Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Cost-Effectiveness of Single-Inhaler Triple Therapy (FF/UMEC/VI) versus Tiotropium Monotherapy in Patients with Symptomatic Moderate-to-Very Severe COPD in the UK
Authors Kendall R , Martin AA , Shah D, Shukla S , Compton C , Ismaila AS
Received 8 December 2022
Accepted for publication 24 July 2023
Published 21 August 2023 Volume 2023:18 Pages 1815—1825
DOI https://doi.org/10.2147/COPD.S400707
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Robyn Kendall,1 Alan A Martin,2 Dhvani Shah,3 Soham Shukla,4 Chris Compton,5 Afisi S Ismaila4,6
1ICON Health Economics, ICON plc, Vancouver, BC, Canada; 2Value Evidence and Outcomes, GSK, London, UK; 3ICON Health Economics, ICON plc, New York, NY, USA; 4Value Evidence and Outcomes, GSK, Collegeville, PA, USA; 5Global Respiratory Franchise, GSK, London, UK; 6Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada
Correspondence: Afisi S Ismaila, Value Evidence and Outcomes, GSK, 1250 South Collegeville Road, Collegeville, PA, 19426-0989, USA, Tel +1 919 315 8229, Email [email protected]
Purpose: For patients with chronic obstructive pulmonary disease (COPD) who remain symptomatic despite maintenance treatment, clinical management guidelines recommend a stepwise escalation from monotherapy to dual therapy, and from dual therapy to triple therapy. However, in clinical practice, patients are often escalated directly from monotherapy to triple therapy based on disease severity. This study evaluated the cost-effectiveness of once-daily, single-inhaler fluticasone furoate, umeclidinium, and vilanterol (FF/UMEC/VI) triple therapy compared with long-acting muscarinic antagonist monotherapy with once-daily tiotropium (TIO) in patients with symptomatic moderate-to-very severe COPD, from a UK National Health Service perspective.
Patients and Methods: The validated GALAXY-COPD disease progression model was populated with patient baseline characteristics and treatment effect data from the 12-week GSK Study 207626 comparing FF/UMEC/VI with TIO in patients with moderate-to-very severe COPD. UK unit costs and drug costs (British Pound, 2021) were applied to healthcare resource utilization and treatments. The base case analysis was conducted over a lifetime horizon, and costs and health outcomes (except for life years [LYs]) were discounted at 3.5% per year. Model outputs included exacerbation rates, healthcare costs, LYs, quality-adjusted LYs (QALYs), and incremental cost-effectiveness ratios.
Results: Overall, treatment with FF/UMEC/VI resulted in increased clinical benefit (reduction in total exacerbations and increased overall survival and QALYs), coupled with cost savings (derived from lower maintenance and exacerbation healthcare costs) compared with TIO monotherapy. In the base case analysis, FF/UMEC/VI provided an additional 0.393 LYs (95% range: 0.176, 0.655) and 0.443 QALYs (0.246, 0.648), at a cost saving of £ 880 (£ 54, £ 1608) versus TIO. FF/UMEC/VI remained the cost-effective (dominant) treatment option across sensitivity and scenario analyses.
Conclusion: FF/UMEC/VI offers greater clinical benefits and is a cost-effective treatment option compared with TIO for the treatment of adult patients with COPD with persistent symptoms and/or who are at risk of exacerbation in the UK.
Keywords: chronic obstructive pulmonary disease, health technology assessment, economic evaluation, real-world, single-inhaler triple therapy
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide.1,2 In England, approximately 1.2 million people (1.9% of the population) were living with diagnosed COPD in 2021.3 The prevalence of COPD in England and Scotland has risen over the past decade,4 with further increases anticipated over the coming years.5 The economic burden of COPD is substantial,6 making it one of the most expensive inpatient conditions treated by the UK National Health Service (NHS).7 The total healthcare cost of COPD was estimated to be £2 billion in 2021,8 and is predicted to rise annually with increasing disease prevalence.
Current clinical management guidelines recommend a stepwise escalation from monotherapy to dual therapy, and from dual therapy to triple therapy, for patients with COPD who continue to experience symptoms, or have a high risk of exacerbation, despite maintenance treatment.1 The product license for single-inhaler triple therapies (SITTs) for COPD in the UK and certain other markets is restricted to step-up from dual therapy with inhaled corticosteroid (ICS)/long-acting β2-agonist (LABA) or long-acting muscarinic antagonist (LAMA)/LABA. However, in clinical practice, patients are often escalated directly from monotherapy to triple therapy.9 In a retrospective analysis of 13,541 patients with COPD recently initiated on LAMA monotherapy, ICS/LABA, or LAMA/LABA, 1130 (28%) patients on monotherapy progressed directly to triple therapy within 1 year.9
Several studies have demonstrated the clinical benefits of escalating from LAMA monotherapy to ICS/LAMA/LABA triple therapy (administered using multiple inhalers) in patients with symptomatic COPD, in terms of improvement in lung function, reduction in moderate/severe exacerbations, and improvement in health-related quality of life.10–15 Evidence also suggests that the use of single inhalers in patients with COPD is associated with improved cost-effectiveness and decreased healthcare resource use compared with multiple inhalers.16–18
The clinical benefit of once-daily SITT with fluticasone furoate, umeclidinium, and vilanterol (FF/UMEC/VI) versus LAMA monotherapy with once-daily tiotropium (TIO) was recently demonstrated in patients with symptomatic moderate-to-very severe COPD, with both therapies demonstrating a similar safety profile (NCT03474081; GSK Study 207626).19 Given the proven clinical benefits of SITT FF/UMEC/VI over TIO monotherapy, and the fact that escalation from monotherapy to triple therapy is often seen in a real-world setting, the potential economic impact of prescribing FF/UMEC/VI in the UK needs to be assessed, to determine the economic value associated with FF/UMEC/VI SITT over TIO monotherapy. This analysis aimed to evaluate the cost-effectiveness of FF/UMEC/VI SITT compared with TIO monotherapy in patients with symptomatic COPD with moderate-to-very severe airflow limitation, from a UK NHS perspective, using data from GSK Study 207626.19
Materials and Methods
Study 207626 Design
The design and findings of Study 207626 (NCT03474081) have been published previously.19 Briefly, this was a 12-week, Phase IV, double-blind, double-dummy, randomized, multicenter study comparing once-daily SITT FF/UMEC/VI with TIO monotherapy in patients with symptomatic moderate-to-very severe COPD (N=800).19 The primary endpoint of the study was lung function measured as change from baseline in trough forced expiratory volume in 1 second (FEV1) at 12 weeks.
Cost-Effectiveness Model
This analysis was conducted using the validated GALAXY-COPD disease progression model,20–22 adapted using patient characteristics and treatment effects from Study 20762619 and UK healthcare costs. GALAXY-COPD uses linked risk equations to model associations between patient baseline characteristics, disease attributes, treatment effects, disease progression, and outcomes. The linked risk equations for clinical outcomes were based on data from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) study.23,24
The base case analysis was conducted over a lifetime horizon, and costs and health outcomes (except for life years [LYs]) beyond 1 year were discounted at 3.5% per year, in accordance with National Institute for Health and Care Excellence (NICE) guidelines.25 Treatment discontinuation was assumed to be zero.
Model Inputs
Patient Population
Full eligibility criteria for Study 207626 have been published previously.19 Patients were eligible to participate if they had a post-bronchodilator FEV1 of <50% predicted and COPD Assessment Test (CAT) score ≥10 or FEV1 <80% predicted with a documented history of ≥2 moderate exacerbations or ≥1 severe exacerbation (requiring hospitalization) in the previous 12 months and a CAT score ≥10. Patients were required to receive daily maintenance treatment with TIO monotherapy for ≥3 months prior to screening.
Data for pooled baseline characteristics, based on the intent-to-treat (ITT) population of Study 2076260 (N=800)19 were included in the model (Table 1). The mean age of the study population was 66.2 years and 32% were female.19 Baseline FEV1% predicted was 50.0%.
 |
Table 1 Model inputs |
Model risk equations include, as covariates, baseline values for age, sex, body mass index, cardiovascular disease and other comorbidities, smoking status, fibrinogen level, modified Medical Research Council (mMRC) dyspnea scale score, FEV1% predicted, prior exacerbation history, St George’s Respiratory Questionnaire (SGRQ) score, 6-minute walk test (6MWT), and height.
For parameters that were not available directly from Study 207626 data (ie baseline mMRC, fibrinogen, and 6MWT), estimates were made using risk equations or analogous data collected in the trial. Further details on the methods used to derive values for these parameters are provided in Supplementary Appendix 1.
Treatment Effects
Treatment effects used in the model were based on data for the ITT population of Study 207626 and are summarized in Table 1. These included change from baseline in post-bronchodilator FEV1 and change from baseline in total SGRQ score. Treatment effect on exacerbation reduction was set to “no difference” because the relative risk reduction of exacerbations was not included as an endpoint in Study 207626; therefore, the sample size and study duration did not allow robust estimates to be made.
As the GALAXY-COPD model predicts changes in SGRQ total score based on changes in FEV1, adjustments were made to prevent double counting of treatment effects. This required first inputting the FEV1 treatment effect into the model. The difference in model-predicted change from baseline in SGRQ score (based on FEV1) between FF/UMEC/VI and TIO was then adjusted so that it matched the observed change in SGRQ score in Study 207626.
Costs
Drug costs (British Pound, 2021) were obtained from the British National Formulary26 and are provided in Table 2. Drug dose, pack size, and cost per pack were used to calculate the cost per day. Health state and exacerbation costs were sourced from literature and publicly available data27 and are provided in Table 2.
 |
Table 2 Model Inputs: Drug Unit and Health States and Exacerbation Costs (2021, GBP) |
For costing purposes, three health states were described, based on categories of COPD severity defined by lung function: moderate to severe (FEV1 80% to 50% predicted), severe (FEV1 <50% to 30% predicted), and very severe (FEV1 <30% predicted). The model calculated the proportion of the patient cohort in each health state in each cycle, and appropriate annual costs were applied. Events included moderate or severe exacerbations and were costed individually. Healthcare resource utilization estimates and unit costs for each health state were obtained from a published NICE economic model report (2018).30 Costs were inflated to 2021 values using the Consumer Price Index data obtained from the Office for National Statistics.29
Additional drug treatments for moderate exacerbations outside of physician encounters were included in the analysis. The costs of medication used at the time were assigned on the basis of an estimated cost per course of antibiotic treatment and cost per course of oral corticosteroid (OCS) treatment (based on NICE guidelines)27,31 and a reported frequency of OCS and antibiotic use from the InforMing the PAthway of COPD Treatment (IMPACT) GALAXY-COPD model32 (using values for the ITT population pooled across the three treatment arms). The IMPACT trial reported that for each moderate exacerbation, the frequency of medication use was 73.0% for OCS and 78.0% for antibiotics.33 Combining the estimated cost per course together with the frequency of medication use, the net cost per exacerbation was £9.14 (Table S1).
The mean duration of exacerbations was not collected in Study 207626; therefore, to calculate patient productivity costs (for scenario analyses), the model incorporated data from a comparable FF/UMEC/VI study (INvestigation of TRelegy Effectiveness: usual PractIce Design [INTREPID]).34 Based on INTREPID data, the mean duration of moderate exacerbations was 12.20 (standard error [SE] 0.42) days and the mean duration of severe exacerbation was 15.68 (SE 1.33) days (calculated weighted average across both treatment arms). The duration of exacerbation was assumed to be the same as the number of days absent from usual activities. Indirect costs included productivity losses incurred by sick leave. This was estimated according to the human capital approach, which can be broadly interpreted as estimating the lost gross value during time absent from usual activities.35 Hence, although a proportion of COPD patients may be unemployed or retired, they will still not be able to continue their usual activities and may need to hire help. Average UK earnings per day were used to calculate the cost of each day absent from usual activities, estimated at £106.38 by the Office for National Statistics, 2021.36
Model Assumptions
It was assumed that the Study 207626 population was representative of the UK population with symptomatic moderate-to-very severe COPD likely to receive FF/UMEC/VI or TIO, and that treatment effects were maintained for the duration of treatment. Treatment effect on exacerbation reduction was set to “no difference” because the relative risk reduction of exacerbations was not included as an endpoint in Study 207626. Therefore, any predicted effects on exacerbations are solely an indirect effect of differences in treatment effect on lung function and SGRQ scores.
Model Outputs
Outputs of the model were cumulative total exacerbations (moderate and severe), average annual exacerbations per person per year, healthcare costs, LYs, quality-adjusted LYs (QALYs), and incremental cost-effectiveness ratios (ICERs). QALYs were calculated by translating model-predicted SGRQ scores to a utility value for each annual cycle, using a published and validated algorithm.37
Scenario and Sensitivity Analyses
Scenario analyses were performed to examine the impact of alternative assumptions and model settings on the base case model results. In the scenario analyses with treatment discontinuation, as no subsequent therapy data were collected in Study 207626, patients who discontinued FF/UMEC/VI were assumed to receive the same subsequent treatment classes and in the same proportions as patients in a comparable FF/UMEC/VI study, INTREPID (84.2% continued on ICS/LABA/LAMA, and 6.9%, 8.1%, and 0.81% switched to ICS/LABA, LAMA/LABA, and LAMA, respectively). The efficacy of subsequent treatment was assumed to be the same as FF/UMEC/VI for the remaining duration of the analysis. For patients who discontinued TIO, the same subsequent treatment approach and assumptions as in the umeclidinium (LAMA) arm of a comparable TIO study, Early MAXimisation of bronchodilation for improving COPD stability (EMAX),38,39 were used (where discontinuation was not due to “no efficacy” or exacerbation [as was the case for Study 207626], 100% of patients remained on LAMA). The efficacy of subsequent treatment was assumed to be the same as TIO for the remaining duration of the analysis. The effect of including an exacerbation treatment effect was tested by including effects on either separate moderate and severe exacerbation rates from the 207626 trial (relative risk reduction [RRR]: moderate: 0.56 [95% confidence interval (CI) 0.34, 0.92]; severe 1.67 [95% CI 0.40, 6.93]) or on combined moderate and severe exacerbation rates (RRR: 0.63 [95% CI 0.40, 1.00]).
One-way sensitivity analyses were conducted to test the robustness of the base case results for baseline covariate values that were not available from Study 207626 (fibrinogen, 6MWT, and mMRC score) and FF/UMEC/VI treatment effects on SGRQ change and FEV1 increment (Table S2). A description of the calculation of upper and lower values used for the sensitivity analyses and further details regarding probabilistic analysis are provided in the Supplementary Methods and Table S3 (Supplementary Appendix 1).
Results
Base Case Analysis
Results of the base case analysis for FF/UMEC/VI versus TIO are presented in Table 3. Compared with TIO, FF/UMEC/VI was the dominant treatment option (meaning greater benefit at a lower cost). Over a lifetime horizon, FF/UMEC/VI provided an additional 0.393 LYs (95% range: 0.176, 0.655) and 0.443 QALYs (0.246, 0.648), at a cost saving of £880 (£54, £1608) versus TIO. Although drug costs were higher for FF/UMEC/VI, they were offset by the lower exacerbation and maintenance healthcare costs, resulting in lower total costs relative to TIO.
 |
Table 3 Base Case Analysis, Lifetime Horizon |
The analysis predicted a slightly higher total number of moderate exacerbations for FF/UMEC/VI (8.403) compared with TIO (8.382) over the lifetime horizon. This was due to patients receiving FF/UMEC/VI surviving 0.393 LYs longer than those receiving TIO.
Scenario and Sensitivity Analyses
Compared with TIO, FF/UMEC/VI remained a dominant treatment option across all scenario analyses except for the scenario with separate moderate and severe treatment effect values where there were incremental costs associated with FF/UMEC/VI, giving an ICER of £6444 per QALY. Cost savings with FF/UMEC/VI versus TIO were highest for the scenario with the combined moderate/severe treatment effect value included (£2205).
FF/UMEC/VI also remained the dominant treatment option compared with TIO across all one-way sensitivity analyses (Table S4).
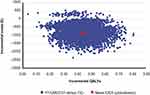
In probabilistic analyses, compared with TIO, FF/UMEC/VI showed higher QALYs across all simulations and was less costly than TIO across the majority (98%) of the 5000 simulations (Figure 1); thus, FF/UMEC/VI was the dominant treatment option in 98% of the simulations. The net benefit acceptability curve showed that at a willingness-to-pay threshold of £20,000 per QALY, the probability that FF/UMEC/VI was cost-effective compared to TIO was 100% (Figure 2).
 |
Figure 2 Net benefit acceptability curves, FF/UMEC/VI versus TIO. Abbreviations: FF, fluticasone furoate; TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol. |
Discussion
In real-world practice, a substantial proportion of patients with COPD are escalated from monotherapy directly to triple therapy,9 despite the lack of clinical guidelines currently supporting this approach,1 and the fact that the product license for SITT in the UK and certain other markets is restricted to step-up from dual therapy with ICS/LABA or LAMA/LABA.
Triple therapy with ICS, LABA, and LAMA traditionally required the use of multiple inhalers40; however, SITTs have recently been developed.34 In a recent trial, once-daily FF/UMEC/VI SITT significantly improved lung function and health status versus once-daily TIO in patients with symptomatic moderate-to-very severe COPD, with both treatments demonstrating a similar safety profile.19
To the authors’ knowledge, this is the first study to assess the cost-effectiveness of SITT with FF/UMEC/VI versus LAMA monotherapy. Overall, treatment with FF/UMEC/VI resulted in an increase in clinical benefit (ie reduction in total exacerbations and increased overall survival and QALYs), coupled with cost savings (derived from lower maintenance and exacerbation healthcare costs) when compared with TIO monotherapy. FF/UMEC/VI remained a dominant treatment option across all sensitivity analyses. In the scenario analyses, FF/UMEC/VI also remained the dominant treatment option (except for the scenario including separate moderate and severe exacerbation treatment effects). Together, these findings suggest that the results of the model are robust when considering relevant uncertainties. ICERs for all analyses were either dominant or well below the recognized UK cost-effectiveness threshold of £20,000 per QALY,25 suggesting that FF/UMEC/VI is a cost-effective treatment option for patients with symptomatic COPD with moderate-to-very severe airflow limitation in the UK.
These findings are consistent with previous economic analyses that have demonstrated the cost-effectiveness of SITT compared with dual therapy for patients with COPD. Two studies using data from the IMPACT trial, one conducted from the UK NHS perspective and the other from the Canadian healthcare perspective, found once-daily FF/UMEC/VI SITT to be more cost-effective when compared with dual therapy FF/VI or UMEC/VI.32,41 Three studies based on data from the Lung Function and Quality of Life Assessment in Chronic Obstructive Pulmonary Disease with Closed Triple Therapy (FULFIL) trial, conducted from a UK NHS perspective (two studies using different models) and a Spanish National Health System perspective, also demonstrated that once-daily FF/UMEC/VI SITT was a cost-effective option for the treatment of patients with symptomatic COPD compared with twice-daily budesonide/formoterol.18,42–44
As previously mentioned, in the UK, European Union, and certain other countries, escalation to triple therapy is only licensed from dual therapy with ICS/LABA or LAMA/LABA and not from LAMA monotherapy.45 In addition, the ICS class-related increased risk of pneumonia, particularly in certain subgroups, needs to be included in any therapeutic decision.46
Limitations of the current study include the potential lack of generalizability of the results to real-world practice, as is the case for all analyses of cost-effectiveness conducted using clinical trial data. Only 12-week discontinuation data were collected in Study 207626 and longer-term treatment discontinuation was therefore difficult to estimate and assumed to be zero in the first and subsequent years. However, FF/UMEC/VI remained dominant in the sensitivity analyses where treatment discontinuation was assumed. No treatment effect for exacerbations was included in the analyses because this was not included as an endpoint in Study 207626, and any effects on exacerbations are solely an indirect effect of differences in treatment effect on lung function and SGRQ scores. However, FF/UMEC/VI remained dominant in scenario analyses exploring the inclusion of direct exacerbation treatment effect.
Conclusion
The results of these analyses show that FF/UMEC/VI offers greater clinical benefits at lower cost, making it a dominant treatment option compared with TIO for the treatment of adult patients with symptomatic moderate-to-very severe COPD and a history of exacerbation. The use of FF/UMEC/VI may therefore improve health outcomes and reduce the economic burden of COPD and should be considered by clinicians as a preferred treatment option, within the context of the product license, where this does not mandate treatment escalation from ICS/LABA or LAMA/LABA.
Abbreviations
6MWT, 6-minute walk test; BMI, body mass index; CAT, COPD Assessment Test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; ECLIPSE, Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points; EMAX, Early MAXimisation of bronchodilation for improving COPD stability; FEV1, forced expiratory volume in 1 second; FF, fluticasone furoate; FULFIL, Lung Function and Quality of Life Assessment in Chronic Obstructive Pulmonary Disease with Closed Triple Therapy; GBP, British Pound; ICER, incremental cost-effectiveness ratio; ICS, inhaled corticosteroid; IMPACT, InforMing the PAthway of COPD Treatment; INTREPID, INvestigation of TRelegy Effectiveness: usual PractIce Design; ITT, intent-to-treat; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LY, life year; mMRC, modified Medical Research Council; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; OCS, oral corticosteroid; PPPY, per person per year; QALY, quality-adjusted life year; RRR, relative risk reduction; SD, standard deviation; SE, standard error; SGRQ, St George’s Respiratory Questionnaire; SITT, single-inhaler triple therapy; TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol.
Data Sharing Statement
Anonymized individual participant data and study documents for Study 207626 can be requested for further research from www.clinicalstudydatarequest.com.
Ethics Approval and Informed Consent
Ethics committee approval and patient informed consent was not required for this analysis. The analysis was conducted using patient characteristics and treatment effects from a previously published study; therefore, no direct patient contact or primary collection of individual patient data were required.
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing, and referencing) was provided by Rebecca Cunningham and Abigail Marmont of Aura, a division of Spirit Medical Communications Group Limited, and was funded by GSK. The abstract of this paper was presented at the International Society of Pharmacoeconomic and Outcomes Research (ISPOR) 25th European Congress 2021 as a poster presentation with interim findings: Martin A, Shah D, Shukla S, et al. Cost-effectiveness of single-inhaler triple therapy (FF/UMEC/VI) versus tiotropium monotherapy in patients with COPD in the UK. Value Health. 2022; 25 (Suppl 1): S40.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by GSK (GSK Study 212889). GSK-affiliated authors were involved in study conception and design, data analysis, data interpretation, and the decision to submit the article for publication. The sponsor funded the article processing charges and open access fee.
Disclosure
AAM, SS, CC, and ASI are GSK employees. AAM, CC, and ASI hold shares in GSK. ASI is also an unpaid part-time professor at McMaster University, Hamilton, ON, Canada. RK and DS are ICON employees. ICON received funding from GSK to conduct this analysis, but not for manuscript development.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2022 report; 2022. Available from: https://goldcopd.org/archived-reports/.
2. World Health Organization. The top 10 causes of death; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
3. Office for Health Improvement & Disparities. Inhale - INteractive Health Atlas of Lung conditions in England; 2022. Available from: https://fingertips.phe.org.uk/profile/inhale/data#page/1/gid/8000008/pat/159/par/K02000001/ati/15/are/E92000001/yrr/1/cid/4/tbm/1.
4. British Lung Foundation. Chronic obstructive pulmonary disease (COPD) statistics; 2022. Available from: https://statistics.blf.org.uk/copd.
5. McLean S, Hoogendoorn M, Hoogenveen RT, et al. Projecting the COPD population and costs in England and Scotland: 2011 to 2030. Sci Rep. 2016;6:31893. doi:10.1038/srep31893
6. Gutiérrez Villegas C, Paz-Zulueta M, Herrero-Montes M, Parás-Bravo P, Madrazo Pérez M. Cost analysis of chronic obstructive pulmonary disease (COPD): a systematic review. Health Econ Rev. 2021;11(1):31. doi:10.1186/s13561-021-00329-9
7. National Institute for Health and Care Excellence. Health and social care directorate. Quality standards and indicators briefing paper; 2015. Available from: https://www.nice.org.uk/guidance/qs10/documents/briefing-paper#:~:text=One%20in%20eight%20(130%2C000)%20emergency,be%20readmitted%20within%20three%20months.
8. de Nigris E, McEwan P, Marshall J, Holmgren U, Foos V. POSB65 Economic burden of COPD in the United Kingdom (2021–2040) estimated with the COPD Health Outcome Policy and Intervention (CHOPIN) model. Value Health. 2022;25(Suppl 1):
9. Lane DC, Stemkowski S, Stanford RH, Tao Z. Initiation of triple therapy with multiple inhalers in chronic obstructive pulmonary disease: an analysis of treatment patterns from a U.S. retrospective database study. J Manag Care Spec Pharm. 2018;24(11):1165–1172.
10. Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146(8):545–555. doi:10.7326/0003-4819-146-8-200704170-00152
11. Cazzola M, Andò F, Santus P, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPD. Pulm Pharmacol Ther. 2007;20(5):556–561.
12. Hanania NA, Crater GD, Morris AN, Emmett AH, O’Dell DM, Niewoehner DE. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respir Med. 2012;106(1):91–101. doi:10.1016/j.rmed.2011.09.002
13. Hoshino M, Ohtawa J. Effects of adding salmeterol/fluticasone propionate to tiotropium on airway dimensions in patients with chronic obstructive pulmonary disease. Respirology. 2011;16(1):95–101. doi:10.1111/j.1440-1843.2010.01869.x
14. Jung KS, Park HY, Park SY, et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respir Med. 2012;106(3):382–389.
15. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(8):741–750. doi:10.1164/rccm.200904-0492OC
16. Usmani OS, Hickey AJ, Guranlioglu D, et al. The impact of inhaler device regimen in patients with asthma or COPD. J Allergy Clin Immunol Pract. 2021;9(8):3033–3040. doi:10.1016/j.jaip.2021.04.024
17. Zhang S, King D, Rosen VM, Ismaila AS. Impact of single combination inhaler versus multiple inhalers to deliver the same medications for patients with asthma or COPD: a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:417–438. doi:10.2147/COPD.S234823
18. Halpin DM, Kendall R, Shukla S, et al. Cost-effectiveness of single- versus multiple-inhaler triple therapy in a UK COPD population: the INTREPID trial. Int J Chron Obstruct Pulmon Dis. 2022;17:2745–2755. doi:10.2147/COPD.S370577
19. Bansal S, Anderson M, Anzueto A, et al. Single-inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) triple therapy versus tiotropium monotherapy in patients with COPD. NPJ Prim Care Respir Med. 2021;31(1):29.
20. Briggs AH, Baker T, Risebrough NA, et al. Development of the Galaxy chronic obstructive pulmonary disease (COPD) model using data from ECLIPSE: internal validation of a linked-equations cohort model. Med Decis Making. 2017;37(4):469–480. doi:10.1177/0272989X16653118
21. Exuzides A, Colby C, Briggs AH, et al. Statistical modeling of disease progression for chronic obstructive pulmonary disease using data from the ECLIPSE study. Med Decis Making. 2017;37(4):453–468.
22. Risebrough NA, Briggs A, Baker TM, et al. Validating a model to predict disease progression outcomes in patients with COPD. Value Health. 2014;17(7):A560–A561. doi:10.1016/j.jval.2014.08.1852
23. Agusti A, Calverley PMA, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11(1):122. doi:10.1186/1465-9921-11-122
24. Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J. 2008;31(4):869–873. doi:10.1183/09031936.00111707
25. National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013; 2013. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-The-methods-of-technology-appraisal-2013-pdf-2007975843781.
26. National Institute for Health and Care Excellence. British National Formulary (BNF); 2022. Available from: https://bnf.nice.org.uk/.
27. National Institute for Health and Care Excellence. Chronic obstructive disease in over 16s: diagnosis and management. NICE guideline NG115; 2018. Available from: https://www.nice.org.uk/guidance/NG115.
28. IQVIA [Data on file]. COPD Drug Market Share Data - provided by GSK. 2020.
29. Office for National Statistics. CPIH index 00: all items 2015=100; 2022. Available from: https://www.ons.gov.uk/economy/inflationandpriceindices/timeseries/l522/mm23.
30. National Institute for Health and Care Excellence. Chronic obstructive disease in over 16s: diagnosis and management: economic model report; 2018. Available from: https://www.nice.org.uk/guidance/ng115/documents/economic-report.
31. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease (acute exacerbation): antimicrobial prescribing. NICE guideline NG114; 2018. Available from: https://www.nice.org.uk/guidance/ng114.
32. Ismaila AS, Risebrough N, Schroeder M, et al. Cost-effectiveness of once-daily single-inhaler triple therapy in COPD: the IMPACT trial. Int J Chron Obstruct Pulmon Dis. 2019;14:2681–2695. doi:10.2147/COPD.S216072
33. GlaxoSmithKline [Data on file]. IMPACT clinical trial supplementary materials; 2017.
34. Halpin DMG, Worsley S, Ismaila AS, et al. INTREPID: single- versus multiple-inhaler triple therapy for COPD in usual clinical practice. ERJ Open Res. 2021;7(2):00950–2020.
35. Liljas B. How to calculate indirect costs in economic evaluations. PharmacoEconomics. 1998;13(1 Pt 1):1–7. doi:10.2165/00019053-199813010-00001
36. Office for National Statistics. EARN01: average weekly earnings; 2022. Available from: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/averageweeklyearningsearn01/current.
37. Starkie HJ, Briggs AH, Chambers MG, Jones P. Predicting EQ-5D values using the SGRQ. Value Health. 2011;14(2):354–360. doi:10.1016/j.jval.2010.09.011
38. Maltais F, Bjermer L, Kerwin EM, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res. 2019;20(1):238.
39. ICON plc [Data on file]. Global health economics, and outcomes research and epidemiology. Cost-effectiveness model for UMEC/VI in COPD using the EMAX trial: UK National Health Services perspective. 2021.
40. Gaduzo S, McGovern V, Roberts J, Scullion JE, Singh D. When to use single-inhaler triple therapy in COPD: a practical approach for primary care health care professionals. Int J Chron Obstruct Pulmon Dis. 2019;14:391–401. doi:10.2147/COPD.S173901
41. Martin A, Shah D, Ndirangu K, et al. Is single-inhaler triple therapy for COPD cost-effective in the UK? The IMPACT trial. ERJ Open Res. 2022;8(1):00333–2021. doi:10.1183/23120541.00333-2021
42. Schroeder M, Benjamin N, Atienza L, et al. Cost-effectiveness analysis of a once-daily single-inhaler triple therapy for patients with chronic obstructive pulmonary disease (COPD) using the FULFIL trial: a Spanish perspective. Int J Chron Obstruct Pulmon Dis. 2020;15:1621–1632.
43. Schroeder M, Shah D, Risebrough N, et al. Cost-effectiveness analysis of a single-inhaler triple therapy for patients with advanced chronic obstructive pulmonary disease (COPD) using the FULFIL trial: a UK perspective. Respir Med X. 2019;1:100008.
44. Fenwick E, Martin A, Schroeder M, et al. Cost-effectiveness analysis of a single-inhaler triple therapy for COPD in the UK. ERJ Open Res. 2021;7(1):00480–2020. doi:10.1183/23120541.00480-2020
45. European Medicines Agency. Trelegy Ellipta 92 micrograms/55 micrograms/22 micrograms inhalation powder, pre-dispensed [summary of product characteristics]; 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/trelegy-ellipta-epar-product-information_en.pdf.
46. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.