Back to Journals » Cancer Management and Research » Volume 11
Cost-Effectiveness Analysis Of EGFR Mutation Testing And Afatinib Versus Gemcitabine-Cisplatin As First-Line Therapy For Advanced Non-Small-Cell Lung Cancer In China
Authors You R, Liu J, Wu DBC, Qian X, Lyu B, Zhang Y, Luo N
Received 17 June 2019
Accepted for publication 9 October 2019
Published 5 December 2019 Volume 2019:11 Pages 10239—10248
DOI https://doi.org/10.2147/CMAR.S219722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Ruxu You,1,* Jinyu Liu,2,* David Bin-Chia Wu,3 XinYu Qian,4 Boxiang Lyu,5 Yu Zhang,1 Nan Luo4
1Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China; 2Department of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China; 3School of Pharmacy, Monash University Malaysia, Kuala Selangor, Malaysia; 4Saw Swee Hock School of Public Health, National University of Singapore, Singapore, Singapore; 5Machine Learning Department, Carnegie Mellon University, Pittsburgh, Pennsylvania, USA
*These authors contributed equally to this work
Correspondence: Nan Luo
Saw Swee Hock School of Public Health, National University of Singapore, 16 Medical Drive, Block MD3, Singapore 117597, Singapore
Tel +65 6516 4966 Ext 188
Fax +65 6779 1489
Email [email protected]
Objective: The purpose of this study was to evaluate the cost-effectiveness of the combined use of afatinib and epidermal growth factor receptor (EGFR) testing versus gemcitabine-cisplatin as the first-line treatment for patients with non-small cell lung cancer (NSCLC) in China.
Methods: A decision-analytic model, based on clinical phase III trials, was developed to simulate patient transitions. Direct costs were estimated from the perspective of the Chinese healthcare system. Quality-adjusted life-years (QALYs) and incremental cost-effectiveness ratios (ICER) were calculated over a 5-year lifetime horizon. Model robustness was conducted in sensitivity analyses.
Results: For the base case, EGFR mutation testing followed by afatinib treatment for advanced NSCLC increased 0.15 QALYs compared with standard chemotherapy at an additional cost of $5069.12. The ICER for afatinib maintenance was $33,416.39 per QALY gained. The utility of PFS and the cost of afatinib had the most important impact on the ICER. Scenario analyses suggested that when a patient assistance program (PAP) was available, ICER decreased to $22,972.52/QALY lower than the willingness-to-pay (WTP) threshold of China ($26,508/QALY).
Conclusion: Our results suggest that gene-guided maintenance therapy with afatinib with the PAP might be a cost-effective treatment option compared with gemcitabine – cisplatin in China.
Keywords: Economic analysis, incremental cost-effectiveness ratio, NSCLC, EGER mutation testing, Afatinib
Introduction
Worldwide, lung cancer is the most common malignancy and the most common cause of cancer deaths in the past few decades.1 In China, the incidence (48.32 per 100,000) and mortality rate (39.27 per 100,000) of lung cancer are both high.2 Non-small cell lung cancer (NSCLC) is the most common histological subtype, accounting for 80–85% of all lung cancers, and approximately 60% of NSCLC patients have advanced stage at newly diagnosed.3
The standard first-line therapy for advanced NSCLC is systemic platinum-based doublet chemotherapy, including cisplatin or carboplatin, combined with taxanes, pemetrexed, and gemcitabine.4 However, therapeutic efficacy of conventional chemotherapy is poor; the median overall survival (OS) time is nearly 1-year.5,6 Therefore, the development of novel and more effective therapies is needed in advanced NSCLC in order to improve the clinical outcomes of patients.
Owing to genetic technology advancement, the identification of lung tumors harbouring mutations in epidermal growth factor receptor (EGFR) has captured people’s attention on molecularly targeted therapies – EGFR tyrosine kinase inhibitors (TKIs).7 In patients with NSCLC, EGFR mutations found in 10% to 15% of Western patients but as high as 47% in Asian patients play a critical role in the development and progression of lung cancer.8,9 First- and second-generation EGFR-TKIs, such as gefitinib, erlotinib, afatinib and osimertinib, are approved first-line treatments by FDA. As illustrated by the results of several randomized clinical trials, these regimens achieve higher response rates, longer progression-free survival (PFS), and a lower incidence of severe adverse effects than platinum-based chemotherapy in a population harboring EGFR mutation.10–12
Recently, an open-label, randomised phase 3 trial (LUX-lung 6) showed that afatinib significantly delayed tumor growth and improved quality of life compared to the widely used gemcitabine and cisplatin when used to treat patients with EGFR mutation-positive NSCLC. These results suggested that afatinib should be considered as the first choice of therapy in eastern Asian patients, especially in China.4
However, this treatment option approach is substantially more expensive because of the high price of afatinib and the cost of genetic screening. While several pharmacoeconomic analyses reveal gefitinib and erlotinib as first-line treatment,13–20 economic assessments for afatinib therapy are limited in China.
Whether EGFR mutation screening and individualized therapy with afatinib are cost-effective in China is unclear. Hence, health policymakers, patients and physicians do not know the relative value for money of available of these potential first-line therapies. The objective of this study was to develop a decision-analytic model and use it to evaluate the cost-effectiveness analysis of EGFR mutation testing followed by targeted individualized first-line afatinib treatment compared to no test and treatment with conventional chemotherapy from the perspective of Chinese payers.
Methods
LUX-Lung 6 Trial
This analysis was based on the results of the LUX-lung 6 trial, an open-label, randomised phase III trial comparing first-line afatinib (40 mg per day) with gemcitabine plus cisplatin (GemCis) chemotherapy (1000 mg/m2gemcitabine on day 1 and day 8 and 75 mg/m2cisplatin on day 1 once every 21 days for up to six cycles) in patients with EGFR mutation-positive advanced NSCLC. Gemcitabine and cisplatin is a widely used and approved first-line chemotherapeutic regimen in Asian countries. Baseline patient characteristics were generally similar and well-balanced across treatment arms. Approximately 90% of patients were Chinese, 65% were women and 77% were never smoked. The primary endpoint was progression-free survival by independent review. Key secondary endpoints were designated as the response proportion and overall survival.4 Afatinib significantly improved the median PFS when compared with those who received GemCis (11.0 vs 5.6 months, HR 0.28, 95% CI 0.20–0.39, p<0.0001).4 Overall survival did not significantly differ between treatment groups (HR 0.93, 95% CI 0.72–1.22, p=0.61).21 The most frequently reported serious adverse events (SAEs, grade≥3) in the afatinib group were rash or acne, diarrhoea, and stomatitis or mucositis, compared with vomiting, anaemia, neutropenia, and thrombocytopenia in the GemCis group.
Model Structure And Outcomes
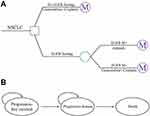
We developed a mathematical model using TreeAge Pro 2018 (TreeAge Software, Williamstown, MA) to estimate the clinical and economic outcomes of afatinib and GemCis. The model consisted of two components: a decision model and a Markov model. A decision tree model was used to present the two alternative strategies (Figure 1A). In the testing strategy, EGFR mutation-positive patients received first-line afatinib treatment, and those who tested negative were treated with GemCis. We estimated that 50.2% of patients whose tests were positive.22 In the no-testing group, screening testing was not performed, and conventional chemotherapy of GemCis was given to all patients regardless of EGFR mutation status.
A Markov model including three mutually exclusive disease-related health states: progression-free survival (PFS), progressive disease (PD), and death were used to simulate NSCLC progression in both the testing and no-testing strategies (Figure 1B). It was assumed that the population cohort entered the model in the PFS state and then either remained in the same state when they responded to treatment or transited to the other state in subsequent Markov cycles. The transition cycle length of the model was set to 3 weeks, which accorded with one chemotherapy cycle. A time horizon of 5 years was chosen to reflect the limited remaining lifetime of the patients.
Primary model outcomes were corresponding costs of the two therapies, quality-adjusted life years (QALYs), and the incremental cost-effectiveness ratio (ICER). Both costs and health benefits were discounted at 5% for the base-case analysis, as recommended for health economic evaluations in China.23 As there is no official cost-effectiveness threshold in China, we used three times the per capita gross domestic product (GDP) value of China in 2017 ($26,508) as the willingness-to-pay (WTP) threshold.23
Clinical Efficacy Data
Transition probabilities for the different health states were estimated from LUX-lung 6 trial.4 Weibull curves were extrapolated to fit the PFS and OS curves, on the basis of survival data extracted from the published Kaplan-Meier curves, by using R statistical software (version 3.4.3; R Foundation, Wien, Austria). The Weibull distribution provided better fits to survival data than did other models according to Akaike’s information criterion.24 The scale parameter (λ) and shape parameter (γ) of Weibull distribution are used to estimate the probabilities of transition at the time of cycle t. It was calculated as follows25
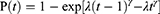
The estimated scale and shape parameters, adjusted R2 are described in Supplemental Table 1.
Costs
Total costs were calculated from the perspective of Chinese healthcare payers. Direct medical costs were associated with the costs of drugs, EGFR testing, maintenance and second-line chemotherapy, consultation visit and monitoring, and management of serious adverse events (SAEs, grade≥3) (Table 1).
 |
Table 1 Unit Costs Included In The Model |
The costs of afatinib and GemCis were based on different brand prices and market share of each brand in China. To calculate the dosages of chemotherapeutic agents, we assumed a body surface area of 1.72 m2. For each chemotherapy cycle, the costs of drug include injection fee and chemotherapy preparation fee by pharmacists. The duration of chemotherapy was six cycles in line with LUX-lung 6, and the pemetrexed maintenance therapy could be continued until disease progression. Once the disease progressed, patients were assumed to receive salvage chemotherapy. The resource utilization related to maintenance and salvage chemotherapy was derived from previously published studies.26,27
Costs for consultation, computed tomography scan, laboratory tests and EGFR testing were sourced from the health system or the National Development and Reform Commission of China. SAEs management strategies were based on clinical practice and expert opinions. The incidences of SAEs were sourced from LUX-lung 6 clinical trials (Supplemental Table 2). The unit costs of treating SAEs were estimated based on patient records in local hospitals.
Utility
As quality of life measurements was not estimated in LUX-lung 6 trial, the utility scores of PFS and PD for each treatment arm were obtained from the literature.28,29 Utility values were weighted by the routes of drug administration and disutility of SAEs in this study (Table 2).
 |
Table 2 Utility Values For The Health States And Disutility Values Associated With Adverse Events And Route Of Administration |
Scenario And Sensitivity Analyses
Scenario, deterministic sensitivity, and probabilistic sensitivity analyses were conducted to assess the impact of uncertainty in model inputs on the outcomes. Two scenario analyses were carried out. First, Mainland China is currently divided into 31 provinces and provincial-level municipalities and the socioeconomic status in these regions differs significantly. We assessed the cost-effective probabilities of treatments for these provinces with different WTP (3×local per capita GDP). Second, due to the challenge of affording afatinib in China, a Patient Assistance Program (PAP) was introduced by the pharmaceutical manufacturer to make the treatment available to eligible patients. Under the PAP, all eligible NSCLC patients would require to pay for 7 months of afatinib, after which patients would then get the free afatinib until the disease has progressed. Thus, we assessed the impact of PAP scenario for afatinib. The variation of price discounts ranging from 10% to 30% for afatinib was also tested to simulate the potential cost savings to patients.
One-way sensitivity analyses were conducted by varying each key model parameter, while keeping all other variables constant at their base-case values, over a range of values derived from 95% confidence intervals or the range informed in the relevant article. Probabilistic sensitivity analyses were performed to assess the effects of uncertainty in all model parameters simultaneously using Monte-Carlo simulations. In this method, all parameters were randomly drawn for 1000 iterations from distributions of their probabilities. We applied beta distributions to probability and utility estimates and triangle distribution to cost estimates. We constructed a cost-effectiveness acceptability curve (CEAC) to estimate the joint impact of parameter uncertainty and potential variability at the decision-maker’s WTP threshold.
Results
Base-Case Analysis
The results of a base-case analysis with a 5-year time horizon, as well as economic and health outcomes estimated by the model, are shown in Table 3. Total costs were estimated to be $13,042.55 for the Afatinib treatment arm and $7973.42 for the GemCis chemotherapy treatment arm. Afatinib generated a gain of 0.15 QALYs over GemCis chemotherapy, resulted in an ICER of 33,416.39/QALY gained. In a scenario where the PAP was included, the ICER declined to $22,972.52.
 |
Table 3 Summary Of The Cost And Outcome Results In Base-Case Analysis |
Scenario And Sensitivity Analyses
Supplemental Tables 3 and 4 showed the outcome of relevant additional scenario analyses. The WTP threshold, of different province-level administrative units, extended from $24,928 (3×$8309) to $57,318 (3×19,106) per QALY gained. Afatinib strategy might be the optimal alternative option in Beijing, Shanghai, Tianjin, Jiangsu, Zhejiang, which have over 95% chance to be cost-effective (Supplemental Table 3). The pricing analyses demonstrated that decreasing the price of afatinib by 10% to 30% lowered the ICER to $30,714.93-$24,562.93 per QALY. When reducing afatinib by 30%, the probability that gene screening followed by afatinib treatment was cost-effective was nearly 80%. The probability that screening followed by afatinib treatment was cost-effective remained high at 90% when PAP was available. We have also conducted the sensitivity analysis and confirmed that extending the time horizon to life years will not affect the results (Supplemental Table 4).
Results of the deterministic (one-way) sensitivity analysis are displayed as tornado plots showing the influence of extreme variations in each key parameter (Figure 2). In spite of the PAP, the study shows that the most impactful parameters were the utility of progression-free survival and the price of afatinib. The utility of PD, the costs of salvage therapy, discount rate and the cost of EGFR testing had a medium impact on the ICER results. Varying the probability of SAEs or disutility of SAEs basically did not affect the model outputs.
When no PAP was available, the probabilistic sensitivity analysis demonstrated that there was almost no cost-effective probability at a threshold of $26,508. With PAP, nearly 90% likelihood for the advanced NSCLC cohort became cost-effectiveness, respectively (Figure 3A). Correspondingly, the acceptability curves showed that the probability of cost-effectiveness also increased with an increase in the WTP threshold, which was sensitive to the thresholds from approximately $30,000 to $48,000 in the no-PAP setting and from approximately $18,000 to $36,000 in the PAP setting (Figure 3B).
Discussion
In China, Afatinib and other TKIs are increasingly used for the treatment of NSCLC. Because of their significantly improved PFS, less adverse drug reactions and oral route afford patients' convenience and enhanced satisfaction compared to those who receive chemotherapy.30 However, the high price and the long-term utilization of afatinib resulted in an unaffordable burden on health resources, which concerns the health policy decision-makers.31 Therefore, it is critical to have a precise pharmacoeconomic evaluation of maintenance afatinib therapy in a health resource-limited setting. To the best of our knowledge, this is the very first study to address the cost-effectiveness of first-line afatinib treatment with EGFR testing for patients with NSCLC from the perspective of Chinese healthcare payers.
According to our analysis results, compared with the “no test” strategy (only GemCis chemotherapy), the “test-treat” approach (EGFR mutation screening followed by afatinib therapy) yielded an ICER of $33,416.39/QALY. The result is primarily due to the high price of afatinib, whereas other costs, such as the costs of EGFR mutation testing and disease management, had minimal impact. For a threshold value of $26,508 per QALY gained in China, the afatinib strategy was not a cost-effective therapeutic approach. The acceptability curve also supported this finding, which showed a paucity of certainty that afatinib was the preferred option at this WTP threshold. In the scenario of afatinib PAP, the gene-guided afatinib treatment for patients with advanced EGFR mutation-positive NSCLC may be the cost-effective option because their ICERs were lower than the threshold, and afatinib had an 80% probability of being cost-effectiveness at the threshold. Although several interesting results were reported in this study, researchers need to be cautious when generalising the results of clinical trials to real-world outcomes.32
In 2014, the UK’s National Institute for Health and Care Excellence (NICE) has recommended the use of afatinib as an option, for treating adults with NSCLC whose tumour tests positive for EGFR-TK mutation and who have not previously received EGFR-TK inhibitor and the manufacturer provides afatinib with a patient access scheme.33 A similar result, based on the afatinib PAP in a Chinese setting, was observed in the current analysis.
To the best of our knowledge, there are few studies reporting the cost-effectiveness of afatinib versus chemotherapy for first-line treatment of NSCLC.34,35 In Singapore, a partitioned survival model was designed to assess the cost-effectiveness of afatinib versus pemetrexed-cisplatin for EGFR mutation-positive NSCLCs. On the base of the results of the LUX-Lung 3 trial results, with an ICER SG$137,648 per QALY gained and SG$109,172 per life-year gained from the Singapore healthcare payer’s perspective, afatinib did not appear cost-effective versus pemetrexed-cisplatin as first-line treatment for EGFR mutation-positive NSCLCs' patients.34 Ting J et al developed a Markov model to compare the cost-effectiveness of erlotinib, afatinib, and pemetrexed-cisplatin in the United States. In the base case, an individual strategy with afatinib was shown to be dominant (less costly and better outcomes) relative to the pemetrexed-cisplatin.35
Different methodological approaches, such as the model structure, time horizon, the measurement of costs and health utilities, lead to the findings of two published reports were inconsistent. Compared with those previous economic evaluations, one principal difference of current research is the comparator. We compared afatinib with gemcitabine-cisplatin in Asian patients based on the LUX-Lung 6 trial. Gemcitabine is the widely accepted and approved in combination with cisplatin for first-line treatment in Eastern Asian where pemetrexed-based chemotherapy was not yet approved.
In one-way sensitivity analysis (Figure 3), we found that the utility values of PFS are the most significant parameter that could change the ICER value. Generally, utility is influenced by the social and cultural values of the stakeholders, and therefore it might differ across countries.36–38 However, in the present study, the utilities were not directly obtained from the LUX-lung 6 trial or a Chinese population. An updated study should be conducted when such data are available in the Chinese NSCLC patients. The price of afatinib was another influential factor. Afatinib treatment became cost-effective given the current WTP threshold if a reduction in the price of afatinib by 30% or PAP plan.
Modern personalized medicine based on genetic information is an efficient way to provide “the right drug, with the right dose at the right time to the right patient”, which might improve patients health using the targeted therapeutic pharmaceutical interventions.39 However, the introduction of genetic screening may, in turn, increase the utilization of health resource.
Potential limitations may, however, be related to the study design. First, due to lack of enough survival data, we used the Weibull distribution to extrapolate the results beyond the follow-up duration of the RCTs, which may not accurately reflect the true survival. An updated cost-effective analysis should be carried out when long-term follow-up of survival data become available. Second, the current analysis did not evaluate the impact of some new EGFR mutation testing techniques, such a next-generation sequencing and immunotherapy, which had a higher cost with a better specificity and sensitivity, on the outcome of afatinib treatment. Third, our analysis did not evaluate the influence of different treatments after disease progression. We assumed patients from both treatment groups received docetaxel after disease progression, which may not always mimic the treatment in the real world because patients might switch to subsequent therapy upon the further progressions, for example the use of platinum-based combination chemotherapy is also possible after the failure of EGFR inhibitors. Fourth, our value frameworks rely on efficacy measures based on improvements in survival rate of median patients, and hence did not take into account of the improvements in “tail of the curve survival”, which may impact the current treatment decisions.38 Finally, we have identified for healthcare policymakers in China whether afatinib strategy is of the best value for money. However, we have not attempted to address issues of affordability (i.e. budget impact), and afatinib doses' modifications in this analysis, these would be an area of important future research.
Conclusion
Our analysis showed that the combined use of EGFR testing and afatinib is not a cost-effective option in most provinces of China. Local governments, with different socioeconomic status, however, could take fully into account covering maintenance afatinib therapy. Because for wealthy provinces (the per capita GDP>$12,289), the new treatment seems to be a reasonable choice. Decreasing the price of afatinib by 30% or the use of the Patient Assistance Program, should be considered as a cost-effective treatment in China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi:10.3322/caac.21208
2. Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China 2011. Chin J Cancer Res. 2015;27:2. doi:10.1186/s40880-015-0001-2
3. Besse B, Adjei A, Baas P, et al. 2nd ESMO consensus conference on lung cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25:1475–1484. doi:10.1093/annonc/mdu123
4. Wu Y-L, Zhou C, Hu C-P, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi:10.1016/S1470-2045(13)70604-1
5. John G, Christina L, Ellis PM, Ung YC, Evans WK. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol. 2010;5:260–274. doi:10.1097/JTO.0b013e3181c6f035
6. Ettinger DS, Wood DE, Akerley W, et al. NCCN guidelines insights: non-small cell lung cancer, version 4. J Natl Compr Canc Netw. 2016;14:255. doi:10.6004/jnccn.2016.0031
7. Reinhard B, Jürgen W, Thomas RK, et al. Lessons learned from lung cancer genomics: the emerging concept of individualized diagnostics and treatment. J Clin Oncol. 2013;31:1858–1865. doi:10.1200/JCO.2012.45.9867
8. Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol. 2006;11:190–198. doi:10.1007/s10147-006-0583-4
9. Sekine I, Yamamoto N, Nishio K, et al. Emerging ethnic differences in lung cancer therapy. Br J Cancer. 2008;99:1757–1762. doi:10.1038/sj.bjc.6604721
10. Mello RAD, Escriu C, Castelobranco P, et al. Comparative outcome assessment of epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of advanced non-small-cell lung cancer: a network meta-analysis. Oncotarget. 2018;9:11805–11815. doi:10.18632/oncotarget.23668
11. Batson S, Mitchell SA, Windisch R, et al. Tyrosine kinase inhibitor combination therapy in first-line treatment of non-small-cell lung cancer: systematic review and network meta-analysis. Onco Targets Ther. 2017;10:2473–2482. doi:10.2147/OTT
12. Lee J-K, Hahn S, Kim D-W, et al. Epidermal growth factor receptor tyrosine kinase inhibitors vs conventional chemotherapy in non-small cell lung cancer harboring wild-type epidermal growth factor receptor: a meta-analysis. JAMA. 2014;311:1430–1437. doi:10.1001/jama.2014.3314
13. Lim E-A, Lee H, Bae E, et al. Economic evaluation of companion diagnostic testing for EGFR mutations and first-line targeted therapy in advanced non-small cell lung cancer patients in South Korea. PLoS One. 2016;11:e0160155. doi:10.1371/journal.pone.0160155
14. Schremser K, Rogowski WH, Adler-Reichel S, et al. Cost-effectiveness of an individualized first-line treatment strategy offering erlotinib based on EGFR mutation testing in advanced lung adenocarcinoma patients in Germany. Pharmacoeconomics. 2015;33:1215–1228. doi:10.1007/s40273-015-0305-8
15. Lu S, Ye M, Ding L, et al. Cost-effectiveness of gefitinib, icotinib, and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Oncotarget. 2017;8:9996–10006. doi:10.18632/oncotarget.14310
16. Narita Y, Matsushima Y, Shiroiwa T, et al. Cost-effectiveness analysis of EGFR mutation testing and gefitinib as first-line therapy for non-small cell lung cancer. Lung Cancer. 2015;90:71–77. doi:10.1016/j.lungcan.2015.07.006
17. Kumar G, Woods B, Hess LM, et al. Cost-effectiveness of first-line induction and maintenance treatment sequences in non-squamous non-small cell lung cancer (NSCLC) in the US. Lung Cancer. 2015;89:294–300. doi:10.1016/j.lungcan.2015.05.020
18. Vergnenegre A, Massuti B, De Marinis F, et al. Economic analysis of first-line treatment with erlotinib in an EGFR-mutated population with advanced NSCLC. J Thorac Oncol. 2016;11:801–807. doi:10.1016/j.jtho.2016.02.004
19. Lee VW, Schwander B, Lee VH. Effectiveness and cost-effectiveness of erlotinib versus gefitinib in first-line treatment of epidermal growth factor receptor-activating mutation-positive non-small-cell lung cancer patients in Hong Kong. Hong Kong Med J. 2014;20:178–186. doi:10.12809/hkmj133986
20. Zhu J, Li T, Wang X, et al. Gene-guided Gefitinib switch maintenance therapy for patients with advanced EGFR mutation-positive non-small cell lung cancer: an economic analysis. BMC Cancer. 2013;13:39. doi:10.1186/1471-2407-13-39
21. Yang JC-H, Wu Y-L, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi:10.1016/S1470-2045(14)71173-8
22. Shi Y, Li J, Zhang S, et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology – mainland China subset analysis of the PIONEER study. PLoS One. 2015;10:e0143515. doi:10.1371/journal.pone.0143515
23. Liu GE. China Guidelines for Pharmacoeconomic Evaluations.
24. Carroll K. On the use and utility of the Weibull model in the analysis of survival data. Control Clin Trials. 2003;24:682–701. doi:10.1016/S0197-2456(03)00072-2
25. Diaby V, Adunlin G, Montero AJJP. Survival modeling for the estimation of transition probabilities in model-based economic evaluations in the absence of individual patient data: a tutorial. Pharmacoeconomics. 2014;32:101–108. doi:10.1007/s40273-013-0123-9
26. Zeng X, Peng L, Li J, et al. Cost-effectiveness of continuation maintenance pemetrexed after cisplatin and pemetrexed chemotherapy for advanced nonsquamous non-small-cell lung cancer: estimates from the perspective of the chinese health care system. Clin Ther. 2013;35:54–65. doi:10.1016/j.clinthera.2012.12.013
27. Zhao LY, Zhou ML, Zhi-Gang LU, et al. Pharmacoeconomic evaluation of two first-line therapies for advanced non-small cell lung cancer. Chin J New Drugs Clin Remedies. 2012;31:677–681. [in Chinese].
28. Zeng X, Li J, Peng L, et al. Economic outcomes of maintenance gefitinib for locally advanced/metastatic non-small-cell lung cancer with unknown EGFR mutations: a semi-Markov model analysis. Asia Pac J Clin Oncol. 2014;9:e88881.
29. Nafees B, Lloyd AJ, Dewilde S, et al. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13:e195–e203. doi:10.1111/ajco.12477
30. Xu J, Jin B, Chu T, et al. EGFR tyrosine kinase inhibitor (TKI) in patients with advanced non-small cell lung cancer (NSCLC) harboring uncommon EGFR mutations: a real-world study in China. Lung Cancer. 2016;96:87–92. doi:10.1016/j.lungcan.2016.01.018
31. Migliorino MR, Santo A, Romano G, et al. Economic burden of patients affected by non-small cell lung cancer (NSCLC): the LIFE study. J Cancer Res Clin Oncol. 2017;143:783–791. doi:10.1007/s00432-016-2326-x
32. Lakdawalla DN, Shafrin J, Hou N, et al. Predicting real-world effectiveness of cancer therapies using overall survival and progression-free survival from clinical trials: empirical evidence for the ASCO value framework. Value Health. 2017;20(7):866–875. doi:10.1016/j.jval.2017.04.003
33. National Institute for Health and Care Excellence. Afatinib for treating epidermal growth factor receptor mutation-positive locally advanced or metastatic non-small-cell lung cancer; 2014. Available from: https://www.nice.org.uk/guidance/ta310
34. Tan PT, Aziz MIA, Pearce F, et al. Cost effectiveness analysis of afatinib versus pemetrexed-cisplatin for first-line treatment of locally advanced or metastatic EGFR mutation positive non-small-cell lung cancer from the Singapore healthcare payer’s perspective. BMC Cancer. 2018;18:352. doi:10.1186/s12885-018-4223-y
35. Chouaid C, Luciani L, LeLay K, et al. Cost-effectiveness analysis of afatinib versus gefitinib for first-line treatment of advanced EGFR-mutated advanced non-small cell lung cancers. J Thorac Oncol. 2017;12:1496–1502. doi:10.1016/j.jtho.2017.07.013
36. Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. doi:10.1186/1477-7525-6-84
37. MacEwan JP, Doctor J, Muliqan K, et al. The value of progression-free survival in metastatic breast cancer: results from a survey of patients and providers. MDM Policy Pract. 2019;4(1):1–14. doi:10.1177/2381468319855386
38. Shafrin J, Schwartz TT, Okoro T, et al. Patient versus physician valuation of durable survival gains: implications for value framework assessments. Value Health. 2017;20(2):217–223. doi:10.1016/j.jval.2016.11.028
39. Larry Jameson J, Longo DLJO, Survey G. Precision medicine – personalized, problematic, and promising. N Engl J Med. 2015;70:612–614.
40. Xiaohui Z, Jianhe L, Liubao P, et al. Economic outcomes of maintenance gefitinib for locally advanced/metastatic non-small-cell lung cancer with unknown EGFR mutations: a semi-markov model analysis. PLoS One. 2014;9:e88881. doi:10.1371/journal.pone.0088881
41. Beauchemin C, Letarte N, Mathurin K, et al. A global economic model to assess the cost-effectiveness of new treatments for advanced breast cancer in Canada. J Med Econ. 2016;19:619–629. doi:10.3111/13696998.2016.1151431
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.