Back to Journals » OncoTargets and Therapy » Volume 12
Crossover safety study of aprepitant: 2-min injection vs 30-min infusion in cancer patients receiving emetogenic chemotherapy
Received 16 January 2019
Accepted for publication 10 March 2019
Published 30 April 2019 Volume 2019:12 Pages 3277—3284
DOI https://doi.org/10.2147/OTT.S201609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Video abstract presented by Rudolph M Navari.
Views: 433
Rudolph M Navari,1 Michael C Mosier2
1Department of Medicine, University of Alabama Birmingham, Birmingham, AL, USA; 2Biostatistics, EMB Statistical Solutions, LLC, Overland Park, KS, USA
Introduction: HTX-019 (CINVANTI®) is a novel injectable emulsion formulation of the neurokinin 1 receptor antagonist (RA) aprepitant, approved for preventing acute and delayed chemotherapy-induced nausea and vomiting (CINV). HTX-019 has demonstrated a tolerable safety profile when administered via 30-min intravenous (IV) infusion and 2-min IV injection in healthy volunteers. This prospective study evaluated the safety of HTX-019 administered via 30-min IV infusion and 2-min injection (IV push) in patients with cancer.
Materials and methods: This prospective single-center, randomized, safety, 2-sequence, 2-period, crossover study evaluated HTX-019 130 mg within a guideline-recommended 3-drug regimen for CINV prophylaxis in patients receiving highly (HEC) or moderately emetogenic chemotherapy (MEC). Treatment-emergent adverse events (TEAEs) were assessed at 0–30 (primary endpoint), 30–60, and >60 mins (chemotherapy administration period) following the initiation of the HTX-019 administration, focusing on infusion-site adverse events and hypersensitivity reactions (dyspnea, anaphylaxis).
Results: Among 135 patients (35 MEC, 100 HEC), the most common diagnoses were ovarian (32), lung (17), endometrial (17), and colorectal (15) cancer. Patients were randomized 1:1 to a 2-min injection and a 30-min infusion of HTX-019 (sequence AB or BA), followed by a 5-hydroxytryptamine type 3 RA IV (palonosetron 0.25 mg for 30 s or ondansetron 8–16 mg for 5–10 mins), dexamethasone IV (8–12 mg for 15 mins), and the chemotherapy regimen. Both administration methods were generally well tolerated. No TEAEs occurred within 30 mins after start of HTX-019 administration. All TEAEs occurred during chemotherapy administration; 2 patients experienced 2 TEAEs following injection, and 5 experienced 8 TEAEs following infusion. Three adverse events following infusion (2 dyspnea, 1 throat closing) were considered serious. No TEAEs were considered related to HTX-019.
Conclusion: Short injection of HTX-019 has a tolerable safety profile in patients with cancer, and represents an alternative method of HTX-019 administration for CINV prevention.
Keywords: aprepitant, bag shortage, CINV, HTX-019, real world, short injection
Plain language summary
- This study evaluated the safety of HTX-019, a treatment for preventing a common side effect of chemotherapy known as chemotherapy-induced nausea and vomiting (CINV)
- HTX-019 is an IV formulation of aprepitant—a drug recommended by treatment guidelines for use in combination with other drugs to prevent CINV; HTX-019 was approved in 2017 as a 30 min infusion for CINV prevention
- Some study results have shown that HTX-019 is also safe and well tolerated when given as a short (2 min) IV injection in healthy volunteers; this is an advantage in reducing the need for IV bags and in decreasing administration times, compared to 30 min infusions
- This real-world study in patients with cancer is of clinical value because it demonstrated that HTX-019 was safe when given as a 2 min injection and 30 min infusion in patients receiving chemotherapy
- Short injection of HTX-019 is a viable method of delivery for preventing CINV within a guideline-recommended drug regimen
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is associated with a significant decrease in quality of life and may lead to delay or discontinuation of chemotherapy.1–3 Antiemetic guidelines provide comprehensive recommendations for combination antiemetic regimens to prevent CINV.1,4–8
The current National Comprehensive Cancer Network (NCCN) guidelines recommend the inclusion of a substance P/neurokinin 1 (NK-1) receptor antagonist (RA) as part of an antiemetic regimen for patients receiving highly emetogenic chemotherapy (HEC) and for appropriate patients receiving moderately emetogenic chemotherapy (MEC).1 HTX-019 (CINVANTI®; Heron Therapeutics, Inc., San Diego, CA, USA), approved by the Food and Drug Administration (FDA) in November 2017, is a novel injectable emulsion formulation of the NK-1 RA aprepitant. HTX-019 is indicated in adults for administration in combination with other antiemetic agents for the prevention of acute and delayed CINV associated with initial and repeat courses of HEC including high-dose cisplatin, and nausea and vomiting associated with initial and repeat courses of MEC.9 Safety concerns exist with intravenous (IV) formulations of other NK-1 RAs (fosaprepitant and rolapitant), such as hypersensitivity reactions (HSRs) including anaphylaxis and anaphylactic shock, and infusion-site adverse events (ISAEs), which may be attributed to their synthetic surfactants (polysorbate 80 and Koliphor HS 15, respectively).10,11 HTX-019 is an NK-1 RA formulation free of polysorbate 80 and other synthetic surfactants to improve the safety profile; unlike fosaprepitant and rolapitant, HTX-019 contains only natural excipients. The formulation delivers 130 mg of aprepitant (150 mg fosaprepitant equivalent) per 18 mL of emulsion.9 HTX-019 utilizes lipid components with a long history of use in parenteral products, such as IV nutrition.12 The NCCN guidelines list HTX-019 130 mg IV as a category 1 recommendation, in combination with other antiemetics, for the prevention of CINV in patients receiving HEC or MEC.1 This recommendation, as well as the FDA approval, was based on the findings of 2 bioequivalence studies of HTX-019 130 mg and fosaprepitant,13,14 in which single-dose HTX-019 130 mg IV (30-min infusion) was bioequivalent to single-dose fosaprepitant 150 mg IV, infused over 20 or 30 mins.13,14 Furthermore, HTX-019 was well tolerated and demonstrated a consistent safety profile in the 200 healthy subjects included across both studies. HTX-019 displayed a more tolerable safety profile than fosaprepitant, as the percentage of subjects who reported any TEAEs and the total number of TEAEs, including those within the first 30 mins after infusion, were lower following HTX-019 treatment compared with fosaprepitant.13,14
The two initial bioequivalence studies evaluated HTX-019 infused for 30 mins; however, an acute shortage of IV fluids and bags, along with the desire to reduce administration times, has prompted the administration of small-volume parenteral solutions as short injections (5 mins or less) whenever possible.15 A third bioequivalence study in 50 healthy volunteers showed that administration of HTX-019 130 mg as an IV push (2-min injection) was comparable to 30-min infusion in terms of pharmacokinetics and safety.16 HTX-019 was well tolerated when administered as either an injection or an infusion.16 The crossover design of that study allowed comparison of IV infusion and IV push in the same subjects, but a limitation was that it was conducted in healthy volunteers. The objective of the present prospective study was to evaluate the safety of HTX-019 administered as 30-min IV infusion or 2-min IV push, in a crossover manner, as part of a guideline-recommended 3-drug regimen for CINV prophylaxis in patients with cancer receiving emetogenic chemotherapy (HEC or MEC).
Materials and methods
Study design and objectives
This study was conducted in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice and the Declaration of Helsinki. The study was approved by the institutional review board at the University of Alabama Birmingham. Written informed consent was obtained from each patient.
This prospective study evaluated the safety profile of HTX-019 administered as part of a guideline-recommended 3-drug antiemetic prophylactic CINV regimen in patients receiving either HEC or MEC, with chemotherapy cycles ranging from 14 to 28 days. Patients were treated in a randomized sequence in crossover fashion (AB or BA) across 2 treatment periods as follows: treatment A—2-min IV injection of HTX-019 130 mg; treatment B—30-min IV infusion of HTX-019 130 mg (in a 130-mL bag). There was a washout period between the 2 treatment periods for each patient (≥14 days corresponding to the next chemotherapy cycle). The primary objective was the assessment of adverse events for 30 mins from the start of HTX-019 administration as either a 2-min injection or a 30-min infusion while patients received the chemotherapy regimen.
Patients
Enrolled patients were adult men and women aged 20–92 with cancer who were receiving at least two cycles (14–28 days for each cycle) of the same chemotherapy regimen (HEC or MEC) and were eligible to receive a guideline-recommended 3-drug antiemetic regimen of an NK-1 RA (HTX-019), 5-hydroxytryptamine type 3 (5-HT3) RA, and dexamethasone. Patients were excluded if they were pregnant; were lactating; had evidence or history of clinically significant allergic hematologic, endocrine, pulmonary, gastrointestinal, cardiovascular, hepatic, psychiatric, or neurologic disease; or had any contraindication or known or suspected hypersensitivity/idiosyncratic reaction to aprepitant or any component of HTX-019, or any drug in the NK-1 RA class.
Treatment regimens
Patients received the antiemetic treatment regimen starting at least 60 mins prior to chemotherapy in the following order: HTX-019 130 mg (2-min injection or 30-min infusion), followed by a 5-HT3 RA (palonosetron 0.25 mg IV for 30 s, or ondansetron 8 mg IV [MEC] or 8–16 mg IV [HEC] for 5–10 mins), followed by dexamethasone 8–12 mg IV for 15–20 mins, followed by the chemotherapy regimen (at least 60 mins after start of HTX-019 administration). Patients scheduled to receive a taxane chemotherapy were given IV diphenhydramine and IV ranitidine (for 30 mins) following the dexamethasone and prior to the chemotherapy.
Study assessments
All adverse events, regardless of causality or seriousness, were recorded from the time the patient signed the informed consent (24–48 hrs prior to the start of treatment) through the end of the study period. Any adverse events occurring at 0–30 (primary endpoint) and 30–60 mins following the start of HTX-019 administration during the first and second cycles were recorded. Any adverse events noted after the 60-min period and during the administration of the chemotherapy were also recorded. Serious adverse events (SAEs) were defined as resulting in the following outcomes: death, life-threatening adverse event, inpatient hospitalization or prolongation of existing hospitalization, or persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions. Other assessments included cancer diagnosis, baseline Eastern Cooperative Oncology Group performance status, baseline physical examination, review of current and past medications, standard blood tests, emetogenic potential of the chemotherapy regimen administered (HEC or MEC), cycle of chemotherapy received based on the crossover design (1 or 2), method of administration of HTX-019 (injection or infusion via a peripheral line or central line), and additional antiemetics administered during the study.
Statistical methods
Sample size justification
This crossover study was designed to demonstrate that delivery of HTX-019 via IV push was noninferior to delivery of HTX-019 via IV infusion in terms of infusion-site reaction (ISR) rate. Assuming an expected ISR rate of 5% for both delivery methods (ie, equivalence), and that an absolute difference of 4% was clinically acceptable as not significantly worse, a sample size of 130 patients was required. This sample size for comparison of paired proportions was computed using an assumed 1% random chance of discordant pairs (ie, an ISR with one method but not with the other), and provided 80% power to demonstrate the noninferiority of IV push to IV infusion using a 95% upper 1-sided confidence limit, meaning the upper confidence limit for the difference in rates would be <4%.
Statistical analysis
The primary endpoint was the evaluation of adverse events occurring during the 30 mins following the start of a 2-min injection or a 30-min infusion of HTX-019. Of particular interest was the occurrence of either ISAEs or HSRs, such as dyspnea or anaphylaxis. These are collectively referred to as ISRs. The difference in the ISR rate between methods was estimated using a 1-sided upper 95% confidence limit, computed using Newcombe’s method 10.17 If this upper confidence limit for the difference in proportions was ≤0.04 (the chosen noninferiority margin), it would be concluded that the 2-min IV injection (IV push method) was noninferior to the 30-min IV infusion method.
Results
Patients
A total of 139 patients were enrolled and randomized, 4 of whom did not complete the study as a result of declining to participate after signing the consent (n=1), significant delay in treatment (n=1), and change in chemotherapy agents (n=2). The demographics and baseline characteristics of the remaining 135 patients are summarized in Table 1. Of 135 patients, 35 received a MEC regimen and 100 received a HEC regimen, with the most common chemotherapy regimens including carboplatin area under the concentration-time curve (AUC) >4, taxanes, anthracycline+cyclophosphamide, and cisplatin. The most common diagnoses were ovarian, lung, endometrial, and colorectal cancer (Table 1). Each patient received a 2-min injection and a 30-min infusion of HTX-019 130 mg according to the 2-treatment, 2-period, 2-sequence crossover design (Figure 1).
 | 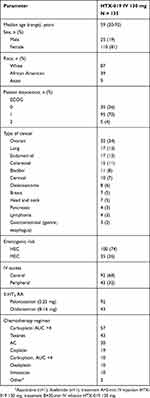 |  | Table 1 Demographics and baseline characteristics |
 | Figure 1 Study design. Abbreviation: IV, intravenous. |
Safety and tolerability of HTX-019 injection and infusion
HTX-019 administered as either an injection or an infusion was generally well tolerated. The number of treatment-emergent adverse events (TEAEs) of any cause and the percentage of patients experiencing a TEAE are shown in Table 2. None of the TEAEs occurred within 30 mins or within the 30–60 mins following HTX-019 injection or infusion. All 10 TEAEs reported in 7 patients occurred at least 60 mins following HTX-019 administration in patients who received HTX-019 infusion or injection followed by IV diphenhydramine, ranitidine, dexamethasone, and palonosetron or ondansetron, then followed by chemotherapy (diphenhydramine and ranitidine were administered as premedications for patients receiving taxane chemotherapy). Given their timing, none of these TEAEs were considered by the investigator to be related to HTX-019 administration.
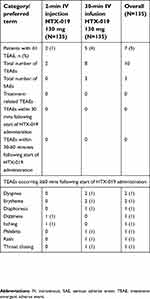 | Table 2 Summary of treatment-emergent adverse events of any cause occurring in either group |
Safety and tolerability of HTX-019 2-min injection
In the 2-min HTX-019 IV injection group (IVP), 2 TEAEs were reported after chemotherapy administration in 2 of 135 patients (1%) (patients IVP1 and IVP2), dizziness (n=1) and itching (n=1). Both patients received HEC via central venous access. Patient IVP1 was 65 years old with ovarian cancer receiving doxorubicin and carboplatin (AUC >4) who reported mild transient dizziness occurring 50 mins after the start of chemotherapy (≥110 mins after start of HTX-019 administration in period 2, lasting 5 mins and resolving spontaneously. Patient IVP2 was 48 years old with lung cancer receiving cisplatin who reported itching of the arms and legs with no rash, occurring 30 mins after the start of chemotherapy (≥90 mins after start of HTX-019 administration) in period 2 and resolving after 15–20 mins with additional IV diphenhydramine.
Safety and tolerability of HTX-019 30-min infusion
In the 30-min HTX-019 infusion group (IVI), 8 TEAEs were reported after the start of chemotherapy administration in 5 of 135 patients (4%) (patients IVI1-5), the most common being erythema (n=2) and dyspnea (n=2) (Table 2). Four of the 5 patients who had TEAEs were receiving HEC (peripheral venous access, n=3; central venous access, n=1); 1 of the 5 received MEC via central venous access. Patient IVI1 was 63 years old with bladder cancer receiving cisplatin and gemcitabine who developed phlebitis at the site of the peripheral line 30 mins after the start of chemotherapy (≥90 mins after start of HTX-019 administration) in period 1, which resolved in 72 hrs with no additional treatment. Patient IVI2 was 49 years old with ovarian cancer receiving paclitaxel and carboplatin (AUC >4) who developed a rash at the site of the peripheral line 30 mins after the start of chemotherapy (≥90 mins after start of HTX-019 administration) in period 1, which resolved in 72 hrs with no additional treatment. Patient IVI3 was 68 years old with ovarian cancer receiving docetaxel and carboplatin (AUC >4) who developed erythema at the site of the peripheral line 20 mins after the start of chemotherapy (≥80 mins after start of HTX-019 administration) in period 2. The erythema persisted for 10 days and slowly resolved without any additional treatment. Patient IVI4 was 48 years old with endometrial cancer treated with carboplatin (AUC >4) and docetaxel via a central line who developed dyspnea, total-body erythema, and diaphoresis 15 mins after beginning docetaxel (≥75 mins after start of HTX-019 administration) in period 2. Following additional treatment with IV diphenhydramine and IV dexamethasone, symptoms resolved within 20–30 mins; this was thought to be a taxane reaction. Patient IVI5 was 29 years old with rectal cancer treated with folinic acid, 5-fluorouracil, and oxaliplatin (FOLFOX) via a central line who developed dyspnea and throat closing 10 mins after starting oxaliplatin (≥70 mins after start of HTX-019 administration) in period 2. Following additional treatment with IV diphenhydramine and IV dexamethasone, symptoms resolved within 30 mins.
Patients IVI4 and IVI5 had 3 SAEs (dyspnea n=2; throat closing n=1) (Table 2), none of which was fatal or led to study drug or chemotherapy discontinuation or withdrawal. Of the 5 patients in the 30-min infusion group who had at least 1 TEAE, 3 received carboplatin plus docetaxel or paclitaxel; they experienced a total of 5 TEAEs, all related to taxanes.
TEAEs in first 30 mins after the start of HTX-019 administration
As outlined above, 6 patients (injection, n=1; infusion, n=5) experienced a total of 9 TEAEs 10–30 mins after start of chemotherapy administration, which was at least 70–90 mins after start of HTX-019 administration. None of these TEAEs occurred within the first 30 mins after start of HTX-019 administration, and none were deemed by the investigator to be related to HTX-019 treatment. All of the TEAEs were resolved by the end of the study.
Noninferiority analysis of ISRs with HTX-019 injection and infusion
No ISRs or other TEAEs were reported in the first 30 mins following the start of HTX-019 130 mg administration with either 2-min injection or 30-min infusion. The adverse events reported with either 2-min injection or 30-min infusion of HTX-019 130 mg were not considered to be ISRs. Therefore, statistical analysis to demonstrate noninferiority with regard to ISRs was not conducted. Instead, the analysis was performed using a “conservative” approach, and included any adverse event reported at any time after the start of HTX-019 treatment. There were 2 such TEAEs reported with the 2-min injection and 5 TEAEs reported with the 30-min infusion, all which occurred more than 60 mins following the start of HTX-019 administration. None of the patients reported a TEAE on both treatments. The point estimate for the difference in TEAE rates was −0.022, and the upper 1-sided 95% confidence limit for the difference in TEAE rates was 0.0127. Since this was well below the 0.04 noninferiority margin set for ISR rates, the 2-min injection would be declared noninferior to the 30-min infusion of HTX-019 130 mg, for the occurrence of TEAEs at any time following the start of HTX-019 administration.
Discussion
HTX-019 130 mg administered as a 2-min injection or a 30-min infusion in a crossover fashion in patients with cancer was well tolerated. No TEAEs occurred within 30 mins after start of HTX-019 administration. Overall, there were 10 TEAEs, with 2 TEAEs occurring in 2 patients (1%) receiving the 2-min injection and 8 TEAEs occurring in 5 patients (4%) receiving the 30-min infusion. All these TEAEs were seen following administration of chemotherapy and were therefore not considered related to HTX-019. The only TEAEs reported following the 2-min injection were itching and dizziness. The most common TEAEs reported with the 30-min infusion were erythema and dyspnea. Six patients experienced a total of 9 TEAEs in the first 30 mins after start of chemotherapy administration (≥70–90 mins after HTX-019 administration). There were 3 SAEs (dyspnea n=2; throat closing n=1) that occurred following the start of chemotherapy administration in 2 patients receiving the 30-min infusion of HTX-019. Given the timing of the TEAEs during chemotherapy administration, none were deemed by the investigators to be related to HTX-019 treatment. All TEAEs were observed in patients receiving taxane-based chemotherapy who had been pretreated with IV diphenhydramine, ranitidine, and palonosetron after HTX-019. The 5 TEAEs reported in 3 patients who received a 30-min infusion were considered taxane-related. All TEAEs resolved by end of the study. In addition, none of the TEAEs in the study were considered ISRs, and administration via the 2-min injection demonstrated noninferiority to the 30-min infusion for TEAEs that occurred at any time following the start of HTX-019 administration.
HTX-019 was approved by the FDA as a 30-min infusion and, more recently, as a 2-min injection for use in adults, in combination with other antiemetics, for the prevention of acute and delayed nausea and vomiting with initial and repeat courses of HEC and MEC.9 The approval of HTX-019 was based on the findings of 2 bioequivalence studies (104 and 106).13,14 A pooled analysis of the 2 studies showed that headache (in 3% of subjects) and fatigue (2%) were the most commonly reported TEAEs after HTX-019 administration, whereas ISAEs (10%) and headache (7%) were the most commonly reported TEAEs after fosaprepitant administration. No serious or severe TEAEs or deaths were reported; all TEAEs were resolved by the end of the studies. Taken together, the percentage of subjects who reported any TEAEs and the number of TEAEs were much less following treatment with HTX-019 130 mg (30-min infusion) compared with fosaprepitant 150 mg (20- or 30-min infusion). Similar results were seen with the percentage of subjects reporting treatment-related TEAEs and the number of treatment-related TEAEs, which were lower in subjects treated with HTX-019.13,14 In addition, the number of TEAEs reported within the first 30 mins following the start of infusion was much lower after HTX-019 (2.6%) compared with fosaprepitant (15%) treatment.13,14
The approved dosage of HTX-019 is 100 (MEC) or 130 mg (HEC) infused for 30 mins.9 In November 2017, the FDA announced a significant acute shortage of small-volume parenteral solutions, including those used for HTX-019 dilution, referring to the American Society of Health-System Pharmacists (ASHP) recommendation that clinicians switch parenteral administration to IV push (injection of 5 mins or less) whenever possible.15 A recent Phase I study (study 108) addressed the ASHP recommendation by showing that a 2-min injection and a 30-min infusion of HTX-019 130 mg had comparable pharmacokinetic and safety profiles. The most common treatment-related TEAEs were headache (4%) and fatigue (6%) in the 2-min injection group and 30-min infusion group, respectively. Only 1 patient (2%) for each method of administration experienced a TEAE (feeling hot) within the first 30-min posttreatment.16 The results of the current prospective study in patients with cancer receiving chemotherapy are consistent with the study 108 findings16 in that HTX-019 administration was well tolerated, especially in patients receiving the 2-min injection. These results are of clinical relevance, as they provide safety information on administering HTX-019 as a short injection within a guideline-recommended 3-drug regimen for CINV prophylaxis in patients receiving emetogenic chemotherapy.
Conclusion
These findings demonstrate that a short injection of HTX-019 has a tolerable safety profile in patients with cancer, and may be used as a potential alternative method of administration in CINV prevention while decreasing the need for IV bags.
Acknowledgments
Medical writing support was provided by Phillip Giannopoulos, PhD, of SciStrategy Communications, and funded by Heron Therapeutics, Inc. The authors would like to thank the University of Alabama Birmingham pharmacy administration and staff as well as the nursing infusion center staff for their assistance with the study.
Author contributions
Rudolph M Navari was responsible for the conception and design, and collection and assembly of data. Michael C Mosier was responsible for the data analysis and interpretation. Rudolph M Navari and Michael C Mosier were responsible for the writing and critical revisions of the manuscript, read and approved the final manuscript, and agree to be accountable for all aspects of the work.
Disclosure
Rudolph M Navari and Michael C Mosier have served in a consultant/advisory role for Heron Therapeutics, Inc. Dr Michael C Mosier reports personal fees from Heron Therapeutics, Inc., during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. NCCN clinical practice guidelines in oncology: antiemesis―version 1.2019.Available from:
2. Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24(27):4472–4478. doi:10.1200/JCO.2006.05.6382
3. Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15(5):497–503. doi:10.1007/s00520-006-0173-z
4. Herrstedt J, Roila F, Warr D, et al. 2016 Updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following high emetic risk chemotherapy. Support Care Cancer. 2017;25(1):277–288. doi:10.1007/s00520-016-3313-0
5. Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(28):3240–3261. doi:10.1200/JCO.2017.74.4789
6. Roila F, Warr D, Hesketh PJ, et al. 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following moderately emetogenic chemotherapy. Support Care Cancer. 2017;25(1):289–294. doi:10.1007/s00520-016-3365-1
7. Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20(1):107–117. doi:10.1007/s00520-010-1073-9
8. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE Study. J Oncol Pract. 2014;10(1):68–74. doi:10.1200/JOP.2012.000816
9. CinvantiTM (Aprepitant) Injectable Emulsion, for Intravenous Use. San Diego, CA: Heron Therapeutics; 2019.
10. Emend (Fosaprepitant) for Injection, for Intravenous Use [Prescribing Information]. Whitehouse Station, NJ: Merck & Co; 2019.
11. Varubi (Rolapitant) Tablets, for Oral Use. Varubi® (Rolapitant) Injectable Emulsion, for Intravenous Use [Prescribing Information]. Waltham, MA: Tesaro Inc; 2018.
12. Raman M, Almutairdi A, Mulesa L, Alberda C, Beattie C, Gramlich L. Parenteral nutrition and lipids. Nutrients. 2017;9(4):388. doi:10.3390/nu9040388
13. Ottoboni T, Keller MR, Cravets M, Clendeninn N, Quart B. Bioequivalence of HTX-019 (aprepitant IV) and fosaprepitant in healthy subjects: a phase I, open-label, randomized, two-way crossover evaluation. Drug Des Devel Ther. 2018;12:429–435. doi:10.2147/DDDT.S155875
14. Ottoboni T, Lauw M, Keller MR, et al. Safety of HTX-019 (intravenous aprepitant) and fosaprepitant in healthy subjects. Future Oncol. 2018;14(27):2849–2859. doi:10.2217/fon-2018-0311
15.
16. Ottoboni T, Lauw M, Keller MR, et al. HTX-019 via 2-min injection or 30-min infusion in healthy subjects. Future Oncol. 2019;15(8):865-874. doi:10.2217/fon-2018-0809
17. Newcombe RG. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med. 1998;17(22):2635–2650.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
