Back to Journals » Vascular Health and Risk Management » Volume 19
Cross-Sectional Relationship Between Atrial Conduction Delay and Arterial Stiffness in Patients with Obstructive Sleep Apnea
Authors Ueda A, Kasagi S, Maeno K, Naito R, Kumagai T, Kimura Y, Kato M, Kawana F, Tomita Y , Narui K, Kasai T
Received 10 July 2023
Accepted for publication 5 November 2023
Published 13 November 2023 Volume 2023:19 Pages 733—740
DOI https://doi.org/10.2147/VHRM.S428713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Daniel Duprez
Azusa Ueda,1,2 Satoshi Kasagi,2 Ken-ichi Maeno,2,3 Ryo Naito,4– 6 Takiko Kumagai,1,2 Yuka Kimura,1,2 Mitsue Kato,1,2,4 Fusae Kawana,1,2,4 Yasuhiro Tomita,2,5,6 Koji Narui,2,4 Takatoshi Kasai2,4– 6
1Clinical Physiology, Toranomon Hospital, Tokyo, Japan; 2Department of Sleep Respiratory Medicine, Toranomon Hospital, Tokyo, Japan; 3Department of Cardiovascular Medicine, Japanese Red Cross Ise Hospital, Mie, Japan; 4Cardiovascular Respiratory Sleep Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan; 5Department of Cardiovascular Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan; 6Sleep and Sleep Disordered Breathing Center, Juntendo University Hospital, Tokyo, Japan
Correspondence: Satoshi Kasagi
Sleep Respiratory Medicine, Toranomon Hospital, 2-2-2 Toranomon, Minato-ku, Tokyo, 105-8470, Japan
, Tel +81-3-3588-1111
, Fax +81-3-3560-7815
, Email [email protected]
Aim: Prolonged P-wave duration (PWD), which indicates atrial conduction delay, is a potent precursor of atrial fibrillation (AF) that may be induced by obstructive sleep apnea (OSA). The cardio-ankle vascular index (CAVI), which is an arterial stiffness parameter, is elevated in patients with OSA; moreover, an increased CAVI is associated with atrial conduction delay through left atrium enlargement in association with left ventricular diastolic dysfunction. We aimed to examine the relationship between the CAVI and PWD in patients with OSA.
Methods: We included patients with a sinus rhythm who underwent overnight polysomnography. We measured the PWD and CAVI on standard 12-lead electrocardiograms; further, we analyzed the relationship between PWD and CAVI.
Results: We analyzed data from 300 participants (men, 89.0%; mean age, 52.3 ± 13.1 years; and body mass index, 26.2 ± 3.9 kg/m2). The mean PWD was 104.4 ± 10.4 ms while the mean CAVI was 7.5 ± 1.5. PWD was significantly correlated with CAVI (r = 0.478, p < 0.001); additionally, PWD and CAVI were directly associated with OSA severity (p = 0.002 and p = 0.002, respectively). Multivariate regression analysis revealed an independent significant correlation of PWD and CAVI with OSA severity.
Conclusion: In patients with OSA, an increase in arterial stiffness is associated with atrial conduction delay.
Keywords: atrial fibrillation, cardio-ankle vascular index, obstructive sleep apnea, P-wave duration
Introduction
The P-wave duration (PWD) on an electrocardiogram is generally accepted as a reliable and noninvasive indicator of atrial conduction.1 Prolonged PWD, which indicates atrial conduction delay, is a potent precursor of atrial fibrillation (AF).2,3 Obstructive sleep apnea (OSA) may cause atrial conduction delay, which can be modified by OSA treatment through continuous positive airway pressure (CPAP).4 The possible mechanisms underlying the relationship between OSA and atrial conduction delay include mechanical and autonomic cardiovascular effects such as elevated cardiac wall stress related to exaggerated intrathoracic pressure oscillations,5,6 elevated sympathetic nerve activity,7 activation of systemic inflammation,8 oxidative stress associated with the production of reactive oxygen species,9 and the related endothelial dysfunction.10 These pathophysiological effects of OSA may directly contribute to vascular damage and left atrial remodeling, which are strongly associated with subsequent cardiovascular events, including new-onset AF.3,11
Additionally, OSA may increase arterial stiffness.12 Specifically, the cardio-ankle vascular index (CAVI), which is among the arterial stiffness parameters, is higher in patients with severe OSA and is considered a clinically useful index for vascular damage progression.13 Although the underlying mechanisms remain unclear, PWD is associated with arterial stiffness parameters, which could involve left ventricular diastolic dysfunction.14 In patients with OSA, the left atrial diameter is associated with arterial stiffness.12 However, the relationship between PWD and arterial stiffness in patients with OSA remains unclear.
This study aimed to examine the relationship of OSA severity with PWD and CAVI as well as the relationship between PWD and CAVI in patients with OSA.
Methods
Participants
We included all consecutive participants was taken informed consent who had undergone an overnight sleep study at the Sleep Center of the Toranomon Hospital (Tokyo, Japan) from January to July 2007. The inclusion criteria were as follows: age ≥ 20 years and normal sinus rhythm, where every P-wave was positive in leads I, II, and aVF with uniform morphologies; every QRS complex was preceded by a P-wave; and every P-wave was followed by a QRS. The exclusion criteria were as follows: 1) a history of AF, atrial flutter, or sodium-channel blocker administration; 2) frequent episodes of atrial and ventricular ectopies on electrocardiogram and/or during CAVI measurements; 3) having a pacemaker or implantable cardioverter-defibrillator; 4) evidence of heart failure; 5) a history of ischemic heart disease; having significant valvular heart disease, aortic disease, peripheral vascular disease, and severe chronic pulmonary disease; 6) being on dialysis; 7) ankle-brachial index < 0.9 during CAVI measurements; and 8) having central sleep apnea; 9) have experienced psychological severe stress before measuring the CAVI. All the patients provided informed consent for participation in the study. The study complied with the Declaration of Helsinki and was conducted in accordance with the ethics policies of the involved institution.
Sleep Study
All participants underwent an overnight sleep study using a digital polygraph (SomnoStar a Sleep System; Sensor Medics, Yorba Linda, CA, USA) at our sleep laboratory, with the use of standard definitions and scoring methods.15 OSA severity was evaluated using the apnea-hypopnea index (AHI), which was calculated as the total number of apnea and hypopnea events divided by the total sleep time, expressed as the number of events per hour. Patients with central sleep apnea were defined as those with an AHI of ≥ 5 events/h, with > 50% being central events.16–18
CAVI
Technologists who were blinded to the polysomnographic and electrocardiogram data measured the CAVI using a Vasera VS-1000 device (Fukuda Denshi, Tokyo, Japan) while the participants were awake between 8:00 AM and 12:00 noon on the same day.
Further, blood pressure measurements, anthropometric data, and blood samples were collected. Cuffs were applied to the four extremities; moreover, electrocardiographic electrodes were attached to the upper arm. Phonocardiography was performed using a microphone placed at the sternal angle. The participants rested in a supine position for 5 minutes. Details regarding CAVI and CAVI measurements have been previously described.13,19–21
P-Wave Duration
On the day of the sleep study, a standard 12-lead electrocardiogram was obtained using the same electrocardiogram device (PageWriter Touch; Philips, Amsterdam, the Netherlands) at 25 mm/s with 1 mV/cm standardization after supine rest for ≥ 5 minutes. The data were digitally stored on a server at our institution. The PWD was manually measured by an experienced observer who was blinded to the polysomnographic data and CAVI value using software for the digital caliper (Hakarun, version 0.7.0; Onegland, Shizuoka, Japan) with fourfold magnification. The PWD was obtained as the mean duration of three consecutive beats in lead II. Each measurement included identifying the initiation of the P-wave onset and its termination at the P-wave offset. The P-wave onset was identified as the junction of the T and P isoelectric line with the beginning of the positive deflection. Additionally, the P-wave offset was identified as the junction between the end of the P-wave deflection and PR segment.22 To determine intraobserver reproducibility, we obtained the coefficients of variation (CV) from triplicate measurements obtained on different days in 10 randomly selected patients. The average CV was 2.2%.22
Blood Samples and Other Data
Venous blood samples with complete blood count and biochemistry were obtained in the early morning after overnight fasting and immediately after the sleep study and analyzed serum were separated by centrifugation at central laboratory in the Toranomon Hospital. We calculated the estimated glomerular filtration rate (eGFR) based on serum creatinine levels.23 Additionally, we measured the height and weight using exclusive measuring instrument at the same time. Blood pressure and heart rate were measured on the same day as in the sleep study. Specifically, blood pressure was measured using a sphygmomanometer with an appropriately sized cuff after the participants had rested for ≥ 5 min. Triplicate measurements were performed, with the mean of the last two values being recorded. The mean blood pressure was calculated as follows: mean blood pressure = diastolic blood pressure + 1/3 × (systolic blood pressure − diastolic blood pressure). Hypertension was defined as systolic blood pressure of ≧ 140 mmHg, diastolic blood pressure of ≧ 90 mmHg, or treatment with antihypertensive medications. A habitual drinker was defined as an individual who ingested alcohol at least thrice per week at the time of the sleep study. A current smoker was defined as an individual who smoked at the time of the sleep study or had quit smoking within 1 year before the sleep study.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation or median (interquartile range) while categorical variables are expressed as numbers and percentages. Correlations among the CAVI, other variables, and PWD were analyzed. To identify factors independently correlated with PWD, we performed multivariable regression analysis, with PWD as the dependent variable and significant variables (ie, P < 0.1) in the univariate analyses as the independent variables. In the regression analyses, we used the natural log-transformed AHI since AHI showed a non-normal distribution. Statistical analyses were performed using a statistical software package (StatView, version 5.0, for Windows; SAS Institute, Cary, NC, USA). Statistical significance was set at p < 0.05.
Results
During the study period, 412 participants underwent a sleep study. We analyzed data from 300 eligible participants. Table 1 analyzes the characteristics of the participants. The participants were generally middle-aged, predominantly male, and mildly obese. Among the participants, 22.3% and 65.7% were current smokers and habitual drinkers, respectively. The mean P-wave duration and CAVI were 104.4 ± 10.4 ms and 7.5 ± 1.5, respectively. Table 2 summarizes the sleep study data. The natural log-transformed AHI showed a modest but significantly positive correlation with CAVI and PWD (correlation coefficient, 0.162; P = 0.005 and 0.157; P = 0.007). As shown in Table 3, age, mean blood pressure, eGFR, and mean SO2 showed a significant univariate relationship with PWD. Additionally, CAVI showed a significant positive correlation with PWD (Figure 1). Multivariate regression analysis, which included PWD as the dependent variable and age, mean blood pressure, eGFR, mean SO2, log-transformed AHI, and CAVI as independent variables, showed a significant independent association of mean blood pressure and CAVI with PWD (Table 4).
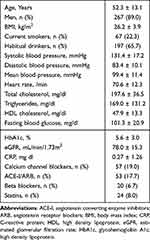 |
Table 1 Characteristics of the Participants |
 |
Table 2 Sleep Study Data |
 |
Table 3 Significant Correlates with the PWD Other Than CAVI in Univariable Analyses |
 |
Table 4 Significant Correlates with the PWD in Multivariable Analysis |
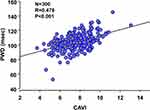 |
Figure 1 Scatter plots of the PWD and CAVI. |
Discussion
PWD and CAVI showed a significant independent correlation with OSA severity. This is the first report to show that an increase in arterial stiffness is associated with atrial conduction delay in patients with OSA.
Patients with OSA may have increased arterial stiffness; however, the relationship between OSA severity and arterial stiffness remains unclear. Generally, increased arterial stiffness reflects the degree of structural and functional vascular damage, with the latter being associated with endothelial dysfunction and sympathetic nervous system overactivation.24,25 Specifically, endothelial dysfunction and sympathetic overactivation may affect muscular arterial stiffness; further, patients with moderate-to-severe OSA have endothelial dysfunction, which could be reversed by treatment with CPAP.10,26
The CAVI represents arterial stiffness and can predict atherosclerosis development. Since the CAVI reflects the stiffness of the aorta, femoral arteries, and tibial arteries, arterial stiffness assessed by CAVI comprises both elastic and muscular arterial stiffness. The vascular stiffness of muscular vessels is strongly affected by blood pressure, sympathetic nerve activity, and physiological and psychological stress.27 Compared with other parameters of arterial stiffness, the CAVI is less dependent on blood pressure during measurement since it is calculated using simultaneously measured systolic and diastolic blood pressures.28,29 Taken together, the CAVI allows calculation of the degree of structural and functional vascular damage based on the non-invasive measurement and is less susceptible to blood pressure variations during the measurement. Our findings demonstrated that OSA severity was associated with increased arterial stiffness since the CAVI was higher in patients with more severe OSA.
The mechanism underlying the relationship between arterial stiffness and PWD in patients with OSA remains unclear. Prolonged PWD, which indicates delayed atrial conduction, is associated with atrial enlargement and abnormality in left atrial function, with subsequent atrial arrhythmias such as AF. PWD is significantly correlated with the left atrial pressure and size30,31 As an indirect mechanism, OSA is associated with left ventricular diastolic dysfunction, which can cause impaired left ventricular filling and increased left atrial pressure.32,33 As a direct mechanism, negative intrathoracic pressure against an obstructed upper airway in patients with OSA increases the left ventricular afterload and left ventricle transmural pressure. We previously reported that OSA may cause atrial conduction delays, which can be modified by CPAP treatment of OSA.4
Arterial stiffness is associated with left atrial size in patients with hypertension;34,35 moreover, the left atrial diameter is significantly correlated with arterial stiffness parameters, including pulse wave velocity, independent of age, sex, body mass index, ventricular remodeling, and filling pressure.34 Furthermore, since arterial stiffness of the aorta is positively correlated with left ventricular afterload,36 increased arterial stiffness in patients with OSA could be an independent factor associated with atrial remodeling.12 Notably, long-term intermittent hypoxia in patients with OSA may have a direct effect on the heart and aorta; additionally, increased arterial stiffness may affect prolonged PWD. Since we observed a significant independent correlation of CAVI with PWD in patients with OSA, there could be an independent mechanism through which OSA promotes arterial stiffness, which causes left atrial and ventricular afterload and left ventricular diastolic dysfunction, and ultimately leads to prolonged PWD, which indicates delayed atrial conduction.
OSA severity was significantly correlated with prolonged PWD and CAVI; moreover, CAVI showed a significant independent correlation with PWD. This indicates that OSA may cause left atrial overload and remodeling, which causes various adverse effects through arterial stiffness, including an increased risk of AF development.
Limitations
This study had several limitations. First, we included a small number of women given the low prevalence of moderate-to-severe OSA in women;37 moreover, we could not identify eligible women during the study period. Additionally, this was not a population-based study. The low proportion of participants with no or mild OSA decreases the generalizability of our findings. Second, we did not obtain structural or functional information regarding the left atrium, which could allow elucidation of the mechanism underlying the association between atrial conduction delay and arterial stiffness in patients with OSA.
Conclusion
We observed a significant correlation of OSA severity with prolonged PWD and CAVI; additionally, CAVI showed a significant independent correlation with PWD. These findings suggest that increased arterial stiffness is associated with atrial conduction delay in patients with OSA, which leads to various adverse effects, including an increased risk of AF development.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Ethics Approval and Informed Consent
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by ethics board of the Toranomon Hospital and informed consent was taken from all the patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was partially supported by Okinawa Memorial Foundation.
Disclosure
Drs. Takatoshi Kasai, Yasuhiro Tomita, Ryo Naito, Fusae Kawama, and Mitsue Kato are affiliated with an endowed department from Philips, ResMed and Fukuda Denshi. The other authors have no conflicts of interest to declare in this work.
References
1. Buxton AE, Calkins H, Callans DJ, et al.; American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology)/American Heart Association Task Force on Clinical Data Standards, ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS writing committee to develop data standards on electrophysiology). Circulation American Heart Association Task Force on Clinical Data Standards ACC/AHA. J Am Coll Cardiol. 2006;114(23):2534–2570.
2. Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. doi:10.1161/01.CIR.0000136587.68725.8E
3. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49(5):565–571. doi:10.1016/j.jacc.2006.08.060
4. Maeno K, Kasagi S, Ueda A, et al. Effects of obstructive sleep apnea and its treatment on signal-averaged P-wave duration in men. Circ Arrhythm Electrophysiol. 2013;6(2):287–293. doi:10.1161/CIRCEP.113.000266
5. Buda AJ, Pinsky MR, Ingels NB, Daughters GT, Stinson EB, Alderman EL. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med. 1979;301(9):453–459. doi:10.1056/NEJM197908303010901
6. Zamagni M, Sforza E, Boudewijns A, Petiau C, Krieger J. Respiratory effort. A factor contributing to sleep propensity in patients with obstructive sleep apnea. Chest. 1996;109(3):651–658. doi:10.1378/chest.109.3.651
7. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi:10.1172/JCI118235
8. Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107(8):1129–1134. doi:10.1161/01.CIR.0000052627.99976.18
9. Alonso-Fernández A, García-Río F, Arias MA, et al. Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: a randomised trial. Thorax. 2009;64(7):581–586. doi:10.1136/thx.2008.100537
10. Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169(3):348–353. doi:10.1164/rccm.200306-767OC
11. Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117(17):2270–2278. doi:10.1161/CIRCULATIONAHA.107.741512
12. Drager LF, Bortolotto LA, Pedrosa RP, Krieger EM, Lorenzi-Filho G. Left atrial diameter is independently associated with arterial stiffness in patients with obstructive sleep apnea: potential implications for atrial fibrillation. Int J Cardiol. 2010;144(2):257–259. doi:10.1016/j.ijcard.2009.01.018
13. Kumagai T, Kasai T, Kato M, et al. Establishment of the cardio-ankle vascular index in patients with obstructive sleep apnea. Chest. 2009;136(3):779–786. doi:10.1378/chest.09-0178
14. Celik T, Yuksel UC, Bugan B, et al. P-wave dispersion and its relationship to aortic elasticity in young prehypertensive patients. Am J Hypertens. 2009;22(12):1270–1275. doi:10.1038/ajh.2009.157
15. Berry RB, Brooks R, Gamaldo C, et al. AASM Scoring Manual Updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–666. doi:10.5664/jcsm.6576
16. Kasai T, Narui K, Dohi T, et al. Efficacy of nasal bi-level positive airway pressure in congestive heart failure patients with Cheyne–Stokes respiration and central sleep apnea. Circ J. 2005;69(8):913–921. doi:10.1253/circj.69.913
17. Suda S, Kasai T, Matsumoto H, et al. Prevalence and clinical correlates of sleep-disordered breathing in patients hospitalized with acute decompensated heart failure. Can J Cardiol. 2018;34(6):784–790. doi:10.1016/j.cjca.2018.03.006
18. Darien II. American Academy of Sleep Medicine: International Classification of Sleep Disorders.
19. Kato M, Kumagai T, Naito R, et al. Change in cardio-ankle vascular index by long-term continuous positive airway pressure therapy for obstructive sleep apnea. J Cardiol. 2011;58(1):74–82. doi:10.1016/j.jjcc.2011.03.005
20. Kasai T, Inoue K, Kumagai T, et al. Plasma PENTRAXIN3 and arterial stiffness in men with obstructive sleep apnea. Am J Hypertens. 2011;24(4):401–407. doi:10.1038/ajh.2010.248
21. Tomita Y, Kasai T. Relationship between cardio-ankle vascular index and obstructive sleep apnea. Rev Cardiovasc Med. 2020;21(3):353–363. doi:10.31083/j.rcm.2020.03.67
22. Maeno K, Kasai T, Kasagi S, et al. Relationship between atrial conduction delay and obstructive sleep apnea. Heart Vessels. 2013;28(5):639–645. doi:10.1007/s00380-012-0288-8
23. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi:10.1053/j.ajkd.2008.12.034
24. Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107(22):2864–2869. doi:10.1161/01.CIR.0000069826.36125.B4
25. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–943. doi:10.1161/01.ATV.0000160548.78317.29
26. Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100(23):2332–2335. doi:10.1161/01.CIR.100.23.2332
27. Shimizu K, Takahashi M, Shirai K. A huge earthquake hardened arterial stiffness monitored with cardio-ankle vascular index. J Atheroscler Thromb. 2013;20(5):503–511. doi:10.5551/jat.16097
28. Hayashi K, Handa H, Nagasawa S, Okumura A, Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech. 1980;13(2):175–184. doi:10.1016/0021-9290(80)90191-8
29. Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101–107. doi:10.5551/jat.13.101
30. Faggiano P, D’Aloia A, Zanelli E, Gualeni A, Musatti P, Giordano A. Contribution of left atrial pressure and dimension to signal-averaged P-wave duration in patients with chronic congestive heart failure. Am J Cardiol. 1997;79(2):219–222. doi:10.1016/S0002-9149(96)00720-5
31. Ishimoto N, Ito M, Kinoshita M. Signal-averaged P-wave abnormalities and atrial size in patients with and without idiopathic paroxysmal atrial fibrillation. Am Heart J. 2000;139(4):684–689. doi:10.1016/S0002-8703(00)90048-6
32. Arias MA, García-Rio F, Alonso- Fernández A, Mediano O, Martínez I, Villamor J. Obstructive Sleep Apnea Syndrome Affects Left Ventricular Diastolic Function: effects of Nasal Continuous Positive Airway Pressure in Men. Circulation. 2005;112(375):383. doi:10.1161/CIRCULATIONAHA.104.501841
33. Baguet JP, Barone-Rochette G, Lévy P, et al. Left ventricular diastolic dysfunction is linked to severity of obstructive sleep apnoea. Eur Respir J. 2010;36(6):1323–1329. doi:10.1183/09031936.00165709
34. Lantelme P, Laurent S, Besnard C, et al. arterial stiffness is associated with left atrial size in hypertensive patients. Arch Cardiovasc Dis. 2008;101(1):35–40. doi:10.1016/S1875-2136(08)70253-5
35. Janwanishstaporn S, Boonyasirinant T. Correlation between aortic stiffness and left atrial volume index in hypertensive patients. Clin Exp Hypertens. 2016;38(2):160–165. doi:10.3109/10641963.2015.1081211
36. Belz GG. Elastic properties and Windkessel function of the human aorta. Cardiovasc Drugs Ther. 1995;9(1):73–83. doi:10.1007/BF00877747
37. Ip MSM, Lam B, Tang LCH, Lauder IJ, Ip TY, Lam WK. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest. 2004;125(1):127–134. doi:10.1378/chest.125.1.127
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
