Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Cost-Effectiveness of Once-Daily Single-Inhaler COPD Triple Therapy in Spain: IMPACT Trial
Authors Paly VF , Vallejo-Aparicio LA, Martin A , Izquierdo JL , Riesco JA , Soler-Cataluña JJ , Abreu C, Biswas C , Ismaila AS
Received 16 March 2022
Accepted for publication 30 November 2022
Published 16 December 2022 Volume 2022:17 Pages 3097—3109
DOI https://doi.org/10.2147/COPD.S366765
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Victoria Federico Paly,1 Laura Amanda Vallejo-Aparicio,2 Alan Martin,3 José Luis Izquierdo,4 Juan Antonio Riesco,5 Juan José Soler-Cataluña,6 Catarina Abreu,7 Chandroday Biswas,8 Afisi S Ismaila9,10
1ICON plc, Philadelphia, PA, USA; 2GSK, Madrid, Spain; 3GSK, Brentford, UK; 4Hospital Universitario de Guadalajara, Guadalajara, Spain; 5Hospital San Pedro de Alcántara, Cáceres, Spain; 6Hospital Arnau de Vilanova, Valencia, Spain; 7ICON Plc, New York, NY, USA; 8ICON Plc, Bengaluru, Karnataka, India; 9Value Evidence and Outcomes, GSK, Collegeville, PA, USA; 10Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada
Correspondence: Afisi S Ismaila, Value Evidence and Outcomes, GSK, 1250 South Collegeville Road, Collegeville, PA, 19426-0989, USA, Tel +1 919 315 8229, Email [email protected]
Purpose: Given between-country differences in healthcare systems, treatment costs, and disease management guidelines, country-specific cost-effectiveness analyses are important. This study evaluated the cost-effectiveness of once-daily fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) versus FF/VI and UMEC/VI among patients with symptomatic chronic obstructive pulmonary disease (COPD) at risk of exacerbations from a Spanish healthcare system perspective.
Patients and Methods: Baseline data and treatment effects from the IMPACT trial were populated into the validated GALAXY COPD progression model. Utilities were estimated using Spanish observational data. Direct healthcare costs (2019 €) were informed by Spanish public sources. A 3% discount rate for costs and benefits was applied. The time horizon and treatment duration were 3 years (base case). One-way sensitivity, scenario, and probabilistic sensitivity analyses were performed.
Results: FF/UMEC/VI treatment resulted in fewer exacerbations over 3 years (4.130 vs 3.648) versus FF/VI, with a mean (95% confidence interval [CI]) incremental cost of € 444 (€ 149, € 713) per patient and benefit of 0.064 (0.053, 0.076) quality-adjusted life years (QALYs), resulting in an incremental cost-effectiveness ratio (ICER) of € 6887 per QALY gained. FF/UMEC/VI was a dominant treatment strategy versus UMEC/VI, resulting in fewer exacerbations (4.130 vs 3.360), with a mean (95% CI) incremental cost of –€ 450 (–€ 844, –€ 149) and benefit of 0.054 (0.043, 0.064) QALYs. FF/UMEC/VI was cost-effective versus FF/VI and UMEC/VI across all analyses.
Conclusion: FF/UMEC/VI was predicted to be a cost-effective treatment option versus FF/VI or UMEC/VI in symptomatic COPD patients at risk of exacerbations in Spain, across all scenarios and sensitivity analyses.
Keywords: chronic obstructive pulmonary disease, cost-effectiveness, single-inhaler triple therapy, Spain, triple inhaled therapy
Plain Language Summary
Why was this study done?
- Chronic obstructive pulmonary disease (COPD) is associated with large costs for both the patient and their healthcare systems, and these costs differ between countries.
- The goal of this study was to understand the cost-effectiveness of once-daily treatment with fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) compared with FF/VI and UMEC/VI among patients with COPD who experience symptoms and are at risk of exacerbations, from a Spanish healthcare system perspective.
What did the researchers do/find?
- Data from the IMPACT trial, which included a Spanish subgroup, and from Spanish public sources and literature were entered into the GALAXY COPD progression model.
- FF/UMEC/VI was cost-effective compared with FF/VI and UMEC/VI across all analyses and improved quality of life.
- Treatment with FF/UMEC/VI resulted in fewer exacerbations over 3 years compared with FF/VI and UMEC/VI, with a small additional cost of €444 per patient and an additional saving of €450 per patient, respectively.
- This led to improvements in quality-adjusted life years, whereby incremental cost-effectiveness ratios for FF/UMEC/VI versus dual therapies were well below the Spanish willingness-to-pay threshold of €30,000 and remained below this threshold in all analyses.
What do these results mean?
- These results show that treatment with FF/UMEC/VI improves patient health outcomes and is a cost-effective treatment option in Spain compared with FF/VI or UMEC/VI for patients with COPD experiencing symptoms or who are at risk of exacerbations.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of mortality worldwide and the fourth most common cause of death in Spain.1,2 Healthcare utilization and cost burden associated with COPD are substantial, with severity of symptoms and frequency of exacerbations being primary drivers.3,4 The Confronting COPD survey showed that over the course of 1 year, Spanish patients with COPD undergo a mean of 5.10 scheduled primary care visits, 1.11 scheduled specialist visits, and 1.00 inpatient hospitalizations.5 The cost associated with COPD management in Spain represents 0.2% of its Gross Domestic Product.6
COPD treatment objectives include reducing symptoms and lowering exacerbation risk.1 Both Spanish guidelines and the Global Initiative for COPD (GOLD) report recommend inhaled corticosteroid/long-acting muscarinic antagonist/long-acting β2-agonist (ICS/LAMA/LABA) triple therapy in patients who experience exacerbations or persistent symptoms despite ICS/LABA or LAMA/LABA therapy.1,7 The 52-week, Phase III, global IMPACT trial in 10,355 symptomatic COPD patients at risk of exacerbations demonstrated that once-daily single-inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) triple therapy reduced moderate/severe exacerbation rates, and improved lung function and health-related quality of life (HRQoL) versus once-daily single-inhaler UMEC/VI and FF/VI dual therapy.8
Although the efficacy of triple versus dual therapy for symptomatic COPD patients is well established, its cost-effectiveness is an important consideration from a payer perspective. Cost-effectiveness analyses should be country-specific given different healthcare systems, costs of treatment, and disease management guidelines between countries. A few studies have investigated the cost-effectiveness of dual therapies for COPD in Spain.9–11 Additionally, FF/UMEC/VI was shown to reduce healthcare-associated costs and to be cost-effective versus budesonide/formoterol (BUD/FOR) in symptomatic COPD patients at risk of exacerbations from the Spanish National Healthcare System perspective, using data from the Phase III FULFIL trial.12–14 Nevertheless, data on the cost-effectiveness of single-inhaler ICS/LAMA/LABA triple therapy versus ICS/LABA or LAMA/LABA dual therapy in Spain are limited. This study assessed the cost-effectiveness of FF/UMEC/VI versus FF/VI and UMEC/VI in patients with symptomatic COPD at risk of exacerbations from a Spanish healthcare system perspective, using data from the large-scale IMPACT trial.
Material and Methods
Analysis methods are described below and additional details can be found in the Supplementary Materials and Supplementary Figure 1.
Study Design
The validated GALAXY COPD progression model15–17 was used in this analysis, and populated using data from the IMPACT trial (study CTT116855, NCT02164513) and from Spanish public sources and literature. Briefly, the GALAXY COPD progression model uses limited risk equations which predict status for lung function, exacerbation frequency, dyspnea, cough/sputum, exercise capacity, and HRQoL. These equations were developed using data from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study and use covariates from the above and other patient characteristics, as well as modifiers reflecting treatment effects, to predict disease progression, HRQoL, and survival, measured as quality-adjusted life years (QALYs) for each treatment.15,18 IMPACT methods and results have been previously published.8
Population Characteristics
The analysis population reflected the population of the IMPACT trial, which enrolled 10,355 patients ≥40 years of age with symptomatic COPD (COPD Assessment Test [CAT] score ≥10) and post-bronchodilator forced expiratory volume in 1 second (FEV1) <50% predicted and ≥1 moderate/severe exacerbations in the prior year, or FEV1 ≥50% to <80% predicted and ≥2 moderate exacerbations or ≥1 severe exacerbation in the prior year.8 Baseline characteristics included a combination of those from the IMPACT Spanish subgroup (n=499; Table 1) and those from the global intent-to-treat (ITT) population, as per a similar FULFIL study analysis.14 The approach was validated by Spanish clinical experts. A separate analysis was conducted using baseline characteristics from the overall ITT population only (Supplementary Table 1).
 |
Table 1 Model Input Parameters for the Spanish Population of the IMPACT Trial |
Treatment Effects
Treatment effects were based on the IMPACT ITT population (Table 1), and were assumed to begin at the onset of the analysis and be maintained as long as patients remained on treatment. Treatment discontinuation rates of 18.3%, 25.2% and 27.3% per year for FF/UMEC/VI, FF/VI and for UMEC/VI, respectively, were applied, based on discontinuation rates in the IMPACT ITT population.8 Following discontinuation, all patients were assumed to receive subsequent treatment with efficacy assumed to be the same as the reference treatment. Based on IMPACT data, all patients (regardless of initial treatment) were assumed to receive ICS/LABA (25.1%), ICS/LABA/LAMA (57.9%), LAMA/LABA (10.3%), or LAMA (6.7%).
Utilities
Utility values for each cycle were estimated using a linear regression equation developed using data from a Spanish observational study, with EuroQol-5D utility index as the dependent variable,9,15 which was modified for the current analysis in two ways: the modelled values for “dyspnea several days per week” and “dyspnea most days per week” were used in place of “modified Medical Research Council (mMRC) 2–3” and “mMRC 4”, respectively, and a calibration factor was added, consistent with a previous Spanish cost-effectiveness analysis using FULFIL study data.14
Costs
Direct healthcare costs (2019 €) were informed by Spanish public sources as detailed previously.9,14,19 Drug costs included initial treatment, subsequent treatment and rescue medication. For a 30-day supply, FF/UMEC/VI (100/62.5/25 mcg), FF/VI (100/25 mcg) and UMEC/VI (62.5/25 mcg) costs were €83.52, €51.52, and €70.25, equating to €2.78, €1.72, and €2.34 daily costs, respectively. Non-drug costs were determined using a health-state costing approach, assessing the proportion of patients in each of nine specified health states (based on three lung function and three symptom frequency ratings) and applying costs sourced from the literature, adjusted for inflation (Table 2).9,14,20 Moderate and severe exacerbation events were costed individually.
 |
Table 2 Costs by Health State and Exacerbation Event |
Drug costs post-discontinuation were calculated as weighted average costs using published prices from the Spanish Ministry of Health, Equality and Social Policy catalogue (March 2019), and based on market shares within each subsequent treatment class, with market share data obtained from the Real Life Data, as previously detailed.9,14,19 Estimated daily costs of ICS/LABA, ICS/LABA + LAMA, LAMA/LABA, and LAMA as subsequent treatment were €1.56, €2.94, €2.69, and €1.40 (monthly costs: €46.90, €88.23, €80.81, and €41.93). Rescue medication costs were only applied when patients were on study treatment and were calculated based on salbutamol cost (€2.51 per 200 doses of 100 mcg) and observed usage in IMPACT (FF/UMEC/VI: 1.75 uses/day, FF/VI: 2.03 uses/day; UMEC/VI: 2.05 uses/day).
Model Outputs
Model outputs included cumulative number of exacerbations, survival (including accumulated life years [LY] and QALY), costs (including drug costs and non-drug costs) and incremental cost-effectiveness ratios (ICERs), presented as incremental cost per QALY gained.
Base Case, Scenario, and Sensitivity Analyses
The base case time horizon and duration of treatment effect were 3 years to align with prior analyses.9 An annual discount rate of 3% was applied to costs and benefits, in line with Spanish guidelines for economic evaluation of health technologies.21 Analyses were also performed in subgroups based on exacerbation history (≥2 moderate or ≥1 severe or <2 moderate and no severe exacerbations in the previous year).
One-way sensitivity and scenario analyses were performed to test the robustness of base case results. A probabilistic sensitivity analysis (PSA) was conducted to address the uncertainty in the parameters used within the model by assigning distributions to input parameters (Supplementary Table 2) and randomly sampling from these distributions over 5000 Monte Carlo simulations. Further details on sensitivity, scenario and PSA analyses can be found in the Supplementary Materials.
Based on Spanish literature, the willingness-to-pay threshold was assumed to be €30,000 per QALY gained.22,23
Results
FF/UMEC/VI vs FF/VI in the Spanish IMPACT Population
Base Case
Over a 3-year time horizon, FF/UMEC/VI was associated with fewer exacerbations and slightly higher total costs per patient versus FF/VI, gaining 0.064 QALYs at an additional cost of €444 per patient, resulting in an ICER of €6887 (Table 3).
 |
Table 3 Base Case Results with FF/UMEC/VI versus FF/VI and versus UMEC/VI (Spanish IMPACT Population) |
In patients with ≥2 moderate or ≥1 severe exacerbations in the previous year, FF/UMEC/VI was associated with fewer exacerbations and slightly higher total costs per patient versus FF/VI, gaining 0.059 QALYs with an incremental cost of €414 per patient, resulting in an ICER of €7017 (Supplementary Table 3). Results were similar for patients with <2 moderate and no severe exacerbations in the previous year, with 0.059 QALYs gained and an incremental cost of €308 per patient, resulting in an ICER of €5259 (Supplementary Table 3).
Sensitivity, Scenario and Probabilistic Sensitivity Analyses
ICERs with FF/UMEC/VI versus FF/VI remained below the Spanish willingness-to-pay threshold of €30,000 for all sensitivity and scenario analyses. Across sensitivity analyses, ICERs ranged from €2780 to €25,534; results were most sensitive to changes in duration of treatment effect and exacerbation treatment effects (Figure 1). In scenario analyses, ICERs ranged from €3459 to €9135 and were most affected by increasing the time horizon to 25 years and assuming an ongoing treatment effect (50% decrease from base case) and estimating utilities from SGRQ-C using the original GALAXY approach (33% increase from base case) (Figure 1). The ICER when pneumonia clinical events were included was €7309 (Figure 1).
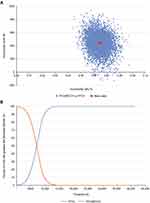
Across all PSA iterations, FF/UMEC/VI was associated with higher QALYs compared with FF/VI and was more costly across most iterations (Figure 2A). The PSA estimated the probability of FF/UMEC/VI being cost-effective versus FF/VI to be 100% at a willingness-to-pay threshold of €30,000 per QALY (Figure 2B).
Outputs from the analysis using data from the IMPACT ITT population were consistent with those using data from the Spanish IMPACT population (Supplementary Tables 4 and 5).
FF/UMEC/VI vs UMEC/VI in the Spanish IMPACT Population
Base Case
Over a 3-year time horizon, FF/UMEC/VI was a dominant treatment strategy (less costly and more effective) versus UMEC/VI, and was associated with fewer moderate or severe exacerbations and slightly lower total costs per patient, resulting in 0.054 QALYs gained at a cost saving of €450 per patient (Table 3).
In patients with ≥2 moderate or ≥1 severe exacerbations in the previous year, FF/UMEC/VI was associated with fewer exacerbations and lower total costs per patient versus UMEC/VI, resulting in 0.059 QALYs gained with a cost saving of €675 per patient and giving a dominant ICER. Patients with <2 moderate and no severe exacerbations in the previous year were also predicted to experience fewer exacerbations with FF/UMEC/VI versus UMEC/VI, with slightly higher total costs per patient, gaining 0.032 QALYs with an incremental cost of €252 per patient, and resulting in an ICER of €7817 (Supplementary Table 3).
Scenario, Sensitivity, and Probabilistic Analyses
Across sensitivity and scenario analyses, FF/UMEC/VI was dominant versus UMEC/VI in all but one case (data not shown owing to dominant outcome). FF/UMEC/VI was only non-dominant with a 1-year treatment effect, where the ICER was €1109, below the Spanish accepted threshold of €30,000 per QALY. FF/UMEC/VI remained dominant versus UMEC/VI after the inclusion of pneumonia events.
In the PSA, FF/UMEC/VI was more effective and less costly than UMEC/VI in all iterations (Figure 3). FF/UMEC/VI versus UMEC/VI was estimated to be 100% cost-effective across all willingness-to-pay thresholds (data not shown).
Outputs from the analysis using data from the IMPACT ITT population were consistent with those using data from the Spanish IMPACT population (Supplementary Tables 4 and 5).
Discussion
In this analysis, FF/UMEC/VI was predicted to reduce moderate and severe exacerbation rates and provide improvements in QALYs over a 3-year time horizon versus FF/VI and UMEC/VI, with either moderate or no cost increment. The probability that FF/UMEC/VI would be cost-effective compared with FF/VI or UMEC/VI over the 3-year period was 100% at a willingness-to-pay threshold of €30,000. As such, FF/UMEC/VI was shown to be a cost-effective treatment option in patients with symptomatic COPD at risk of exacerbations from a Spanish healthcare perspective. Results were consistent when using baseline data from the ITT population rather than from the subgroup of patients from Spain in IMPACT.
As expected, drug costs were higher for FF/UMEC/VI versus FF/VI and UMEC/VI. For FF/VI, these costs were partly offset by lower non-drug costs with FF/UMEC/VI, owing to fewer exacerbations and associated hospital stays and less time spent in the more costly state with poorer lung function, leading to a relatively small incremental cost of €444 per patient over 3 years. Savings in non-drug costs with FF/UMEC/VI versus UMEC/VI led to an overall cost saving of €450. Notably, FF/UMEC/VI remained cost-effective across all sensitivity and scenario analyses, demonstrating the robustness of the results across the range of values, accounting for uncertainty in factors that may contribute to treatment effectiveness and/or costs.
Importantly, FF/UMEC/VI remained cost-effective versus dual therapy across exacerbation frequency subgroups, indicating consistent benefits in patients with a range of exacerbation histories as found in real-world populations. The highest ICER (€7817) was seen for FF/UMEC/VI versus UMEC/VI in patients with <2 moderate and no severe exacerbations in the prior year, with a gain of 0.032 QALYs at an incremental cost of €252. The lowest ICER, which was dominant, was seen in patients with ≥2 moderate or ≥1 severe exacerbation. This suggests that previous exacerbation history, and thus risk of further exacerbations, is a factor in the degree of benefit from escalation to triple therapy. Nonetheless, the ICER remained well below the willingness-to-pay threshold of €30,000 and FF/UMEC/VI can therefore be considered cost-effective in both subgroups versus UMEC/VI. Interestingly, the ICER for FF/UMEC/VI versus FF/VI was lower in patients with a history of less frequent exacerbations, seemingly driven by a greater between-treatment difference in non-drug costs. However, as for the other treatment comparison, ICERs in both subgroups remained below the willingness-to-pay threshold for FF/UMEC/VI versus FF/VI.
ICERs in all analyses were below the commonly used Spanish threshold of cost-effectiveness for chronic medicines of €30,000–€45,000 per QALY gained.23 More recently, it has been suggested that this threshold should vary between €25,000 and €60,000, depending on the disease.24 With just one exception, all ICERs suggest that FF/UMEC/VI would still be cost-effective if the threshold was reduced to €25,000 per QALY gained. In the sensitivity analysis in which the duration of FF/UMEC/VI treatment effect was adjusted to 1 year (for a 3-year time horizon analysis), there was a substantial increase in ICER versus base case (FF/UMEC/VI vs FF/VI: from €6887 to €25,534; FF/UMEC/VI vs UMEC/VI: from dominant to €1109). UPLIFT study data have shown that LAMA treatment effects were maintained over a 4-year period; therefore, it is likely that the duration of FF/UMEC/VI treatment effect would last beyond 1 year if patients continued their treatment.25 Adjusting the FEV1 treatment effect to the upper and lower 95% CI values had minimal impact. However, the ICER for FF/UMEC/VI versus FF/VI was sensitive to changes in exacerbation risk (range: €2780 to €13,419), indicating that exacerbation treatment effects were important value drivers. This is unsurprising since exacerbations are known drivers of costs associated with COPD.3,4 Only small increases in ICERs were seen when pneumonia events were included, with ICER remaining well below the Spanish willingness-to-pay threshold.
These results are consistent with a Spanish cost-effectiveness analysis of the FULFIL study, which also used the GALAXY model and demonstrated the cost-effectiveness of FF/UMEC/VI versus BUD/FOR in a population representative of Spanish symptomatic COPD patients at risk of exacerbations, with an ICER of €642 per QALY.13,14 Results are also consistent with a Canadian cost-effectiveness analysis based on IMPACT data that demonstrated the cost-effectiveness of FF/UMEC/VI versus FF/VI and UMEC/VI (ICERs: C$18,989 and C$13,776 per QALY, respectively, against a Canadian willingness-to-pay threshold of C$50,000 per QALY).26 Owing to the unique nature of each country’s healthcare system, country-specific analyses are necessary, and the present results confirm the cost-effectiveness of FF/UMEC/VI versus FF/VI or UMEC/VI from a Spanish perspective.
COPD economic models are often based around disease attributes such as predicted FEV1.27,28 By accounting for treatment effects on a broader range of COPD parameters, and their ongoing inter-relatedness over time, the validated GALAXY model better reflects the multifactorial nature of COPD.15,29,30 Furthermore, use of data from the 52-week IMPACT trial provided a strong basis for extrapolation of treatment effects beyond the first year, based on the cycle length of 1 year used in the GALAXY model. However, there are some study limitations to consider. Pneumonia events are not explicitly modelled, although a scenario analysis was included in which the cost and utility impact of pneumonia events were accounted for separately. Furthermore, impact of single versus multiple inhalers on cost-effectiveness could not be evaluated due to the IMPACT trial design, as the ELLIPTA inhaler was used to administer all three treatments. Ease of inhaler use has been identified as a key patient preference in COPD, which may improve treatment compliance; thus, the type of inhaler is an important factor in the assessment of cost-effectiveness and should be included in future analyses.31 Finally, this analysis was designed to compare triple therapy with dual therapy as a reference. While differences in exacerbation rates for FF/VI versus UMEC/VI were observed in the IMPACT trial,8,32 a direct comparison between these two therapies based on absolute treatment effects was not conducted.
One of the strengths of the GALAXY model is that it uses a wide range of patient baseline characteristics to parameterize the modelled target population, thus maximizing the information upon which long-term disease progression is predicted. However, this potentially becomes a limitation when the full set of baseline parameters are not available for the population of interest. In IMPACT, baseline fibrinogen, 6MWD, and mMRC data were not available, and were either predicted by risk equations or derived from analogous data. Importantly, the proportion of patients with mMRC score ≥2 was estimated based on the proportion of patients with a CAT score ≥21 in the IMPACT ITT population. However, there is evidence to show that the correlation between CAT and mMRC score may be variable, with a weak relationship between the constructs measured.33 As such, an analysis in which the proportion of patients with an mMRC score ≥2 has been accurately measured would be beneficial to further validate results. Nevertheless, while these data are assumed to be important clinical factors in the GALAXY model, the resulting ICER is not necessarily sensitive to variations in these assumptions. Finally, while these results are consistent with those seen in other countries, healthcare systems, costs of treatment, and disease management guidelines differ between countries, therefore, the results of this analysis may not be generalizable outside of Spain.
Conclusion
In conclusion, FF/UMEC/VI versus FF/VI or UMEC/VI reduced exacerbations and increased QALYs gained, with a moderate or no cost increment. ICERs for FF/UMEC/VI versus dual therapies were well below the Spanish willingness-to-pay threshold of €30,000 and remained below this threshold in all sensitivity and scenario analyses. Overall, these results show that FF/UMEC/VI delivers improvements in health outcomes relative to UMEC/VI and FF/VI and is a cost-effective treatment option in Spain for symptomatic COPD patients at risk of exacerbations.
Abbreviations
BUD, budesonide; CAT, COPD Assessment Test; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FF, fluticasone furoate; FOR, formoterol; GOLD, Global Initiative for COPD; ICER, incremental cost-effectiveness ratio; ICS, inhaled corticosteroid; ITT, intent-to-treat; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LY, life years; mMRC, modified Medical Research Council; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life years; UMEC, umeclidinium; VI, vilanterol.
Data Sharing Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Ethics Approval and Informed Consent
Ethical approval and patient informed consent were not applicable for this analysis as anonymized patient aggregated (summary) data were used for a subset of patients from the IMPACT primary trial. For the IMPACT primary trial, all patients provided written informed consent; the study was conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki and received approval by local ethics review boards of the participating sites (Supplementary Table 6).
Acknowledgments
Editorial support in the form of preparation of the first draft based on input from all authors, and collation and incorporation of author feedback to develop subsequent drafts, was provided by Chrystelle Rasamison, of Fishawack Indicia Ltd. part of Fishawack Health, UK, and was funded by GSK. ELLIPTA is owned by or licensed to the GSK Group of Companies.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study (GSK ID: HO-17-17596) was funded by GSK. The funders of the study had a role in study design, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data and the final responsibility to submit for publication.
Disclosure
LA Vallejo-Aparicio, A Martin, and AS Ismaila are employees of GSK and A Martin and AS Ismaila own stocks/shares in GSK. AS Ismaila is an unpaid part-time professor at McMaster University. VF Paly and C Abreu are ICON employees and C Biswas was an ICON employee at the time of this analysis. ICON received funding from GSK to conduct this study but not payment for manuscript development. JL Izquierdo, JA Riesco and JJ Soler-Cataluña received consulting fees from GSK to conduct this study but did not receive payment for manuscript development. JJ Soler-Cataluña also reports grant and personal fees from GSK; grant, personal fees and non-financial support from Boehringer Ingelheim and Laboratorios Esteve; personal fees and non-financial support from Bial, Menarini and Novartis; and personal fees from AstraZeneca, Chiesi, Faes, Rovi and Ferrer. The authors report no other conflicts of interest in this work.
References
1. GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020. Available from: www.goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
2. Soriano JB, Rojas-Rueda D, Alonso J, et al. The burden of disease in Spain: results from the global burden of disease 2016. Med Clin. 2018;151(5):171–190. doi:10.1016/j.medcli.2018.05.011
3. Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. doi:10.1111/resp.12660
4. Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic burden of Chronic Obstructive Pulmonary Disease (COPD): a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:439–460. doi:10.2147/copd.s234942
5. Izquierdo Alonso J. The burden of COPD in Spain: results from the Confronting COPD survey. Respir Med. 2003;97(Suppl C):S61–69. doi:10.1016/S0954-6111(03)80026-4
6. Ministerio de Sanidad y Politica Social. Estrategia en EPOC del Sistema Nacional de Salud; 2019. Available from: www.mscbs.gob.es/organizacion/sns/planCalidadSNS/docs/EstrategiaEPOCSNS.pdf.
7. Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish Guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53(6):324–335. doi:10.1016/j.arbres.2017.03.018
8. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
9. Miravitlles M, Galdiz JB, Huerta A, Villacampa A, Carcedo D, Garcia-Rio F. Cost-effectiveness of combination therapy umeclidinium/vilanterol versus tiotropium in symptomatic COPD Spanish patients. Int J Chron Obstruct Pulmon Dis. 2016;11:123–132. doi:10.2147/copd.s94006
10. Driessen MT, Whalen J, Seewoodharry Buguth B, et al. Cost-effectiveness analysis of umeclidinium bromide/vilanterol 62.5/25 mcg versus tiotropium/olodaterol 5/5 mcg in symptomatic patients with chronic obstructive pulmonary disease: a Spanish National Healthcare System perspective. Respir Res. 2018;19(1):224. doi:10.1186/s12931-018-0916-7
11. Capel M, Mareque M, Alvarez CJ, Lindner L, Oyaguez I. Cost-effectiveness of fixed-dose combinations therapies for chronic obstructive pulmonary disease treatment. Clin Drug Investig. 2018;38(7):611–620. doi:10.1007/s40261-018-0646-0
12. Atienza L, Benjamin N, Schroeder M, et al. Impact of once-daily single inhaler triple therapy on healthcare resource utilization and associated costs in COPD patients in Spain. Eur Respir J. 2018;52:PA3155.
13. Benjamin N, Huerta A, Biswas C, et al. Cost-effectiveness of a new single inhaler triple therapy versus a dual inhaled corticosteroid plus long-acting beta agonist in patients with COPD in Spain. Value Health. 2018;21(Suppl 3):CE2. doi:10.1016/j.jval.2018.09.014
14. Schroeder M, Benjamin N, Atienza L, et al. Cost-effectiveness analysis of a once-daily single-inhaler triple therapy for patients with Chronic Obstructive Pulmonary Disease (COPD) Using the FULFIL trial: a Spanish perspective. Int J Chron Obstruct Pulmon Dis. 2020;15:1621–1632. doi:10.2147/COPD.S240556
15. Exuzides A, Colby C, Briggs AH, et al. Statistical modeling of disease progression for chronic obstructive pulmonary disease using data from the ECLIPSE study. Med Decis Making. 2017;37(4):453–468. doi:10.1177/0272989x15610781
16. Risebrough NA, Briggs A, Baker TM, et al. Validating a model to predict disease progression outcomes in patients with COPD. Value Health. 2014;17(7):A560–561. doi:10.1016/j.jval.2014.08.1852
17. Tabberer M, Gonzalez-McQuire S, Muellerova H, et al. Development of a conceptual model of disease progression for use in economic modeling of chronic obstructive pulmonary disease. Med Decis Making. 2017;37(4):440–452. doi:10.1177/0272989X16662009
18. Briggs AH, Baker T, Risebrough NA, et al. Development of the Galaxy chronic obstructive pulmonary disease (COPD) model using data from ECLIPSE: internal validation of a linked-equations cohort model. Med Decis Making. 2017;37(4):469–480. doi:10.1177/0272989X16653118
19. Big-Pac RLD. Pacientes en tratamiento farmacológico para la EPOC. Movimiento Total Anual a Q2. Real Life Data SLU; 2018. Available from: www.rlifedata.com.
20. Instituto Nacional de Estadistica (National Institute of Statistics). INEbase- Standard of living and living conditions (CPI)- Consumer price and housing indices; 2019. Available from: www.ine.es/dyngs/INEbase/en/categoria.htm?c=Estadistica_P&cid=1254735976604.
21. Lopez-Bastida J, Oliva J, Antonanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513–520. doi:10.1007/s10198-010-0244-4
22. Sacristan JA, Oliva J, Del Llano J, Prieto L, Pinto JL. ¿Qué es una tecnología sanitaria eficiente en España? [What is an efficient health technology in Spain?]. Gac Sanit. 2002;16(4):334–343. Spanish. doi:10.1016/s0213-9111(02)71933-x
23. De Cock E, Miravitlles M, Gonzalez-Juanatey JR, Azanza-Perea JR. Valor umbral del coste por año de vida ganado para recomendar la adopción de tecnologías sanitarias en España: evidencias procedentes de una revisión de la literatura. Pharmacoecon Span Res Artic. 2007;4:97–107. doi:10.1007/BF03320930
24. Sacristan JA, Oliva J, Campillo-Artero C, et al. ¿Qué es una intervención sanitaria eficiente en España en 2020? [What is an efficient health intervention in Spain in 2020?]. Gac Sanit. 2020;34(2):189–193. Spanish. doi:10.1016/j.gaceta.2019.06.007
25. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800
26. Ismaila AS, Risebrough N, Schroeder M, et al. Cost-effectiveness of once-daily single-inhaler triple therapy in COPD: the IMPACT trial. Int J Chron Obstruct Pulmon Dis. 2019;14:2681–2695. doi:10.2147/copd.s216072
27. Hoogendoorn M, Feenstra TL, Asukai Y, et al. Cost-effectiveness models for chronic obstructive pulmonary disease: cross-model comparison of hypothetical treatment scenarios. Value Health. 2014;17(5):525–536. doi:10.1016/j.jval.2014.03.1721
28. Starkie HJ, Briggs AH, Chambers MG. Pharmacoeconomics in COPD: lessons for the future. Int J Chron Obstruct Pulmon Dis. 2008;3(1):71–88.
29. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322
30. Hoogendoorn M, Feenstra TL, Asukai Y, et al. External validation of health economic decision models for Chronic Obstructive Pulmonary Disease (COPD): report of the third COPD modeling meeting. Value Health. 2017;20(3):397–403. doi:10.1016/j.jval.2016.10.016
31. Lewis HB, Schroeder M, Gunsoy NB, et al. Evaluating patient preferences of maintenance therapy for the treatment of chronic obstructive pulmonary disease: a discrete choice experiment in the UK, USA and Germany. Int J Chron Obstruct Pulmon Dis. 2020;15:595–604. doi:10.2147/COPD.S221980
32. Lipson DA, Barnhart F, Boucot I, et al. Exacerbation outcomes with LAMA/LABA and ICS/LABA in high risk COPD patients in the IMPACT trial. Eur Respir J. 2018;52(Suppl 62):PA4384.
33. Jones PW. The COPD Assessment Test: what have we learned over its first 5 years? Eur Respir J. 2014;44(4):833–834. doi:10.1183/09031936.00125214
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.