li HC, Zhao W, et al. Curcumin attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells via activation of the Akt signaling pathway. Neurochem Res. 2016;41(9):2425–2432. doi:10.1007/s11064-016-1955-4
52. Lv D, Bai Z, Yang L, et al. Lipid emulsion reverses bupivacaine-induced apoptosis of h9c2 cardiomyocytes: PI3K/Akt/GSK-3beta signaling pathway. Environ Toxicol Pharmacol. 2016;42:85–91. doi:10.1016/j.etap.2016.01.004
53. Nakamura T, Popitz-Bergez F, Birknes J, et al. The critical role of concentration for lidocaine block of peripheral nerve in vivo. Anesthesiology. 2003;99:1189–1197. doi:10.1097/00000542-200311000-00028
54. Pichiorri F, Masciullo M, Foti C, et al. Cocaine-related cervical spinal cord infarction: a case report and review of the literature. J Med Case Rep. 2022;16(1):59. doi:10.1186/s13256-021-03223-4
55. Hiroki Iida MI, Iida M. Effects of spinal analgesics on spinal circulation the safety standpoint. J Neurosurg Anesthesiol. 2008;20:180–187. doi:10.1097/ANA.0b013e31817f1861
56. Aps C, Reynolds F. The effect of concentration on vasoactivity of bupivacaine and lignocaine. Br J Anaesth. 1976;48(12):1171–1174. doi:10.1093/bja/48.12.1171
57. Hall EA, Sauer HE, Davis MS, et al. Lidocaine infusions for pain management in pediatrics. Paediatr Drugs. 2021;23(4):349–359. doi:10.1007/s40272-021-00454-2
58. Stamenkovic DM, Bezmarevic M, Bojic S, et al. Updates on wound infiltration use for postoperative pain management: a narrative review. J Clin Med. 2021;10(20):4659. doi:10.3390/jcm10204659
59. Bianconi M, Ferraro L, Traina GC, et al. Pharmacokinetics and efficacy of ropivacaine continuous wound instillation after joint replacement surgery. Br J Anaesth. 2003;91(6):830–835. doi:10.1093/bja/aeg277
60. Benoit PW. Reversible skeletal muscle damage after administration of local anesthetics with and without epinephrine. J Oral Surg. 1978;36(3):198–201.
61. Yagiela JA, Benoit PW, Fort NF. Mechanism of epinephrine enhancement of lidocaine-induced skeletal muscle necrosis. J Dent Res. 1982;61(5):686–690. doi:10.1177/00220345820610051301
62. Moser LB, Bauer C, Jeyakumar V, et al. Hyaluronic acid as a carrier supports the effects of glucocorticoids and diminishes the cytotoxic effects of local anesthetics in human articular chondrocytes in vitro. Int J Mol Sci. 2021;22(21). doi:10.3390/ijms222111503
63. Ishida O, Tanaka Y, Morimoto I, et al. Chondrocytes are regulated by cellular adhesion through CD44 and hyaluronic acid pathway. J Bone Miner Res. 1997;12(10):1657–1663. doi:10.1359/jbmr.1997.12.10.1657
64. D’Agostino G, Saporito A, Cecchinato V, et al. Lidocaine inhibits cytoskeletal remodelling and human breast cancer cell migration. Br J Anaesth. 2018;121(4):962–968. doi:10.1016/j.bja.2018.07.015
65. Li R, Xiao C, Liu H, et al. Effects of local anesthetics on breast cancer cell viability and migration. BMC Cancer. 2018;18(1):666. doi:10.1186/s12885-018-4576-2
66. Zhu J, Han S. Lidocaine inhibits cervical cancer cell proliferation and induces cell apoptosis by modulating the lncRNA-MEG3/miR-421/BTG1 pathway. Am J Transl Res. 2019;11(9):5404–5416.
67. Zhu G, Zhang L, Dan J, et al. Differential effects and mechanisms of local anesthetics on esophageal carcinoma cell migration, growth, survival and chemosensitivity. BMC Anesthesiol. 2020;20(1):126. doi:10.1186/s12871-020-01039-1
68. Zink W, Steinfeldt T, Wiesmann T. Bestandsaufnahme der Lokalanästhetika 2020 [Stocktaking of local anesthetics 2020]. Der Anaesthesist. 2020;69(5):301–313. German. doi:10.1007/s00101-020-00740-7
69. Biki B, Mascha E, Moriarty DC, et al. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109(2):180–187. doi:10.1097/ALN.0b013e31817f5b73
70. Exadaktylos AK, Buggy DJ, Moriarty DC, et al. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105(4):660–664. doi:10.1097/00000542-200610000-00008
71. Bundscherer A, Malsy M, Bitzinger D, et al. Interaktion von Anästhetika und Analgetika mit Tumorzellen [Interaction of anesthetics and analgesics with tumor cells]. Der Anaesthesist. 2014;63(4):313–325. German. doi:10.1007/s00101-014-2310-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.