Back to Journals » Cancer Management and Research » Volume 14
CSTF2 Acts as a Prognostic Marker Correlated with Immune Infiltration in Hepatocellular Carcinoma
Authors Zhang W , Wan Y, Zhang Y, Liu Q, Zhu X
Received 31 January 2022
Accepted for publication 4 September 2022
Published 12 September 2022 Volume 2022:14 Pages 2691—2709
DOI https://doi.org/10.2147/CMAR.S359545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Wang Zhang, Yipeng Wan, Yue Zhang, Qi Liu, Xuan Zhu
Departments of Gastroenterology and Hepatology, First Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China
Correspondence: Xuan Zhu, Departments of Gastroenterology and Hepatology, First Affiliated Hospital of Nanchang University, No. 17, Yongwaizheng Street, Nanchang, 330000, People’s Republic of China, Tel +86 13970090801, Email [email protected]
Background: Cleavage stimulation factor 2 (CSTF2) encodes a nuclear protein that is implicated in the development of various cancers. However, the role of CSTF2 in hepatocellular carcinoma (HCC) has not been understood. This study aims to explore the function of CSTF2 in HCC.
Methods: The expression, diagnostic capability, prognostic value, and immune cell effect of CSTF2 in HCC were explored using various databases. The expression level of CSTF2 were validated in our cell lines. The effect of CSTF2 on hepatocarcinogenesis was explored by CSTF2 silencing.
Results: CSTF2 expression was significantly elevated in HCC and correlated with multiple clinicopathological characteristics. CSTF2 exhibited good diagnostic capability in discriminating HCC samples from nontumorous samples. High CSTF2 expression was significantly related to poor overall survival. Univariate and multivariate Cox regression analyses suggested that CSTF2 expression was an independent risk factor for HCC. These results were validated in ICGC cohorts. In addition, the nomogram based on CSTF2 showed better predictive performance than the AJCC staging system in TCGA and ICGC cohorts. Functional enrichment analysis revealed that CSTF2-related genes were involved in DNA/RNA processing and the cell cycle. In addition, we found that CSTF2 expression was closely related to the levels of various infiltrating immune cells, especially neutrophils. Moreover, some immune checkpoints had positive relationships with CSTF2 expression. CSTF2 silencing inhibited proliferation, invasion and migration, and promoted apoptosis in HepG2 cells. Western blotting analysis revealed that CSTF2 silencing inactivated the Wnt/β-catenin signaling pathway.
Conclusion: High CSTF2 expression not only correlates with unfavorable outcomes but also affects immune cell infiltration and immune checkpoint expression in HCC. CSTF2 silencing can alleviate the malignant phenotypes of hepatic cancer cell by inactivating the Wnt/β-catenin signaling pathway. These results indicate that CSTF2 can serve as a promising prognostic marker and therapeutic target for HCC patients.
Keywords: hepatocellular carcinoma, CSTF2, immune infiltration, prognosis, biomarker
Introduction
Hepatocellular carcinoma (HCC), as a major histological type of primary liver cancer, is the fifth most common cancer and the second most common cause of cancer-related death worldwide, with approximately 800,000 new cases annually.1,2 According to annual projections, the World Health Organization predicts that more than 1 million patients will die as a result of liver cancer in 2030.3 In China, the incidence of liver cancer is higher than that in other countries, and approximately 466,100 new HCC cases were reported in 2015, accounting for more than 50% of newly diagnosed liver cancer cases worldwide.4,5 HCC has become a major public health challenge for the global medical community. The early diagnosis of HCC is difficult because of the unapparent early clinical manifestations; therefore, most HCC patients are diagnosed in a late stage and have lost the opportunity for surgical resection. Although tremendous progress in treatment strategies for HCC has been made over the past decades, the clinical outcome of patients with advanced HCC is still not ideal, with a 5-year recurrence rate of more than 70%.6,7 Hence, identifying novel reliable diagnostic and prognostic markers for HCC is of great value for guiding clinical management and improving patient prognosis.
Cleavage stimulation factor 2 (CSTF2), also known as CSTF64, is a member of the cleavage stimulation factor complex that has an essential role in the accurate polyadenylation of most mRNAs.8–11 CSTF2 encodes a nuclear protein containing a ribonucleoprotein-type RNA binding domain and can regulate biological processes such as endoderm differentiation, intellectual development, and embryonic stem cell development.12–15 In addition, CSTF2 has been found to regulate gene expression in immune cells, including B cells and T cells, which means that CSTF2 might be related to immune cell infiltration.16,17 Recent studies have revealed that aberrant CSTF2 expression is associated with oncogenic processes. CSTF2 is overexpressed in urothelial carcinoma of the bladder and significantly enhances cell proliferation, migration, and invasion.18 In esophageal squamous cell carcinoma, the shortened BID 3ʹUTR induced by CSTF2 contributes to tumor progression by disrupting competing endogenous RNA cross-talk and downregulating the expression of the tumor suppressor gene ZFP36L2.19 CSTF2 may function as an oncogene to regulate the expression of cancer-related genes in lung cancer.20 Additionally, higher expression of CSTF2 is associated with poor prognosis in patients with lung cancer.21 In nonneoplastic diseases, CSTF2 in the peripheral blood of multiple sclerosis patients is significantly lower than that in healthy subjects and can serve as a potential theoretical biomarker for diagnosis.22 However, the diagnostic and prognostic value of CSTF2 in HCC has not been determined.
In this study, we analyzed CSTF2 expression and its correlation with clinicopathological features in patients with HCC using various databases. In addition, the potential diagnostic and prognostic values of CSTF2 in HCC were evaluated. Moreover, an external cohort from the International Cancer Genome Consortium (ICGC) database was used to validate the results of The Cancer Genome Atlas (TCGA) database analysis. The possible pathogenic mechanism of CSTF2 was revealed through enrichment analysis of co-expressed genes of CSTF2. Finally, the relationship between CSTF2 and immune cell infiltration was investigated. We also verified the high expression of CSTF2 in hepatic cancer cells. CSTF2 silencing inhibited proliferation, invasion and migration, and promoted apoptosis in hepatic cancer cells, which was related to inactivating the Wnt/β-catenin signaling pathway. Our study revealed the important role of CSTF2 in the molecular pathogenesis of HCC and its prognostic value.
Materials and Methods
Data Acquisition
The gene expression profiles and clinical data, involving 374 HCC samples and 50 nontumor samples, were downloaded from the TCGA Liver Hepatocellular Carcinoma (TCGA-LIHC) database (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga). Three datasets (GSE43456, GSE84402 and GSE105130) containing HCC tissue and normal liver tissue were obtained from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). We also downloaded the mRNA expression profiles of 243 HCC tissues and 202 normal tissues with clinical data from the International Cancer Genome Consortium (ICGC) database (https://dcc.icgc.org/). The expression matrix was extracted by R4.1.0 software (https://www.r-project.org) or Perl languages (https://www.perl.org/) and was log2 transformed if necessary. Supplementary Figure 1 displays the analysis flow of this study.
CSTF2 Expression and Clinicopathological Characteristics
The Tumor Immune Estimation Resource (TIMER, https://cistrome.shinyapps.io/timer/) is a web-based tool for the comprehensive analysis of immune infiltration according to multiple cancer types and was first used to analyze CSTF2 gene expression in diverse cancers.23 The expression level of CSTF2 was then compared between HCC and nontumorous tissues based on TCGA and GEO data. Next, we merged the expression data and clinicopathological data on the basis of the patient’s unique identification numbers. The HCC patients were divided into high and low expression groups according to the median CSTF2 expression value. The correlation between CSTF2 expression and clinicopathological parameters was analyzed using the chi-square test.
Cell Culture and Transfection
The human hepatocyte cell line LO2 and hepatocellular carcinoma cell line HepG2 were kindly provided by Procell Life Science & Technology Co., Ltd. (Wuhan, China). LO2 cells were cultured in RPMI-1640 (Gibco, USA) containing 10% fetal bovine serum (Gibco, USA). HepG2 cells were cultured in DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA). CSTF2 siRNAs were designed by Gene Pharma (Shanghai, China). The sequences of the siRNAs are listed in Supplementary Table 1. HepG2 cells were cultured in 6-well plates until they reached 50–70% confluence. Then HepG2 cells were transfected with siRNA using LipofectamineTM 3000 (Thermo Fisher Scientific, USA) according to the manufacturer’s manual. The cells were maintained in a humidified 5% carbon dioxide incubator at 37°C.
Cell Proliferation and Apoptosis
Cell proliferation was evaluated by CCK-8 assay. Briefly, the transfected cells (5*10^3 cells/well) were seeded into 96-well plates and cultured overnight. After 0 h, 24 h, 48 h and 72 h of incubation, 10 µL CCK-8 reagent (Elabscience, China) was added to each well and the absorbance at 450 nm was measured. Cell apoptosis was evaluated using the Annexin V-FITC/PI Apoptosis Detection Kit (KeyGEN BioTECH, China). The transfected cells were stained with Annexin V-FITC and Propidium Iodide according to the manufacturer’s manual. Then the cells were analyzed by a flow cytometer (Beckman coulter, USA).
Cell Migration and Invasion Assays
The transfected cells (6*10^4) were resuspended in serum-free medium and seeded into the upper chamber (Corning, USA). Then, the upper chamber was placed in a 24-well plate that contained 600 µL of 10% fetal bovine serum DMEM. In the cell invasion assay, the upper chamber was precoated with Matrigel (Corning, USA). After 48 h of incubation, the migrated and invaded cells were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. The images were captured with a microscope.
Real-Time Quantitative PCR Analysis (RT–qPCR)
Total RNA was extracted from cells using TRIzol reagent (Tiangen, China) according to the manufacturer’s manual. We measured the concentration and purity of RNA by a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). Complementary DNA (cDNA) was synthesized using a TIANScript RT kit (Tiangen, China). The mRNA level of CSTF2 was determined by using 2X M5 HiPer UltraSYBR Mixture (Mei5bio, China) in the QuantStudioTM 5 Real-Time PCR system (Thermo Fisher Scientific, USA). PCR primers were synthesized by SangonBiotech company (Shanghai, China). CSTF2: forward: 5ʹ- CAGCGGTGGATCGTTCTCTAC-3ʹ, reverse: 5ʹ-AACAACAGGTCCAACCTCAGA-3ʹ. GAPDH served as an internal control to normalize CSTF2 expression by using the 2−ΔΔCT method.
Western Blotting Analysis
Cellular protein was prepared using RIPA buffer containing 1X protease inhibitor. We determined the protein concentration by a Bradford Protein Assay kit (Tiangen, China). The protein samples were separated on an SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was blocked with 5% skim milk dissolved in Tris-buffered saline with Tween 20 (TBST) and then incubated with the indicated primary antibodies overnight at 4°C. We used the following primary antibodies: CSTF2 (1:1000) from Santa Cruz, β-catenin (1:5000), c-MYC (1:5000), Cyclin D1 (1:5000), Bax (1:5000) and Bcl-2 (1:2000) from Proteintech, and GAPDH (1:1000) from Abmart. After incubation with HRP-conjugated secondary antibodies, the membrane was treated with chemiluminescence reagent. The signals were acquired with a luminescence image analyzer (Thermo Fisher Scientific, USA).
Diagnostic and Prognostic Values of CSTF2
The diagnostic value of CSTF2 was evaluated in TCGA and ICGC cohorts, and the area under the curve (AUC) value was used to assess the diagnostic efficacy of CSTF2 in discriminating HCC samples from nontumorous samples. Univariate and multivariate Cox regression analyses were conducted in R4.1.0 software to identify independent prognostic factors. A nomogram was developed based on the independent prognostic factors in the TCGA cohort. The concordance index (C-index) and calibration curve were used to evaluate its predictive performance. We compared the predictive performance between the CSTF2-based nomogram and the American Joint Committee on Cancer (AJCC) staging system in TCGA and ICGC cohorts. Gene Expression Profiling Interactive Analysis (GEPIA, http://gepia.cancer-pku.cn/), a web-based tool, was used to analyze the prognostic value of CSTF2 expression in HCC patients. Moreover, we validated the effect of CSTF2 expression on survival in HCC patients from the ICGC database.
Functional Enrichment Analyses
The LinkedOmics database (http://www.linkedomics.org/) is a useful tool that supports multi-omics analysis in a cancer type or pan-cancer analysis.24 We analyzed CSTF2-related genes in the LinkedOmics database. The Pearson correlation test was used for statistical analysis. The top 50 genes positively and negatively correlated with CSTF2 are separately shown by heatmaps. Finally, the top 50 CSTF2-related genes were selected, and Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed with the clusterProfiler package in R (version 4.1.0). Based on the median CSTF2 expression value, the HCC patients in TCGA cohort were classified into CSTF2 high and low expression groups. Then, we conducted gene set enrichment analysis (GSEA) to identify the potential pathway that contributed to the carcinogenesis of CSTF2.
Immune Infiltration Analysis
The relationship between CSTF2 expression and various infiltrating immune cells was analyzed via the TIMER database. CIBERSORT (http://cibersort.stanford.edu/) is an analytical tool to characterize the cell composition of complex tissue based on their gene expression profiles.25 We used CIBERSORT to evaluate the difference of 22 immune cell types in TCGA-LIHC cohort that were categorized into the CSTF2 high and low expression groups based on the median CSTF2 expression value.
Statistical Analysis
R version 4.1.0 software and SPSS 20.0 were used to conduct statistical analyses. Scatter plots were generated by GraphPad Prism 5.0. Statistical differences between two groups were calculated by a two-tailed Student’s t test, paired t test or Mann–Whitney rank sum test. The Pearson chi-square test was used to determine the correlation between CSTF2 expression and clinicopathological parameters. Univariate and multivariate Cox regression analyses were performed in R4.1.0 software. P<0.05 was considered statistically significant.
Results
Transcriptional Level of CSTF2 in HCC
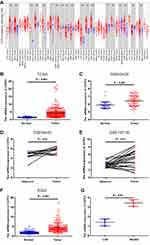
We initially analyzed CSTF2 transcriptional levels in different cancers via the TIMER database. As shown in Figure 1A, the mRNA expression of CSTF2 was remarkably upregulated in LIHC tissue compared to normal tissue. CSTF2 was also highly transcribed in other gastrointestinal cancers, such as cholangiocarcinoma (CHOL), and stomach adenocarcinoma (STAD). However, CSTF2 mRNA downregulation was observed in renal carcinoma, prostate adenocarcinoma and thyroid carcinoma. These results revealed that CSTF2 was aberrantly expressed in various cancers. Increased CSTF2 mRNA expression in patients with HCC was validated by using TCGA, GEO and ICGC data (Figure 1B–F). Furthermore, elevated mRNA levels of CSTF2 were also confirmed in the hepatocellular carcinoma cell line HepG2 compared to the hepatocyte cell line LO2 (Figure 1G).
CSTF2 Protein Was Upregulated in HCC
To understand the CSTF2 protein expression profile in HCC, we utilized The Human Protein Atlas (HPA) (https://www.proteinatlas.org/) to assess CSTF2 protein expression between HCC and nontumorous tissues. The immunohistochemical staining results in HPA indicated that CSTF2 was highly expressed in tumor tissues (Figure 2A). In addition, we observed that the hepatocellular carcinoma cell line HepG2 expressed higher protein levels of CSTF2 than the hepatocyte cell line LO2 (Figure 2B). Altogether, these results suggested that CSTF2 protein expression was significantly upregulated in HCC tissues compared to normal counterparts.
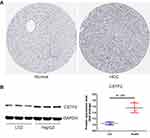 |
Figure 2 CSTF2 protein was upregulated in HCC. (A) The protein expression of CSTF2 in HCC based on the HPA database. (B) The protein expression of CSTF2 in cell lines. |
Relationship Between CSTF2 and Clinicopathological Characteristics of HCC Patients
We analyzed the clinical information of HCC patients from the TCGA-LIHC project to explore the correlation between CSTF2 expression and clinicopathological parameters. Based on the median CSTF2 expression value, the HCC patients were classified into CSTF2 high and low expression groups. As shown in Table 1, CSTF2 expression was significantly correlated with gender (P=0.037), AJCC stage (P=0.003) and survival status (P=0.008). There was no significant correlation between CSTF2 expression and other clinicopathological parameters, including age (P=0.59) and histologic grade (P=0.099).
 |
Table 1 Relationship Between CSTF2 and Clinicopathological Characteristics of HCC Patients |
Diagnostic and Prognostic Values of CSTF2 in HCC
To evaluate the diagnostic value of CSTF2 in HCC, we generated an ROC curve of CSTF2 using TCGA-LIHC data. As indicated in Figure 3A, CSTF2 exhibited good discrimination in diagnosing HCC patients with an AUC of 0.938 (95% CI 0.913–0.964). We also analyzed the prognostic value of CSTF2 in HCC using GEPIA with TCGA data. The Kaplan–Meier curve revealed that high CSTF2 expression was significantly related to poor overall survival (Figure 3B). Additionally, univariate and multivariate Cox regression analyses suggested that high CSTF2 expression was an independent risk factor for poor survival in HCC. Other independent risk factors, including age and AJCC stage, are listed in Table 2.
 |
Table 2 Univariate and Multivariate Cox Regression Analyses Were Conducted to Identify Independent Risk Factors for Poor Survival of HCC |
Establishment and Validation of a Prediction Nomogram
According to the multivariate Cox regression analysis, high CSTF2 expression, age, and AJCC stage are independent risk factors associated with unfavorable survival in HCC. A nomogram incorporating these independent risk factors was established to predict the 1-, 3-, and 5-year overall survival rates of HCC patients in the TCGA-LIHC cohort (Figure 4A). The internal validation indicated that the C-index for the nomogram (C-index=0.700; 95% CI 0.644–0.756) to predict overall survival was higher than that of the AJCC staging system (C-index=0.644; 95% CI 0.582–0.707). In the ICGC cohort, the C-index of the nomogram (C-index=0.759; 95% CI 0.751–0.767) for overall survival was also higher than that of the AJCC staging system (C-index=0.694; 95% CI 0.684–0.704). The calibration plots exhibited satisfactory agreement between the predicted survival probability and actual 1-, 3- and 5-year overall survival rates in the TCGA cohort (Figure 4B) and ICGC cohort (Figure 4C).
Validation of the Diagnostic and Prognostic Values of CSTF2 in the ICGC Cohort
In the ICGC cohort, CSTF2 also showed a good diagnostic ability in distinguishing HCC samples from normal samples with an AUC of 0.932 (95% CI 0.897–0.950) (Figure 5A). The survival curve from the ICGC cohort indicated that the high CSTF2 expression group had unfavorable overall survival compared with the low CSTF2 expression group (Figure 5B). Univariate Cox regression analysis revealed that AJCC stage and CSTF2 expression were associated with overall survival (Figure 5C). Multivariate Cox regression analysis confirmed that high CSTF2 expression was an independent risk factor for unfavorable survival in HCC patients (Figure 5D).
CSTF2-Related Functions and Pathways in HCC
Co-expressed genes usually have similar roles in modulating some pathophysiological processes. To investigate the potential mechanism of CSTF2 in hepatocarcinogenesis, CSTF2-related genes were analyzed in the LinkedOmics database. Heatmaps were used to display the top 50 genes that were positively and negatively correlated with CSTF2 expression (Figure 6A and B). We selected the top 50 positively correlated genes to perform functional enrichment analyses to explore the potential biological functions of CSTF2 in hepatocarcinogenesis. GO biological process (BP) analysis indicated that DNA recombination, DNA replication, double-strand break repair and recombination repair were significantly enriched. According to the cellular component (CC) enrichment analysis, the chromosomal region, telomeric region, DNA replication preinitiation complex and centromeric region were the significantly enriched categories. The molecular function (MF) analysis showed that single-stranded DNA binding, DNA replication origin binding, DNA helicase activity and ATPase activity were mainly enriched (Figure 6C). The KEGG enrichment analysis revealed that DNA replication, RNA transport and the cell cycle were remarkably enriched (Figure 6D). These enrichment analysis results suggested that CSTF2 may play an important role in DNA replication and repair.
Relationship Between CSTF2 Expression and Tumor-Infiltrating Immune Cells
As immune infiltration is closely associated with tumor formation and progression, we wondered whether immune cell infiltration was involved in the pathogenetic mechanism of CSTF2 in HCC. We utilized CIBERSORT to explore the effect of CSTF2 expression on infiltrating immune cells in HCC. As shown in Figure 7A, in 22 immune cell subgroups, memory B cells, M0 macrophages, neutrophils and follicular helper T cells were significantly abundant in the CSTF2 high expression group, whereas naïve B cells and activated CD4+ T cells were remarkably reduced. We further explored the relationship between CSTF2 expression and infiltrating immune cells in HCC using the TIMER database. The results indicated that CSTF2 expression was positively related to B cells (Cor = 0.319, p = 1.36e-09), CD8+ T cells (Cor = 0.234, p = 1.26e-05), CD4+ T cells (Cor = 0.326, p = 5.96e-10), macrophages (Cor = 0.393, p = 4.66e-14), neutrophils (Cor = 0.414, p = 9.46e-16) and dendritic cells (Cor = 0.409, p = 3.92e-15) (Figure 7B). In addition, we also analyzed the correlation between CSTF2 expression and immune cell markers. As shown in Table 3, most immune cell markers had a positive correlation with CSTF2 expression after adjustment based on purity. Immune checkpoint blockade therapy is a popular immunotherapy at present. Some immune checkpoints, such as CD274 (PD-L1), CTLA4, HAVCR2 (TIM3), PDCD1 (PD-1) and TIGIT, were positive correlated with CSTF2 expression (Figure 7C–G). In addition, these immune checkpoint markers were significantly upregulated in the high CSTF2 expression group (Figure 7H).
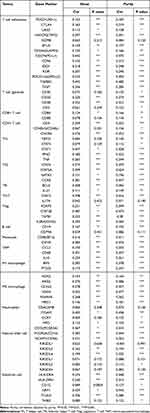 |
Table 3 Correlation Analysis of CSTF2 with Immune Cell Markers in the TIMER Database |
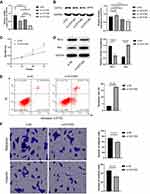
CSTF2 Silencing Alleviated the Malignant Phenotypes of Hepatic Cancer Cells
We knocked down the expression of CSTF2 to explore the effects of CSTF2 silencing on malignant phenotypes in hepatic cancer cells. As shown in Figure 8A and B, the expression of CSTF2 in HepG2 cells was significantly inhibited by si-CSTF2#3 transfection. Then we explored the influence of CSTF2 silencing on proliferation, apoptosis, invasion and migration in HepG2 cells. Figure 8C indicates that CSTF2 silencing inhibited the proliferation of HepG2 cells. CSTF2 gene knockdown downregulated Bcl-2 (anti-apoptotic protein) expression and upregulated Bax (pro-apoptotic protein) expression (Figure 8D). The flow cytometry results verified that CSTF2 gene knockdown increased the apoptosis of HepG2 cells (Figure 8E). In addition, CSTF2 silencing significantly reduced the migration and invasion of HepG2 cells (Figure 8F). These results demonstrated that CSTF2 silencing alleviated the malignant phenotypes in HepG2 cells.
CSTF2 Silencing Inactivated the Wnt/β-Catenin Signaling Pathway
GSEA revealed that the Wnt/β-catenin signaling pathway was significantly enriched in the CSTF2 high expression group, which suggested that the facilitatory effect of CSTF2 in hepatocarcinogenesis might be related to the activation of the Wnt/β-catenin signaling pathway (Figure 9A). Therefore, we evaluated Wnt/β-catenin signaling pathway-related protein expression. As shown in Figure 9B, downregulated expression of β-catenin, c-MYC and Cyclin D1 was observed in the CSTF2 silencing group, which indicated that CSTF2 silencing inactivated the Wnt/β-catenin signaling pathway.
Discussion
HCC is a common solid malignant tumor in the human digestive system. At present, patients with early- or early-stage HCC mainly benefit from curative therapies such as liver resection, radiofrequency ablation and liver transplantation, but radiotherapy and chemotherapy are preferred for patients with unresectable or advanced HCC.26 Recently, immunotherapy has been considered a promising treatment strategy for HCC patients.27 Although great progress has been made in the early diagnosis and clinical integrated treatment of HCC over the past few decades, the prognosis of HCC patients remains unsatisfactory. Aberrant gene expression may be associated with oncogenesis and tumor progression, which contribute to unfavorable prognosis of HCC.28,29 In the present study, we revealed that the mRNA expression of CSTF2 was significantly higher in HCC tissue than in normal tissue. The protein level of CSTF2 was in line with the above mentioned finding, which was verified in the hepatocellular carcinoma cell line HepG2 and hepatocyte cell line LO2. Additionally, CSTF2 expression was significantly correlated with gender, AJCC stage and survival status in HCC patients. The Kaplan–Meier curve indicated that CSTF2 overexpression was associated with adverse overall survival. Moreover, univariate and multivariate Cox regression analyses further showed that CSTF2 served as an independent risk factor for poor prognosis of HCC. CSTF2 also possessed good discrimination ability in diagnosing HCC patients with an AUC of 0.938 in the TCGA cohort. These results were validated in ICGC cohort. Altogether, these results suggest that CSTF2 can serve as a potential prognostic marker for HCC patients with poor outcomes.
As CSTF2 has a good diagnostic ability in discriminating HCC patients and serves as an independent predictor of an unfavorable prognosis in HCC patients, we wonder whether it can be applied to construct a prognostic model. A nomogram based on the independent risk factors was established to predict the overall survival rate of patients with HCC. The predictive efficiency of the nomogram was internally and externally evaluated by the C-index and calibration curve. The AJCC staging system is one of the most commonly used staging systems for HCC in clinical practice. Multiple versions of the AJCC staging system were successively updated, which reflects recent advances in the recognition of HCC therapeutic strategies. The AJCC staging system is accepted worldwide and has been validated by various external studies in terms of utility and clinical applicability.30–32 In this study, the nomogram based on age, CSTF2 expression and AJCC stage showed a better predictive performance in TCGA and ICGC cohorts than the AJCC staging system, which meant that age and CSTF2 expression might be useful to supplement the AJCC staging system. A larger, multicenter cohort is needed to further test the predictive performance of the nomogram.
Co-expressed genes act synergistically to modulate some pathophysiological processes and therefore usually have similar functional roles, which provides an excellent strategy to understand the oncogenic mechanisms of CSTF2. The co-expression analysis showed that the top 3 positively correlated genes of CSTF2 were NONO, CENPI and PHF6. These genes have been reported to contribute to tumorigenesis and progression. NONO, an RNA/DNA binding protein, drives oncogenic transcriptional programs to promote tumorigenesis.33 NONO interacts with the hypoxia inducible factor-1/2 complex and its downstream transcripts to activate the transcription of hypoxia-induced genes, which facilitate HCC progression.34 CENPI, a member of the centromere protein family, has an essential role in accurate chromosome segregation.35 CENPI is upregulated in multiple cancers and serves as an oncogene to promote tumor cell proliferation, migration and invasion.36–38 As a chromatin-binding protein, PHF6 is also elevated in HCC tissues and positively associated with lymph node metastasis, while silencing PHF6 inhibits HCC cell proliferation and migration.39,40 These results suggest that CSTF2 and its related genes may serve as promising prognostic biomarkers for HCC. To further explore the molecular functions and potential mechanisms of CSTF2 in HCC, we performed functional enrichment analyses of CSTF2-related genes. The GO enrichment analysis of the top 50 positively co-expressed genes indicated that DNA replication, RNA localization, double-strand break repair and recombination repair were significantly enriched. KEGG enrichment analysis revealed that CSTF2 was involved in DNA replication, RNA transport and the cell cycle. CSTF2 affects histone RNA processing and participates in cell cycle regulation in the 3’ end processing of replication-dependent histone mRNAs.41 Hence, CSTF2 contributes to HCC development and progression, probably by affecting DNA replication and repair, RNA processing and the cell cycle.
Tumor-infiltrating immune cells are important components of the tumor immune microenvironment and consist of multiple immune cells that influence the response to oncotherapy and patient outcomes. Persistent hepatic inflammation can lead to hepatocarcinogenesis.42 As innate immune cells, neutrophils play an important role in the innate immune response and crucially respond to various noxious stimuli, which trigger chronic inflammation. During the development of HCC, neutrophils drive inflammation to promote tumor progression. In addition, neutrophils affect the clinical therapeutic strategies and outcomes of HCC patients.43 An elevated neutrophil-to-lymphocyte ratio is a credible predictor that can be utilized to predict short survival.44 Dendritic cells (DCs), the most important antigen-presenting cells, trigger an adaptive immune response by presenting various antigens to other immune cells. DCs change their state from immune activation to immunosuppressive in advanced stages of HCC.45 Intratumoral plasmacytoid DCs induce an immunotolerant and inflammatory tumor microenvironment by comprising regulatory T cells and IL-17+ cells, which contributes to the progression of HCC.46 Our results revealed that neutrophils were significantly abundant in the CSTF2 high expression group, whereas the levels of naïve B cells and activated CD4+ T cells were markedly reduced. In addition, the correlation coefficient between neutrophils and CSTF2 was higher than that between other immune cells and CSTF2. These results indicated that CSTF2 might regulate T cell and neutrophil function in HCC. Immune cells can contribute to tumor progression through immune escape by elevating immune checkpoints. Binding of immune checkpoint proteins to their ligands triggers the inactivation of T cells and immunosuppression, which promote tumor immune escape and progression. Immune checkpoint inhibitors restore T cells activation and immune responses by blocking immune checkpoint molecules.47 Immune checkpoint blockade therapies have been shown to be effective treatments, and HCC patients benefit more from immune checkpoint inhibitors combined with other therapies, such as local resection and chemoembolization.48 The expression of immune checkpoints such as PD-1, PD-L1, CTLA4, TIM3 and TIGIT was positively correlated with tumor progression.48 In this study, we found that CSTF2 expression had positive relationships with PD-1, PD-L1, CTLA4, TIM3 and TIGIT expression. Moreover, the expression of these immune checkpoints was significantly upregulated in the high CSTF2 expression group compared with the low CSTF2 expression group. The Wnt/β-catenin signaling pathway participates in multiple pathophysioligical processes that are related with cellular homeostasis and tumorigenesis. When Wnt/β-catenin signaling pathway is activated, β-catenin binds to a series of transcription factors to induce the expression of Wnt/β-catenin target genes, such as c-MYC and Cyclin D1.49 The Wnt/β-catenin signaling pathway is closely associated with hepatocarcinogenesis and has been regarded as a potential therapeutic target.50 Inactivating the Wnt/β-catenin signaling pathway inhibit the malignant phenotypes of liver cancer.51 Our results revealed that CSTF2 silencing inhibited proliferation, invasion and migration, and promoted apoptosis in HepG2 cells. GSEA suggested that the facilitatory effect of CSTF2 in hepatocarcinogenesis might be related with the activation of Wnt/β-catenin signaling pathway, which was validated by Western blotting analysis (Supplementary Figure 2). These results indicated that CSTF2 might be a promising therapeutic target for HCC patients.
Conclusion
In conclusion, we demonstrated that overexpression of CSTF2 correlates with unfavorable outcomes in HCC patients. The nomogram based on CSTF2 expression possesses better predictive performance than the AJCC staging system. High CSTF2 expression affects immune cell infiltration and immune checkpoint expression in the tumor microenvironment of HCC. CSTF2 silencing can alleviated the malignant phenotypes of hepatic cancer cells by inactivating the Wnt/β-catenin signaling pathway. CSTF2 can serve as a promising prognostic marker and therapeutic target for HCC patients.
Data Sharing Statement
The datasets analyzed in the study are available in the following public databases, including TCGA (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga), GEO (https://www.ncbi.nlm.nih.gov/geo/), ICGC (https://dcc.icgc.org/), LinkedOmics (http://www.linkedomics.org/), HPA (https://www.proteinatlas.org/), TIMER (https://cistrome.shinyapps.io/timer/), and GEPIA (http://gepia.cancer-pku.cn/) online tools.
Ethics Approval
Our research was approved by the ethics committee of the First Affiliated Hospital of Nanchang University.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Number: 81960120) and the “Gan-Po Talent 555” project of Jiangxi Province [Grant Number: GCZ(2012)-1]. The authors would like to sincerely appreciate all coordinators, hepatologists, and investigators that participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
2. Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi:10.1038/nrdp.2016.18
3. Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
4. Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res. 2018;30(6):571–579. doi:10.21147/j.issn.1000-9604.2018.06.01
5. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
6. Galle P, Forner A, Llovet J, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
7. Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34(2):153–159. doi:10.1053/j.semdp.2016.12.011
8. Darmon SK, Lutz CS. mRNA 3’ end processing factors: a phylogenetic comparison. Comp Funct Genomics. 2012;2012:876893. doi:10.1155/2012/876893
9. Takagaki Y, Manley JL, MacDonald CC, et al. A multisubunit factor, CstF, is required for polyadenylation of mammalian pre-mRNAs. Genes Dev. 1990;4(12a):2112–2120. doi:10.1101/gad.4.12a.2112
10. Mandel CR, Bai Y, Tong L. Protein factors in pre-mRNA 3’-end processing. Cell Mol Life Sci. 2008;65(7–8):1099–1122. doi:10.1007/s00018-007-7474-3
11. Salisbury J, Hutchison KW, Graber JH. A multispecies comparison of the metazoan 3’-processing downstream elements and the CstF-64 RNA recognition motif. BMC Genomics. 2006;7(1):55. doi:10.1186/1471-2164-7-55
12. Takagaki Y, MacDonald CC, Shenk T, et al. The human 64-kDa polyadenylylation factor contains a ribonucleoprotein-type RNA binding domain and unusual auxiliary motifs. Proc Natl Acad Sci USA. 1992;89(4):1403–1407. doi:10.1073/pnas.89.4.1403
13. Youngblood BA, MacDonald CC. CstF-64 is necessary for endoderm differentiation resulting in cardiomyocyte defects. Stem Cell Res. 2014;13(3 Pt A):413–421. doi:10.1016/j.scr.2014.09.005
14. Grozdanov PN, Masoumzadeh E, Kalscheuer VM, et al. A missense mutation in the CSTF2 gene that impairs the function of the RNA recognition motif and causes defects in 3’ end processing is associated with intellectual disability in humans. Nucleic Acids Res. 2020;48(17):9804–9821. doi:10.1093/nar/gkaa689
15. Youngblood BA, Grozdanov PN, MacDonald CC. CstF-64 supports pluripotency and regulates cell cycle progression in embryonic stem cells through histone 3’ end processing. Nucleic Acids Res. 2014;42(13):8330–8342. doi:10.1093/nar/gku551
16. Takagaki Y, Seipelt RL, Peterson ML, et al. The polyadenylation factor CstF-64 regulates alternative processing of IgM heavy chain pre-mRNA during B cell differentiation. Cell. 1996;87(5):941–952. doi:10.1016/S0092-8674(00)82000-0
17. Chuvpilo S, Zimmer M, Kerstan A, et al. Alternative polyadenylation events contribute to the induction of NF-ATc in effector T cells. Immunity. 1999;10(2):261–269. doi:10.1016/S1074-7613(00)80026-6
18. Chen X, Zhang JX, Luo JH, et al. CSTF2-induced shortening of the RAC1 3ʹUTR promotes the pathogenesis of urothelial carcinoma of the bladder. Cancer Res. 2018;78(20):5848–5862. doi:10.1158/0008-5472.CAN-18-0822
19. Lin A, Ji P, Niu X, et al. CstF64-induced shortening of the bid 3ʹUTR promotes esophageal squamous cell carcinoma progression by disrupting ceRNA cross-talk with ZFP36L2. Cancer Res. 2021;81(22):5638–5651. doi:10.1158/0008-5472.CAN-21-1201
20. Zhang S, Zhang X, Lei W, et al. Genome-wide profiling reveals alternative polyadenylation of mRNA in human non-small cell lung cancer. J Transl Med. 2019;17(1):257. doi:10.1186/s12967-019-1986-0
21. Aragaki M, Takahashi K, Akiyama H, et al. Characterization of a cleavage stimulation factor, 3’ pre-RNA, subunit 2, 64 kDa (CSTF2) as a therapeutic target for lung cancer. Clin Cancer Res. 2011;17(18):5889–5900. doi:10.1158/1078-0432.CCR-11-0240
22. Gharesouran J, Taheri M, Sayad A, et al. A novel regulatory function of long non-coding RNAs at different levels of gene expression in multiple sclerosis. J Mol Neurosci. 2019;67(3):434–440. doi:10.1007/s12031-018-1248-2
23. Li T, Fan J, Wang B, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi:10.1158/0008-5472.CAN-17-0307
24. Vasaikar SV, Straub P, Wang J, et al. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46(D1):D956–D963. doi:10.1093/nar/gkx1090
25. Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi:10.1038/nmeth.3337
26. Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13(2):125–137. doi:10.1007/s12072-018-9919-1
27. Liu Z, Liu X, Liang J, et al. Immunotherapy for hepatocellular carcinoma: current status and future prospects. Front Immunol. 2021;12:765101. doi:10.3389/fimmu.2021.765101
28. Ye Y, Yu F, Li Z, et al. RNA binding protein serine/arginine splicing factor 1 promotes the proliferation, migration and invasion of hepatocellular carcinoma by interacting with RecQ protein-like 4 mRNA. Bioengineered. 2021;12(1):6144–6154. doi:10.1080/21655979.2021.1972785
29. Liang Y, Fan Y, Liu Y, et al. HNRNPU promotes the progression of hepatocellular carcinoma by enhancing CDK2 transcription. Exp Cell Res. 2021;409(1):112898. doi:10.1016/j.yexcr.2021.112898
30. Park S, Choi S, Cho YA, et al. Evaluation of the American Joint Committee on Cancer (AJCC) 8th edition staging system for hepatocellular carcinoma in 1008 patients with curative resection. Cancer Res Treat. 2020;52(4):1145–1152. doi:10.4143/crt.2020.208
31. Abdel-Rahman O. Assessment of the discriminating value of the 8th AJCC stage grouping for hepatocellular carcinoma. HPB. 2018;20(1):41–48. doi:10.1016/j.hpb.2017.08.017
32. Kamarajah SK, Frankel TL, Sonnenday C, et al. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with Hepatocellular Carcinoma (HCC): a Surveillance, Epidemiology, End Results (SEER) analysis. J Surg Oncol. 2018;117(4):644–650. doi:10.1002/jso.24908
33. Wei Y, Luo H, Yee PP, et al. Paraspeckle protein NONO promotes TAZ phase separation in the nucleus to drive the oncogenic transcriptional program. Adv Sci. 2021;8(24):2102653. doi:10.1002/advs.202102653
34. Shen M, Zhang R, Jia W, et al. Nuclear scaffold protein p54nrb/NONO facilitates the hypoxia-enhanced progression of hepatocellular carcinoma. Oncogene. 2021;40(24):4167–4183. doi:10.1038/s41388-021-01848-9
35. Okada M, Cheeseman IM, Hori T, et al. The CENP-H–I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat Cell Biol. 2006;8(5):446–457. doi:10.1038/ncb1396
36. Ding N, Li R, Shi W, et al. CENPI is overexpressed in colorectal cancer and regulates cell migration and invasion. Gene. 2018;674:80–86. doi:10.1016/j.gene.2018.06.067
37. Wang J, Liu X, Chu H, et al. Centromere protein I (CENP-I) is upregulated in gastric cancer, predicts poor prognosis, and promotes tumor cell proliferation and migration. Technol Cancer Res Treat. 2021;20:1180552969. doi:10.1177/15330338211045510
38. Thangavelu PU, Lin C, Vaidyanathan S, et al. Overexpression of the E2F target geneCENPI promotes chromosome instability and predicts poor prognosis in estrogen receptor-positive breast cancer. Oncotarget. 2017;8(37):62167–62182. doi:10.18632/oncotarget.19131
39. Yu Q, Yin L, Jian Y, et al. Downregulation of PHF6 inhibits cell proliferation and migration in hepatocellular carcinoma. Cancer Biother Radiopharm. 2019;34(4):245–251. doi:10.1089/cbr.2018.2671
40. Yu Q, Zhou J, Jian Y, et al. MicroRNA‐214 suppresses cell proliferation and migration and cell metabolism by targeting PDK2 and PHF6 in hepatocellular carcinoma. Cell Biol Int. 2019;44(1):117–126. doi:10.1002/cbin.11207
41. Romeo V, Griesbach E, Schümperli D. CstF64: cell cycle regulation and functional role in 3′ end processing of replication-dependent histone mRNAs. Mol Cell Biol. 2014;34(23):4272–4284. doi:10.1128/MCB.00791-14
42. Leone V, Ali A, Weber A, et al. Liver inflammation and hepatobiliary cancers. Trends Cancer. 2021;7(7):606–623. doi:10.1016/j.trecan.2021.01.012
43. Chen H, Zhou X, Li J, et al. Neutrophils: driving inflammation during the development of hepatocellular carcinoma. Cancer Lett. 2021;522:22–31. doi:10.1016/j.canlet.2021.09.011
44. Kim CG, Kim C, Yoon SE, et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J Hepatol. 2021;74(2):350–359. doi:10.1016/j.jhep.2020.08.010
45. Zhong M, Zhong C, Cui W, et al. Induction of tolerogenic dendritic cells by activated TGF-β/Akt/Smad2 signaling in RIG-I-deficient stemness-high human liver cancer cells. BMC Cancer. 2019;19(1):439. doi:10.1186/s12885-019-5670-9
46. Zhou Z, Xin H, Li J, et al. Intratumoral plasmacytoid dendritic cells as a poor prognostic factor for hepatocellular carcinoma following curative resection. Cancer Immunol Immunother. 2019;68(8):1223–1233. doi:10.1007/s00262-019-02355-3
47. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi:10.1016/j.intimp.2018.06.001
48. Zheng Y, Wang S, Cai J, et al. The progress of immune checkpoint therapy in primary liver cancer. Biochim Biophys Acta Rev Cancer. 2021;1876(2):188638. doi:10.1016/j.bbcan.2021.188638
49. Wen X, Wu Y, Awadasseid A, et al. New advances in canonical wnt/beta-catenin signaling in cancer. Cancer Manag Res. 2020;12:6987–6998. doi:10.2147/CMAR.S258645
50. Xu C, Xu Z, Zhang Y, et al. beta-catenin signaling in hepatocellular carcinoma. J Clin Invest. 2022;132(4). doi:10.1172/JCI154515.
51. Zi Y, Gao J, Wang C, et al. Pantothenate kinase 1 inhibits the progression of hepatocellular carcinoma by negatively regulating Wnt/beta-catenin Signaling. Int J Biol Sci. 2022;18(4):1539–1554. doi:10.7150/ijbs.67842
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.