Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Cutaneous Metastasis from Lung Adenocarcinoma
Authors Zhong L , Mao D, Li H, Chen X , Jin J, Wen G
Received 9 July 2022
Accepted for publication 5 September 2022
Published 12 September 2022 Volume 2022:15 Pages 1869—1872
DOI https://doi.org/10.2147/CCID.S381327
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Lingzhi Zhong, Dandan Mao, Houmin Li, Xue Chen, Jiang Jin, Guangdong Wen
Department of Dermatology, Peking University People’s Hospital, Beijing, People’s Republic of China
Correspondence: Guangdong Wen, Peking University People’s Hospital, No. 11 Xizhimen South Street, Xicheng District, Beijing, People’s Republic of China, Tel +86-10-88325472, Email [email protected]
Abstract: Cutaneous metastases are rare and often portend the aggressive malignancy and poor prognosis. We report a case of a 62-year-old man with a rapidly growing nodule on the left back for 2 months. The patient was diagnosed with lung adenocarcinoma shortly before the skin lesion presented. Physical examination showed a dome-shaped purplish red nodule, with ulceration and hemorrhagic crust. Excision of the skin lesion was performed, and the histopathology showed tumor cells infiltrate with immunohistochemistry (TTF-1+CK7+CD20–) favoring primary lung adenocarcinoma.
Keywords: cutaneous metastases, lung cancer, adenocarcinoma, immunohistochemistry, case report
Introduction
Lung cancer tends to metastasize to hilar nodes, brain, adrenal glands, bone, but rarely to skin. We report a case of a 62-year-old man with a rapidly growing nodule on the left back for 2 months. The patient was diagnosed with lung adenocarcinoma shortly before the skin lesion presented, with no smoking history and systemic symptoms. The histopathology and immunohistochemistry confirmed metastasis from lung adenocarcinoma. This case report highlights that timely biopsy of the lesion is significant for the diagnosis, prognosis, and therapeutic management of cutaneous metastases.
Case Report
A 62-year-old male nonsmoker presented to our department with a rapidly growing skin lesion on the left back. The lesion had been present for 2 months duration, with accompanying pain and bloody discharge. Two months before the lesion presented, the patient was found to have lung adenocarcinoma by regular check-ups. The chest CT showed left upper lobe lung cancer with mediastinum and left hilar lymph node metastases. A biopsy specimen of the left upper lung revealed invasive adenocarcinoma, with positive immunohistochemical results of thyroid transcription factor-1 (TTF-1) and napsin A. In addition, the patient exhibited few symptoms except for weight loss and the skin lesion.
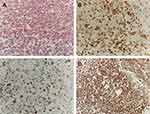
Physical examination showed a dome-shaped nodule, measuring 4cm×4cm on the left back. The skin lesion was purplish red and firm, with ulceration and hemorrhagic crust (Figure 1). A surgery was performed to remove the skin lesion, and the histopathology showed tumor cells infiltrate in the dermis with extensive necrosis. The tumor cells had abundant and light staining cytoplasm, clear nucleolus, and obvious atypia (Figure 2A). Immunohistochemistry showed tumor cells positive for cytokeratin-7 (CK7), TTF-1 and vimentin (Figures 2B–D), and negative for CK20 and CD5/6. The proliferative index, as measured by Ki-67, was over 90% of tumor cells. According to the clinical and pathological features, the diagnosis of cutaneous metastasis of lung adenocarcinoma was made.
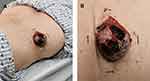 |
Figure 1 A dome-shaped nodule, measuring 4cm×4cm on the left back (A). Purplish red and firm, with ulceration and hemorrhagic crust (B). |
Discussion
Cutaneous metastases are rare and often portend the aggressive malignancy and poor prognosis. The percentage of lung cancer with cutaneous metastases varies between 1% and 12%.1 Histology shows most commonly adenocarcinoma, followed by squamous cell carcinoma, small cell carcinoma, and large cell carcinoma.2,3 Common sites include chest, back, abdomen, head, and neck.1–3 The most common presentation is the nodular type, which is suggested as the result of hematogenous spread.4 Nodules are often described as variable red to pink and violaceous color, fixed, firm, and painless. They usually grow rapidly and may necrotize or ulcerate.4,5 Less common forms may present as papular, plaque-like, ulcerated, vascular, zosteriform, erysipelas-like.1 The skin lesion in our case was a common nodular type on the left back. But it grew rapidly to an unusual big size, accompanied with ulceration and bleeding. It also showed obvious pain, which differs from the described cases.2–4 This case emphasized that a rapidly growing nodule with ulceration is one of the typical presentations of cutaneous metastases. Identifying such typical nodules provides clinical significance for the diagnosis of cutaneous metastases.
The pathogenesis of cutaneous metastases from lung cancer is suggested to be by lymphovascular invasion, with poor differentiation and upper lobe tumors increasing the risk.6 Our patient had left upper lobe lung adenocarcinoma with lymph node metastases, which could increase the risk of cutaneous metastasis. The skin lesions present usually after diagnosis of the primary tumor but sometimes before or concurrently with it. In our case, the skin lesion presented shortly after the discovery of lung cancer by regular check-ups. However, the patient showed no smoking history and systemic symptoms, and the skin lesion occurred as the first and only presentation. This case indicated that new-emerging cutaneous nodules may be the presenting sign of an internal cancer. Timely biopsy of suspicious skin lesions and systemic examination are significant for early diagnosis.
The diagnosis of cutaneous metastases is mainly confirmed by skin biopsy. Immunohistochemical markers can help identify the primary lung cancer. The CK7+/CK20– vs CK20+/CK7– pattern was able to identify lung vs colorectal adenocarcinoma, respectively.7 Besides, both TFF-1 and napsin A are highly sensitive and specific for detecting primary lung adenocarcinoma.8 Skin metastases with glandular differentiation are usually reactive for CK7, TTF-1, Ber-EP4, carcinoembryonic antigen (CEA), and surfactant apoprotein A but not for CK5/6 and CK20.9 In this case, the immunohistochemistry of the lung specimen showed TTF-1+/napsin A+ and the subepidermal specimen showed TTF-1+CK7+CD20–CK5/6–, which confirmed cutaneous metastasis from lung adenocarcinoma. Vimentin was also positive in our case. Researchers found that vimentin was required for lung adenocarcinoma metastasis by heterotypic tumor cell-cancer-associated fibroblast interactions during collective invasion.10 Another study showed that vimentin could lead to metastasis and immune escape in lung adenocarcinoma by triggering TGF-β-signaling.11 These studies showed that vimentin is critical for lung adenocarcinoma metastasis and could be a potential target for anti-metastatic therapies.
Treatment of solitary cutaneous metastases includes surgery, chemotherapy, or radiotherapy. Poor prognostic indicators include non-resectability, small-cell histology and multiple/distant metastases.12 Mean survival is short, usually 5–6 months after diagnosis of cutaneous metastasis.1
Conclusion
Cutaneous metastases are rare and often portend the aggressive malignancy and poor prognosis. We emphasize that cutaneous metastases should be ruled out in patients with suspicious skin lesions, smoking history, or lung cancer. Timely biopsy and systemic examination should be performed to provide implications for diagnosis, prognosis, and treatment management.
Ethics and Consent Statements
Written informed consent was provided by the patient to have the case details and any accompanying images published. Institutional approval was not required to publish the case details.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81402588 and 82103750).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mollet TW, Garcia CA, Koester G. Skin metastases from lung cancer. Dermatol Online J. 2009;15(5):1.
2. Babacan NA, Kiliçkap S, Sene S, et al. A case of multifocal skin metastases from lung cancer presenting with vasculitic-type cutaneous nodule. Indian J Dermatol. 2015;60(2):213. doi:10.4103/0019-5154.152582
3. Dhambri S, Zendah I, Ayadi-Kaddour A, Adouni O, El Mezni F. Cutaneous metastasis of lung carcinoma: a retrospective study of 12 cases. J Eur Acad Dermatol Venereol. 2011;25(6):722–726. doi:10.1111/j.1468-3083.2010.03818.x
4. Liao H, Wu S, Karbowitz SR, Morgenstern N, Rose DR. Cutaneous metastasis as an initial presentation of lung adenocarcinoma with KRAS mutation: a case report and literature review. Stem Cell Investig. 2014;1:6. doi:10.3978/j.issn.2306-9759.2014.03.04
5. McSweeney WT, Tan K. Cutaneous metastases as a presenting sign of metastatic NSCLC. J Surg Case Rep. 2019;2019(10):rjz279. doi:10.1093/jscr/rjz279
6. McGrath RB, Flood SP, Casey R. Cutaneous metastases in non-small cell lung cancer. BMJ Case Rep. 2014;2014:bcr2014205752. doi:10.1136/bcr-2014-205752
7. Kummar S, Fogarasi M, Canova A, Mota A, Ciesielski T. Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer. 2002;86(12):1884–1887. doi:10.1038/sj.bjc.6600326
8. Ye J, Findeis-Hosey JJ, Yang Q, et al. Combination of napsin A and TTF-1 immunohistochemistry helps in differentiating primary lung adenocarcinoma from metastatic carcinoma in the lung. Appl Immunohistochem Mol Morphol. 2011;19(4):313–317. doi:10.1097/PAI.0b013e318205b059
9. Alcaraz I, Cerroni L, Rütten A, Kutzner H, Requena L. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34(4):347–393. doi:10.1097/DAD.0b013e31823069cf
10. Richardson AM, Havel LS, Koyen AE, et al. Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell-cancer-associated fibroblast interactions during collective invasion. Clin Cancer Res. 2018;24(2):420–432. doi:10.1158/1078-0432.CCR-17-1776
11. Jang HR, Shin SB, Kim CH, et al. PLK1/vimentin signaling facilitates immune escape by recruiting Smad2/3 to PD-L1 promoter in metastatic lung adenocarcinoma. Cell Death Differ. 2021;28(9):2745–2764. doi:10.1038/s41418-021-00781-4
12. Ambrogi V, Nofroni I, Tonini G, Mineo TC. Skin metastases in lung cancer: analysis of a 10-year experience. Oncol Rep. 2001;8(1):57–61.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.