Back to Journals » Cancer Management and Research » Volume 13
Cost-Effectiveness Analysis of Camrelizumab Immunotherapy versus Docetaxel or Irinotecan Chemotherapy as Second-Line Therapy for Advanced or Metastatic Esophageal Squamous Cell Carcinoma
Authors Lin YT , Chen Y, Liu TX , Kuang F, Huang P
Received 3 September 2021
Accepted for publication 24 October 2021
Published 2 November 2021 Volume 2021:13 Pages 8219—8230
DOI https://doi.org/10.2147/CMAR.S335515
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Ying-Tao Lin,1 Ying Chen,2 Tian-Xiu Liu,3 Fang Kuang,1 Ping Huang1
1Administration Office of Drug Clinical Trial, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, People’s Republic of China; 2Management Office of Science and Technology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, 350014, People’s Republic of China; 3Department of Thoracic Radiotherapy, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, 350014, People’s Republic of China
Correspondence: Ping Huang
Administration Office of Drug Clinical Trial, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, People’s Republic of China
Tel +86 13960908650
Fax +86 0591 62752517
Email [email protected]
Purpose: The aim of this study was to assess the cost-effectiveness of camrelizumab immunotherapy versus docetaxel or irinotecan chemotherapy as second-line therapy for advanced esophageal squamous cell carcinoma (ESCC), which was evaluated in the ESCORT trial.
Materials and Methods: A partitioned survival model was developed to reflect the costs and effectiveness of the ESCORT trial. The clinical efficacy data, safety data, and health-related costs and utilities were derived from published data from clinical trials or health administration departments in China. Adverse event-related costs, drug administration, and other expenses were derived from a single center of Fujian Medical University Cancer Hospital in 2021. All survival analyses were performed with SPSS software. Overall survival was estimated with the Kaplan–Meier method, and progression-free survival was estimated with the life table method. Sensitivity analyses were conducted to assess the uncertainty of the model. Incremental cost, quality-adjusted life years (QALYs), and the incremental cost-effectiveness ratio (ICER) were calculated.
Results: Camrelizumab therapy had 0.232 QALYs at an incremental cost of USD$9959.44 compared with the chemotherapy group with 0.158 QALYs at an incremental cost of USD$8601.67. The ICER was USD$18393.12/QALY. Probabilistic sensitivity analyses showed that when the willingness-to-pay threshold reached USD$31200/QALY, which is nearly three times the Chinese gross domestic product per capita, camrelizumab had an 80% possibility of being cost-effective versus docetaxel or irinotecan chemotherapy.
Conclusion: Camrelizumab is a cost-effective option compared with docetaxel or irinotecan chemotherapy in patients with advanced ESCC as second-line therapy in China.
Keywords: camrelizumab, chemotherapy, cost-effectiveness, esophageal squamous cell carcinoma
Plain Language Summary
Significant Findings of the Study
Camrelizumab was a more cost-effective treatment option than chemotherapy for patients with advanced esophageal squamous cell carcinoma as second-line therapy from the perspective of Chinese society.
What This Study Adds
Since healthcare-related costs have become one of the most severe problems worldwide, this study provides an alternative, cost-effective treatment option to help alleviate the burden on patients.
Introduction
Esophageal cancer is the seventh most common cancer worldwide and the sixth most common cause of cancer-related deaths.1,2 The incidence, prevalence, and histologic type of esophageal cancer vary between geographic regions, especially among the United States, Europe, and areas commonly referred to as “the esophageal cancer belt,” which is a geographical area that extends from the Caspian Sea to northern China and across Central Asia and East Asia.3 It was estimated that 477,900 people in China will be diagnosed with esophageal cancer, 90% of which will be histologically identified as squamous cell carcinoma and 375,000 of these patients will die from the disease.4,5 Advanced esophageal cancer is a rapidly fatal disease.6 There is no consensus on the optimal second-line treatment for advanced or metastatic esophageal cancer.7,8 Chemotherapy monotherapy is the typical treatment for advanced esophageal cancer, including paclitaxel, docetaxel, or irinotecan.9–11 Some studies have summarized data from retrospective analyses,12–14 and based on these research caveats, the objective response rate and median survival appear to be comparable among patients treated with paclitaxel, docetaxel, and irinotecan.15
With rapid improvements in therapy, immunotherapy has recently become a new method for the treatment of cancer patients; however, these treatments are associated with high costs, which have therefore increased the social financial burden.16 In addition to therapy effectiveness, the economic benefit is also an important consideration in treatment options and healthcare policymaking. Therefore, there is an urgent need to assess the economic impact of these immunotherapeutics on healthcare to ensure the effectiveness of resource use. Camrelizumab, a programmed cell death 1 (PD-1) inhibitor, has been approved for the treatment of advanced esophageal squamous cell carcinoma in China. The published data of the ESCORT17 clinical trial reported encouraging clinical efficacy of treatment with camrelizumab, which conferred longer overall survival (OS) compared with that of patients treated with chemotherapy using docetaxel or irinotecan. The aim of the present study was to evaluate the cost-effectiveness of camrelizumab immunotherapy and docetaxel/irinotecan chemotherapy options by quantifying and comparing the therapy cost and effectiveness from the perspective of Chinese society based on ESCORT trial data.
Methods
Target Population
The target population in the model was the cohort included in the ESCORT clinical trial, which was a randomized, open-label, phase-3 study conducted in China.17 Forty-three hospitals recruited 457 eligible patients who were randomly assigned to a treatment arm. A total of 448 patients who received at least one cycle of treatment were finally enrolled, including 228 patients in the camrelizumab group and 220 patients in the chemotherapy group. Eligible patients were at least 18 years of age (average age of 60 years); with a confirmed histological or cytological diagnosis of esophageal squamous cell carcinoma (ESCC); experienced tumor progression after first-line chemotherapy, including chemoradiotherapy (patients who experienced tumor progression during or within 6 months after radical chemoradiotherapy or [neo]adjuvant therapy were also eligible); had at least one measurable lesion; and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1.17
Model Construction
This study established a partitioned survival model to evaluate the cost-effectiveness of treatment with camrelizumab versus docetaxel or irinotecan chemotherapy. This model has previously been applied for metastatic cancer and has also been used to estimate health outcomes and costs for each regimen in a specified patient population.18,19 We modeled three mutually exclusive health stages (Figure 1). Patients started in the progression-free (PF) stage until disease progression or death (whichever came first). The progressive disease (PD) stage was the encompassing stage after the first progression, which may remain in the PD stage or enter the death stage at the end of each model cycle but could not return to the PF stage. The death state was the final absorbing stage and could not return to the previous stage.
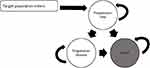 |
Figure 1 Transition diagram for partitioned survival model health outcomes. |
The three mutually exclusive health stages were calculated as follows: progression-free survival (PFS) = proportion of patients with PFS from the PFS curve; PD = proportion of alive patients from the OS curve – the proportion of patients with PFS from the PFS curve; and death = 1 – the proportion of alive patients from the OS curve.18
Cost
In the model, the cost price exchange for the USD was calculated according to the rate of June 2021. The study drug in the ESCORT trial was provided by Jiangsu Hengrui Medicine. The listed drug prices were made available by the National Health Commission of the People’s Republic of China in 2021: camrelizumab cost USD$453 per 200 mg, docetaxel cost USD$46 per 0.5 mL:20 mg and USD$95 per 1.5 mL:60 mg, and irinotecan cost USD$76 per 40 mg and USD$148 per 100 mg. Camrelizumab was administered intravenously at a dose of 200 mg on day 1 of each 14-day cycle. The chemotherapy group was administered docetaxel at a dose of 75 mg/m2 on day 1 of each 21-day cycle or irinotecan at a dose of 180 mg/m2 on day 1 of each 14-day cycle. The chemotherapy group included 43 patients treated with docetaxel and 177 patients treated with irinotecan. Treatment continued until disease progression, defined according to Response Evaluation Criteria In Solid Tumors, version 1.1 (RECIST1.1), unacceptable toxicity, patient withdrawal, or investigator decision, whichever occurred first.
Drug acquisition included the dose received in both the camrelizumab and chemotherapy groups. The cost was calculated for whole vials rounded up at the patient level. Camrelizumab was set at a fixed dose of 200 mg, and the average dose of the chemotherapy group was docetaxel 115 mg per patient and 275 mg irinotecan per patient, based on the average body surface area of 1.53 m2 in the ESCORT trial.
Drug administration costs were assessed separately in the camrelizumab and chemotherapy groups. The prices according to the trial payer’s standard were calculated from the standard fee of Fujian Medical University Cancer Hospital and Fujian Provincial Health Commission in 2021. These costs include costs for preventive medication, hospitalization, nursing, drug infusion, laboratory tests, scans, oncology visits, and other resources used in the different health states.
Adverse event (AE)-related costs were calculated separately for the camrelizumab and chemotherapy groups, and the prices were also taken from Fujian Medical University Cancer Hospital and Fujian Provincial Health Commission in 2021. An AE was defined as any unfavorable and unintended sign, abnormal laboratory results, symptoms, or new or exacerbated disease temporally associated with use of the study drug.20–22 The time horizon for AE analysis was the occurrence or exacerbation on the day or the day after the first administration of the study drug, and not later than 90 days after the last use of the study drug. In the model, we chose 19 patients enrolled in the ESCORT trial of Fujian Medical University Cancer Hospital and calculated their ≥1 grade AE-related treatment cost from the medical records.
Terminal costs were defined as the one-time cost of expenditure on funeral expenses arising from burial, as estimated in the interpretation of the Supreme People’s Court on some issues concerning the application of law to the trial of cases of compensation for personal injury.23,24
Utility Scores
Utility scores were determined based on the global health status quality-of-life scores of the EORTC QLQ-C30 questionnaire.25 The global health status quality-of-life scale was self-evaluated by patients and calculated in accordance with instrument guidelines by the statistical team in the ESCORT trial.17 The standard score at baseline was 65.7 per patient, which was converted to 0.657 and used as the utility score in our model. Deterioration in the quality of life is inevitable during and beyond second-line therapy due to worsening symptoms or decreased functioning.26 Therefore, the utility score will decline linearly from the point of progression to the point of death. During PD, the average utility score from PFS to death was considered, and the utility score of death was defined as 0. Quality-adjusted life years (QALYs) were calculated using the formula utility score × survival duration time.27
Time Horizon and Discount Rate
The time horizon of the model simulated the actual progress of the ESCORT trial, and the length of each model cycle was defined as one month. According to the Second Edition of Cost-Effectiveness in Health and Medicine,28 costs and outcomes had a discounted rate of 3% per year. To conduct the cost-effectiveness assessment, the model was used to project costs, QALYs, and the incremental cost per QALY gained associated with using camrelizumab versus docetaxel or irinotecan as second-line therapy.
Sensitivity Analyses
A deterministic sensitivity analysis was carried out to take all input parameters of the model and change them by 10% in both directions. When one input parameter was changed, the other input parameters were kept constant. Probabilistic sensitivity analysis was performed using Monte Carlo simulation. A total of 1000 simulated iterations were run, and the values for both groups were sampled at random from the normal distribution based on the trial sample means and their standard deviation.
Statistical Analysis
The model was built using Excel 2019 and SPSS 26.0. Excel was used to calculate the cost and utility in the three mutually exclusive health states, as well as to perform the deterministic sensitivity analyses and probabilistic sensitivity analyses. In the ESCORT trial, the survival data were compared between treatment groups using SAS 9.4. OS and PFS were estimated with the Kaplan-Meier method, and 95% CIs were calculated using the Brookmeyer-Crowley method; significance was assessed with the Log rank test at the 0.05 threshold stratified by randomization strata.17 We adopted a similar survival analysis method as used in the ESCORT trial for our model rather than assuming the distribution of survival curves to avoid potential bias. In our model, the survival data were done with SPSS 26.0. OS was estimated with the Kaplan-Meier method; 95% CIs were calculated using Brookmeyer-Crowley method; significance was assessed with the Log rank test at the 0.05 threshold stratified by randomization strata. But, PFS was estimated with the life table method.
Results
Base-Care Analysis
Up to the cut-off point of the ESCORT clinical trial data, the median overall survival was 8.3 months (95% CI 6.8–9.7) in the camrelizumab group and was 6.2 months (95% CI 5.7–6.9) in the chemotherapy group (stratified log-rank p = 0.001). The median PFS was 1.9 months (95% CI 1.9–2.4) in the camrelizumab group and was 1.9 months (95% CI 1.9–2.1) for those treated with docetaxel or irinotecan chemotherapy.17
The survival analysis results of our model demonstrated were very similar to the actual clinical trial data. In the model, the median OS was 8 months (95% CI 6.57–9.42) in the camrelizumab group and was 6 months (95% CI 5.39–6.61) in the chemotherapy group, and the mean OS was 10.05 months (95% CI 9.02–11.08) in the camrelizumab group versus 7.82 months (95% CI 6.97–8.67) in the chemotherapy group (stratified log-rank p = 0.002) (Figure 2). The PFS was 1.95 months in the camrelizumab group compared with 1.97 months in the chemotherapy group (Figure 3).
 |
Figure 2 Estimated overall survival curve for the ESCORT trial. |
 |
Figure 3 Estimated progression survival curve for the ESCORT trial. |
After randomization, patients entered the first stage of the model (camrelizumab group or chemotherapy group). Before the first drug infusion, laboratory tests and scans were performed to confirm the physical condition and assess the tumor, such as hematology, serum chemistry, urinalysis, coagulation, thyroid function, electrocardiogram, computed tomography (CT), and magnetic resonance imaging (MRI). Before the second and other drug infusions, routine medical inspections were performed, including hematology, serum chemistry, and electrocardiogram. Once PD was confirmed by CT or MRI, the patient discontinued the drug infusion. During the disease progression to death, the patient did not have drug acquisition and drug administration costs, but had hematology, serum chemistry, and other routine medical inspections. Thus, cost and outcome data were calculated from the baseline to the terminal stage (Table 1). Treatment-emergent AEs in Fujian Medical University Cancer Hospital are summarized in Table 2.
 |
Table 1 Key Input Data of the Model |
 |
Table 2 Treatment-Emergent Adverse Events in Fujian Medical University Cancer Hospital |
Based on the results of the 2-year lifetime horizon, the camrelizumab group had a mean survival duration of 10 months, while the chemotherapy group had a mean survival of 7.8 months. The camrelizumab group gained an incremental cost and effect of USD$7146.17 and 0.19 life years (LYs) compared with the chemotherapy group. The camrelizumab group also gained an incremental cost and effect of USD$18,393.12 and 0.074 QALYs compared with the chemotherapy group (Table 3).
 |
Table 3 Key Outcome Data of the Model |
Sensitivity Analyses
Deterministic Sensitivity Analyses
The results of the one-way deterministic sensitivity analyses showed that the model was most sensitive to the survival rate of the camrelizumab group. Other parameters influencing the model were survival rate of the chemotherapy group, camrelizumab group lab and scan cost during the PF stage, camrelizumab group drug acquisition cost, utility scores, and chemotherapy group lab and scan cost during the PF stage. Drug administration costs in PD, terminal costs, AE-related costs, and discount rate had a weak influence on the model results (Figure 4).
 |
Figure 4 Tornado diagram for deterministic sensitivity analyses of The ICER per QALY. |
Probabilistic Sensitivity Analyses
The results from the Monte Carlo probabilistic sensitivity analyses showed that camrelizumab versus docetaxel or irinotecan chemotherapy had a 50% probability of being cost effective at a willingness-to-pay (WTP) threshold of USD$185,000/QALY. If the WTP threshold reached the value of USD$31,200/QALY, which is nearly three times the GDP per capita for China, the probability of camrelizumab being cost effective was 80% versus docetaxel or irinotecan chemotherapy (Figures 5 and 6).
 |
Figure 5 Scatter plot of Monte Carlo sensitivity analysis. |
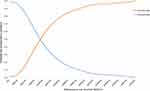 |
Figure 6 Cost-effectiveness acceptability curve for camrelizumab vs docetaxel or irinotecan chemotherapy. |
Discussion
In recent years, healthcare-related costs have become one of the most severe problems worldwide.29 Immunotherapeutic inhibitors have improved survival in ESCC therapy but could also significantly increase healthcare expenditures. Moreover, ESCC is associated with deterioration in the quality of life due to the poor prognosis even with immunotherapeutic treatment in clinical practice. Thus, models have been developed to evaluate the effect of immune inhibitors from an economic perspective. In health economic evaluation models, if a therapy option is to become cost effective, it must have two key attributes: lower cost and higher effectiveness. We modeled these attributes as a reduced incremental cost and increased incremental QALYs in the present study.
In the one-way sensitivity analyses, the most influential parameter was the survival rate of the camrelizumab group, in which a higher survival rate would result in higher treatment costs but also become more cost effective. In recent years, many clinical trial reports have shown that immunotherapeutics are associated with longer overall survival than chemotherapy in the second-line setting of ESCC therapy. The ATTRACTION-330 and KEYNOTE-18131 trials also showed encouraging clinical efficacy in patients with ESCC. Similar to the ESCORT, these two clinical trials demonstrated significantly prolonged OS in patients who received immunotherapy compared with those that received chemotherapy, with a difference of 10.9 versus 8.4 months in the ATTRACTION-3 trial and a difference of 8.4 months versus 5.6 months in the KEYNOTE-181 trial. The ESCORT trial reported clinical benefits with camrelizumab regardless of the level of PD-L1 expression; however, patients with higher PD-L1 expression seemed to benefit more than those with low PD-L1 expression. These results suggested that higher PD-L1 expression might be a useful screening marker to select patients for immunotherapy to achieve more cost-effectiveness in the second-line setting. This finding is comparable to those observed in the ATTRACTION-3 and KEYNOTE-181 studies. Thus, to improve the economic effectiveness of camrelizumab, it is essential to identify the most suitable patients with the best survival benefits for the immunotherapeutic checkpoint.
The present model explored the effect of camrelizumab from the economic profile, and the incremental cost-effectiveness ratio (ICER) for camrelizumab versus docetaxel or irinotecan chemotherapy was USD$18,393.12/QALY in China. This main result showed that camrelizumab is a cost-effective second-line therapy option for patients with advanced ESCC compared to docetaxel or irinotecan chemotherapy in China. In addition, the results of the probabilistic sensitivity analyses indicated that camrelizumab was the advantageous option from a WTP threshold of USD$185,000/QALY. When the WTP threshold reached USD$31,200/QALY, camrelizumab had an 80% probability of becoming the advantageous option. In 2020, China’s per capita GDP was USD$10,504. The ICER is lower than the World Health Organization threshold of three times the GDP per capita for China,32 which is currently at USD$31,512/QALY. These results support that camrelizumab is a cost-effective second-line treatment option for advanced ESCC therapy in China.
The ATTRACTION-3 clinical trial also recently reported an economic evaluation. This trial included 419 patients with advanced ESCC treated with nivolumab or chemotherapy, who were mainly from Japan and South Korea. The results showed that nivolumab monotherapy might not be a more cost-effective option than chemotherapy for these patients at the Chinese WTP threshold.33 The mean incremental effect and cost were 0.107 QALYs and USD$14,627.90 for the nivolumab group, whereas the ICER for nivolumab versus chemotherapy was USD$136,709.35/QALY.
The ATTRACTION-3 trial had many similarities to the ESCORT trial, which included both clinical and economic data. First, the two trials used chemotherapy as the standard treatment and had a similar target population, along with general results with respect to OR, PFS, and hazard ratios, among others. The patients in the ATTRACTION-3 trial were treated with nivolumab at a dose of 240 mg every 2 weeks versus paclitaxel at 100 mg/m2 once per week or docetaxel at 75 mg/m2 every 3 weeks until disease progression or unacceptable toxicity. The patients in the nivolumab group had a significantly longer median OS than those in the chemotherapy group but had shorter PFS. Second, the two studies involved similar docetaxel acquisition, laboratory tests, and scan prices. Third, the two studies resulted in similar incremental effectiveness (0.107 QALYs versus 0.074 QALYs in our study).
However, the economic evaluation results of cost and effectiveness from the ATTRACTION-3 trial are inconsistent with our findings based on the ESCORT trial. The differences between the two studies could be mainly explained by the relatively low cost of camrelizumab. Although the price of nivolumab in China is lower than that in several other countries, camrelizumab still has a 68.35% lower price than nivolumab. In the one-way sensitivity analyses, the model was sensitive to the cost of camrelizumab. If the price of camrelizumab rises to be the same as that of nivolumab, the result of our study will also likely change to indicate that it is not cost effective. In recent years, there has been a deep revolution in medical acquisition in China, which aims to produce drugs with low price and high efficacy. The price of immune inhibitors has declined sharply. A series of Chinese domestic immune inhibitors (toripalimab, sintilimab, and tislelizumab) have also been developed, which are available at much lower prices than pembrolizumab and nivolumab. With the development of the pharmaceutical manufacturing industry, an increasing number of immune inhibitors with lower prices but equal efficacy to pembrolizumab and nivolumab are expected to emerge, making immune inhibitors a more cost-effective option for patients with advanced ESCC in second-line therapy.
Several strengths and limitations of our analyses should be addressed. The major strength of this study is the direct comparison of camrelizumab and docetaxel or irinotecan chemotherapy using the original published trial data, along with clinical costs, financial data, and utility parameters based on real clinical practice and found in the references of the ESCORT trial report. The survival analysis results from our model showed a very similar result to the actual data from the ESCORT based on the Kaplan-Meier method and the life table method. Nevertheless, this study has some limitations. First, the model based on the clinical trial may not fully reflect the disease course in the real world. Second, the AE-related cost was estimated using medical data from one hospital, which might undermine the robustness of the model. Fortunately, the results of the deterministic sensitivity analyses showed that the assessment results were not sensitive to AE-related parameters. Third, utility scores of the baseline cycle in the study were derived from the global health status quality-of-life scores of the EORTC QLQ-C30 questionnaire, which might have led to bias in the model outcomes. Finally, the model results were compared with those of the ATTRACTION-3 trial for interpretation, although patients in the ATTRACTION-3 trial were mainly from Japan and South Korea, which might undermine the robustness of the economic evaluation.
Conclusion
A partitioned survival model was constructed to explore the cost-effectiveness of camrelizumab versus chemotherapy in patients with advanced ESCC. Based on our results, camrelizumab is a cost-effective option compared with docetaxel or irinotecan chemotherapy in patients with advanced ESCC as second-line therapy from the perspective of Chinese society.
Ethical Conduct of Research
The study for investigations involving human subjects and informed consent was obtained from the Ethics Committee of Fujian Cancer Hospital.
Acknowledgments
This study was supported by the Startup Fund for Scientific Research, Fujian Medical University (Grant number: 2019QH1201).
Disclosure
The authors declare no conflicts of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi:10.1002/ijc.31937
3. Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. doi:10.1136/gutjnl-2014-308124
4. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
5. Li M, Wan X, Wang Y, Sun Y, Yang G, Wang L. Time trends of esophageal and gastric cancer mortality in China, 1991–2009: an age-period-cohort analysis. Sci Rep. 2017;7(1):6797. doi:10.1038/s41598-017-07071-5
6. Drahos J, Wu M, Anderson WF, et al. Regional variations in esophageal cancer rates by census region in the United States, 1999–2008. PLoS One. 2013;8(7):e67913. doi:10.1371/journal.pone.0067913
7. Lordick F, Mariette C, Haustermans K, Obermannova R, Arnold D. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v50–v57. doi:10.1093/annonc/mdw329
8. Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi51–vi56. doi:10.1093/annonc/mdt342
9. Thallinger CM, Raderer M, Hejna M. Esophageal cancer: a critical evaluation of systemic second-line therapy. J Clin Oncol. 2011;29(35):4709–4714. doi:10.1200/JCO.2011.36.7599
10. Song Z, Zhang Y. Second-line docetaxel-based chemotherapy after failure of fluorouracil-based first-line treatment for advanced esophageal squamous cell carcinoma. Onco Targets Ther. 2014;7:1875–1881.
11. Burkart C, Bokemeyer C, Klump B, Pereira P, Teichmann R, Hartmann JT. A Phase II trial of weekly irinotecan in cisplatin-refractory esophageal cancer. Anticancer Res. 2007;27(4C):2845–2848.
12. Mizota A, Shitara K, Kondo C, et al. A retrospective comparison of docetaxel and paclitaxel for patients with advanced or recurrent esophageal cancer who previously received platinum-based chemotherapy. Oncology. 2011;81(3–4):237–242. doi:10.1159/000334057
13. Albertsson M, Johansson B, Friesland S, et al. Phase II studies on docetaxel alone every third week, or weekly in combination with gemcitabine in patients with primary locally advanced, metastatic, or recurrent esophageal cancer. Med Oncol. 2007;24(4):407–412. doi:10.1007/s12032-007-0028-6
14. Shirakawa T, Kato K, Nagashima K, et al. A retrospective study of docetaxel or paclitaxel in patients with advanced or recurrent esophageal squamous cell carcinoma who previously received fluoropyrimidine- and platinum-based chemotherapy. Cancer Chemother Pharmacol. 2014;74(6):1207–1215. doi:10.1007/s00280-014-2597-3
15. Grunberger B, Raderer M, Schmidinger M, Hejna M. Palliative chemotherapy for recurrent and metastatic esophageal cancer. Anticancer Res. 2007;27(4C):2705–2714.
16. Tartari F, Santoni M, Burattini L, Mazzanti P, Onofri A, Berardi R. Economic sustainability of anti-PD-1 agents nivolumab and pembrolizumab in cancer patients: recent insights and future challenges. Cancer Treat Rev. 2016;48:20–24. doi:10.1016/j.ctrv.2016.06.002
17. Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, Phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi:10.1016/S1470-2045(20)30110-8
18. Insinga RP, Vanness DJ, Feliciano JL, Vandormael K, Traore S, Burke T. Cost-effectiveness of pembrolizumab in combination with chemotherapy in the 1st line treatment of non-squamous NSCLC in the US. J Med Econ. 2018;21(12):1191–1205. doi:10.1080/13696998.2018.1521416
19. Chouaid C, Bensimon L, Clay E, et al. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer. 2019;127:44–52. doi:10.1016/j.lungcan.2018.11.008
20. Health NIo. Common terminology criteria for adverse events (CTCAE) version 4.03; 2010. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
21. Liu YJ, Zhu GP, Guan XY. Comparison of the NCI-CTCAE version 4.0 and version 3.0 in assessing chemoradiation-induced oral mucositis for locally advanced nasopharyngeal carcinoma. Oral Oncol. 2012;48(6):554–559. doi:10.1016/j.oraloncology.2012.01.004
22. Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). 2021;112(1):90–92. doi:10.1016/j.ad.2019.05.009
23. Wong IO, Kuntz KM, Cowling BJ, Lam CL, Leung GM. Cost effectiveness of mammography screening for Chinese women. Cancer. 2007;110(4):885–895. doi:10.1002/cncr.22848
24. The Supreme People’s Court of The People’s Republic of China. The Supreme People’s Court on some issues concerning the application of law to the trial of cases of compensation for personal injury; 2017. Available from: http://www.court.gov.cn/fabu-xiangqing-73512.html.
25. Fayers PM, Bjordal K, Groenvold M, Curran D, Bottomley A. The EORTC QLQ-C30 Scoring, Manual.
26. van Kleef JJ, Ter Veer E, van den Boorn HG, et al. Quality of life during palliative systemic therapy for esophagogastric cancer: systematic review and meta-analysis. J Natl Cancer Inst. 2020;112(1):12–29.
27. Janmaat VT, Bruno MJ, Polinder S, et al. Cost-effectiveness of cetuximab for advanced esophageal squamous cell carcinoma. PLoS One. 2016;11(4):e0153943. doi:10.1371/journal.pone.0153943
28. Nyman JA. Cost recommendations in the second edition of cost-effectiveness in health and medicine: a review. MDM Policy Pract. 2018;3(1):2381468318765162.
29. Dieleman JL, Campbell M, Chapin A, et al. Future and potential spending on health 2015–40: development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet. 2017;389(10083):2005–2030. doi:10.1016/S0140-6736(17)30873-5
30. Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi:10.1016/S1470-2045(19)30626-6
31. Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi:10.1200/JCO.20.01888
32. Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 2000;9(3):235–251. doi:10.1002/(SICI)1099-1050(200004)9:3<235::AID-HEC502>3.0.CO;2-O
33. Zhang PF, Xie D, Li Q. Cost-effectiveness analysis of nivolumab in the second-line treatment for advanced esophageal squamous cell carcinoma. Future Oncol. 2020;16(17):1189–1198. doi:10.2217/fon-2019-0821
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
