Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Correlation of Serum Clara Cell Secretory Protein 16, Plasma Fibrinogen and Serum Amyloid A with the Severity of Acute Exacerbated COPD and Their Combination in Prognosis Assessment
Authors Hu X, Xu J, Li P, Zheng H
Received 3 March 2023
Accepted for publication 25 June 2023
Published 6 September 2023 Volume 2023:18 Pages 1949—1957
DOI https://doi.org/10.2147/COPD.S410917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Xiaojuan Hu,1,* Jin Xu,1,* Pei Li,2 Hui Zheng3
1Department of Pulmonary and Critical Care Medicine, Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, Shanghai, 200434, People’s Republic of China; 2Department of Nephrology, the Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050004, People’s Republic of China; 3Department of Clinical Laboratory, Fudan University Shanghai Cancer Center, Shanghai, 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaojuan Hu, Department of Pulmonary and Critical Care Medicine, Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, Shanghai, 200434, People’s Republic of China, Tel +86-13524811465, Email [email protected] Hui Zheng, Department of Clinical Laboratory, Fudan University Shanghai Cancer Center, Shanghai, 200032, People’s Republic of China, Tel +86-18017317741, Email [email protected]
Introduction: Chronic obstructive pulmonary disease (COPD) has tremendous detrimental effects on patients’ quality of life, lung function, disease progression and socioeconomic burden. This study aimed to investigate new serum biomarkers for COPD detection. Three recently emerging biomarkers, including Clara cell secretory protein⁃16 (CC16), plasma fibrinogen (FIB) and serum amyloid A (SAA), were investigated for their potential in stratifying the severity of COPD.
Methods: A total of 220 patients with AECOPD were recruited. Multivariate logistical regression was used to analyze odds ratios of an array of characteristic of patients, including age, global initiative for chronic obstructive lung disease (GOLD), diabetes mellitus, heart diseases, PaCO2, CC16, FIB, and SAA. Correlations of CC16, FIB and SAA levels to each other, GOLD, and PaCO2 were also measured using Spearman correlation. Receiver operating characteristic (ROC)/curve analysis was used to assess sensitivity and specificity of CC16, FIB, SAA and the combination of the three markers in identifying AECOPD patients with poor prognosis.
Results: Our data suggested that age, GOLD, diabetes mellitus, heart diseases, PaCO2, CC16, FIB, and SAA are all significant risk factors for poor prognosis of AECOPD. CC16, FIB and SAA were positively correlated to each other and to GOLD and PaCO2 levels. CC16, FIB and SAA all had a high sensitivity and specificity in identifying patients with a poor prognosis. CC16, FIB and SAA are new markers with potentially high predictive value in AECOPD.
Discussion: Our data support further development of these biomarkers to improve clinical management of AECOPD through providing more accurate prognosis of AECOPD patients that enable timely adjustment of treatment plans.
Keywords: AECOPD, CC16, FIB, SAA
Introduction
Chronic obstructive pulmonary disease (COPD) has tremendous detrimental effects on patients’ quality of life, lung function, disease progression and socioeconomic burden.1 COPD is known to be triggered by cold weather, pollutants, or irritants and exacerbated by acute respiratory tract infection.2 Patients in the acute phase of COPD, acute exacerbation COPD (AECOPD), suffer from elevated airway inflammation,3 which in turn progresses into bronchial mucosal congestion and edema, excessive mucus secretion, smooth muscle spasm, and aggravates airway narrowing, leading to restricted expiratory airflow and dynamic hyperinflation of the lungs. Patients may also experience acute changes in shortness of breath, cough or sputum, demanding abrupt change of treatment strategies. As a result, acute exacerbation symptoms could occur, and may lead to a systemic inflammatory response.4 Despite recent advancement in AECOPD treatment, the overall efficacy of AECOPD treatment is still unsatisfactory. There is a pressing unmet need to develop simple but efficient physiological indicators to assess the prognosis of patients with AECOPD, and to take corresponding measures for intervention and treatment to improve the prognosis of patients.5
Serum biomarkers are favored diagnostic tools owing to their cost-effectiveness and convenience. Emerging studies demonstrated a few serum biomarkers, such as C-reactive protein (CRP),6 I-FABP, citrulline, D-Lactate, DAO, and α-GST7 as biomarkers with predictive value in terms of readmission. However, the sensitivity and specificity of the biomarkers remain unsatisfactory. For example, a previous study demonstrated that CRP could achieve a sensitivity of 74.5% and specificity of 81% for readmission events among AECOPD patients.6 Among new biomarkers investigated, serum amyloid A (SAA), which is a polygenic encoded acute temporal response protein, has stood out as patients in the early stage of viral pneumonia,8 early non-invasive bacterial pneumonia,9 COVID19,10 rheumatic diseases,11 etc.
In addition, fibrinogen (FIB) is another promising serum biomarker for AECOPD as suggested in a previous study.12 FIB is a glycoprotein synthesized by hepatocytes and its biological functions are closely related to blood coagulation, cell adhesion, proliferation and extension, phagocytosis and other activities.13 Its molecular weight is the largest among all coagulogens, and it has a chain-like asymmetric structure, which can increase plasma thickening, contribute to the formation of tiny arterial thrombi in the lungs, and aggravate the injury to the lung. Studies suggested that AECOPD patients had a high FIB level at the time of discharge implicating that the patient is still in a state of hypoxia and thus has a higher likelihood of respiratory failure and readmission.14
Another biomarker focused in our study is Clara cell secretory protein-16 (CC16), a major functional protein secreted by Clara cells.15 In COPD, inflammation, oxidative stress and increased alveolar⁃capillary permeability significantly enhance the expression of CC16. CC16 can inhibit phospholipase A2 activity and reduce cell membrane phospholipid catabolism, and also directly inhibit the expression of various inflammatory factors and their biological activities, which play an important role in the development mechanism of asthma, COPD, and acute lung injury.16
In this work, we aimed to characterize the levels of serum CC16, SAA and plasma FIB as a new set of serum biomarkers for AECOPD diagnosis. A retrospective analysis was performed to follow up patients with AECOPD for a period of one year, and the patients were divided into two groups with good and poor prognosis, and to analyze the comparison between the levels of serum CC16, SAA and plasma FIB at admission in AECOPD patients with good and poor prognosis, and to analyze the correlation between the three. The predictive value of the three and their combined tests for prognosis was analyzed. The relationship between the three biomarkers and the severity of AECOPD at the time of admission was then analyzed.
Methods
Subject Enrollment
Our study was approved by the ethics committee of Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, and it was performed in strict accordance with the Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects. All enrolled patients met the diagnostic criteria for AECOPD according to the Guidelines for the Diagnosis and Management of Chronic Obstructive Pulmonary Disease (2013 Revised Edition) developed by the Chinese Medical Association’s Respiratory Diseases Branch in 2007. All patients had acute worsening of respiratory symptoms such as dyspnea, cough or sputum pus and confirmed diagnosis of AECOPD and age ≥18 years. Smoker was defined as who smokes continuously or accumulatively for ≥6 months. The patients gave written informed consent to this study.
Exclusion Criteria
The following patients were excluded: 1) patients with non-acute exacerbation COPD, combined with liver disease, urinary system disease, hematological system disease, tumor; 2) patients with asthma, interstitial lung disease, pneumonia, tuberculosis, lung cancer, pulmonary embolism and other respiratory system diseases; 3) patients suffering from heart failure, acute myocardial infarction, acute cerebral infarction, acute cerebral hemorrhage and other end-stage diseases.
Grading for AECOPD
Pulmonary function classification was defined as airflow impairment with reference to the criteria of the Chinese Expert Consensus on the Diagnosis and Treatment of Acute Exacerbations of Chronic Obstructive Pulmonary Disease, and the severity was divided into 4 grades: Grade I (mild): FEV1% ≥ 80%; Grade II (moderate): 50% ≤ FEV1% < 79%; Grade III (severe): 30% ≤ FEV1% < 50%; Grade IV (very severe): FEV1% < 30%. FEV1 and FVC were measured by Masterscope lung function meter (GOLD: global initiative for chronic obstructive lung disease) from Jäger, Germany.
Patient Follow-Up
Patients discharged from the hospital after treatment for AECOPD were followed up for 1 year. Patients were considered to have a poor prognosis for worsening AECOPD if any of the following occurred: 1) death from respiratory disease during hospitalization or follow-up; 2) intensive care unit admission; 3) invasive or noninvasive mechanical ventilation; 4) COPD-related emergency visits or readmissions during follow-up.
Blood Test
Five milliliters of venous blood were drawn within 24 h of admission, and plasma was separated and stored in a refrigerator at −80°C. 0.13 mol/L sodium citrate was used for anticoagulation. Blood was centrifuged at 3000 r/min for 10 min, and plasma FIB levels were measured by a kit purchased from Fischer Diagnostics Pacific Coagulation Products. Serum CC16 levels were measured by an enzyme-linked immunosorbent assay kit purchased from Emmett BioVendor. SAA was measured by serum using Abbott Biochemistry Analyzer c16000 (Abbott, USA). Fresh arterial blood was used for measuring carbon dioxide levels (PaCO2).
Statistical Analysis
The sample size was determined using established statistical power analysis (probability that it will reject a false null hypothesis). Differences between the means of the two groups were divided by the standard deviation to determine the standardized effect size, then using 5% as the significance level in Student’s t-test and 90% power, the minimum required sample size was calculated. The data are presented with mean ± SD or n (percentage). The comparisons of data were done by Mann–Whitney test, Unpaired t test with Welch’s correction or Fisher’s exact test. Anderson-Darling test, D’Agostino & Pearson test, Shapiro–Wilk test, and Kolmogorov–Smirnov test were used for normality of the data before analysis. For analyzing those with normal distribution, unpaired t test with Welch’s correction. Otherwise, Mann–Whitney test was used. The comparison of categorical data was performed using the chi-square test Fisher’s exact test. Correlation test (Spearman correlation) was used because the analyzed data did not conform to a normal distribution.
Results
Study Design and Subjects
In our trial, we enrolled 220 eligible patients with AECOPD and after one year of follow-up, 151 patients had a good prognosis and 69 patients had a poor prognosis (Table 1). Sixty-nine patients had a poor prognosis, of which 19 (27.5%) died, accounting for 8.6% of all patients. A comparison of serum CC16 plasma FIB and SAA at admission between the 69 patients with poor prognosis who died and those who survived is shown in Figure S1. The comparison revealed no significant differences in each parameter between the two groups. We compared patients’ age, gender, BMI, duration of COPD, pulmonary function class (GOLD class), and whether they had comorbidities including diabetes, hypertension, hyperlipidemia, or coronary artery disease. The patients’ smoking history, partial pressure of carbon dioxide, CC16, FIB, and SAA at the time of admission were compared. Our results showed significant differences between the two groups in terms of patient age, GOLD classification status, presence of diabetes, coronary artery disease, partial pressure of carbon dioxide at admission, and serum CC16, plasma FIB, and SAA.
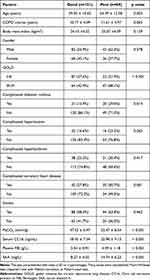 |
Table 1 Analysis of Prognostic Factors in Patients with Acute Exacerbated Chronic Obstructive Pulmonary Disease (AECOPD) |
Multivariate Logistical Regression Analysis to Identify Risk Factors Associated with Poor Prognosis of AECOPD
Table 2 shows a multifactorial regression analysis of risk factors associated with poor prognosis in patients with AECOPD. Among independent variables, age >65 years, GOLD classification of grade III–IV, diabetes mellitus, coronary artery disease, partial pressure of carbon dioxide >50 mmHg, serum CC16 >20 ng/mL, plasma FIB > 4 g/L, and SAA > 10 mg/L were significant risk factors for poor prognosis in patients with AECOPD. The cut-off values were determined using ROC analysis and Youden’s index (Table 3).
 |
Table 2 Multivariate Logistic Analysis for Poor Prognosis in Patients with Acute Exacerbated Chronic Obstructive Pulmonary Disease (AECOPD) |
 |
Table 3 Predictive Values in ROC Analysis |
Correlations of Serum CC16, Plasma FIB and SAA at Admission to Prognosis of AECOPD Patients
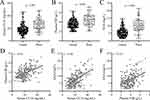
After one year of follow-up, among the 220 patients recruited, 151 patients had a good prognosis and 69 patients had a poor prognosis, and the patients were grouped according to this and then the differences in CC16, FIB, and SAA levels at admission were compared. All three levels were higher in those with poor prognosis (Poor Group) than in those with a good prognosis (Good Prognosis) (p < 0.001 for all comparisons) (Figure 1A–C).
We then analyzed the correlation between the three biomarkers, including CC16, FIB and SAA. As shown in Figure 1D–F, moderate correlations were found between the biomarkers (r=0.43, 0.46, and 0.34 for FIB vs CC16, SAA vs CC16, and SAA vs FIB, respectively).
Correlation of Serum CC16, Plasma FIB and SAA with the Severity of AECOPD
Correlations of CC16, FIB, SAA each other in 220 eligible patients are shown in Figure 2. We first compared the differences in CC16, FIB, and SAA between patients in pulmonary function classes (GOLD classification criteria) I–II and III–IV, showing that CC16, FIB, and SAA were significantly higher in patients in classes III–IV than in patients in classes I–II (Figure 2A–C). In addition, the correlation analysis of partial pressure of carbon dioxide with CC16, FIB and SAA also showed a significant positive correlation between CC16, FIB, and SAA and the severity of AECOPD (Figure 2D–F).
ROC Analysis of Predictive Values of Serum CC16, Plasma FIB, SAA at Admission and Their Combination Test for the Prognosis Assessment of AECOPD in One Year Follow-Up
Figure 3 shows among 220 eligible AECOPD patients, 151 had a good prognosis 69 patients had a poor prognosis after one year of follow-up. For determining the diagnostic values of the biomarkers, we performed ROC analysis using Youden’s index to determine the cutoff values (Table 3). As shown in Table 3, ROC analysis showed that CC16 had a sensitivity of 72.46% and specificity of 69.54%; FIB had a sensitivity of 60.87% and specificity of 72.85%, and SAA had a sensitivity of 76.81% and specificity of 88.74%. Their combined value 0.095 * CC16 + 0.495 * FIB + 0.245 * SAA showed an outstanding sensitivity of 76.81% and 88.74%.
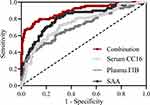 |
Figure 3 ROC analysis of predictive values of serum CC16, plasma FIB, SAA at admission and their combination test for the prognosis assessment of AECOPD in one year follow-up. |
Discussions
It has been proven difficult to identify objective markers for AECOPD severity as suggested in a previous meta-analysis,17 which found that only respiratory rate and arterial CO2 had predictive power for AECOPD severity. To address this challenge, our study validated that SAA is one of the biomarkers for AECOPD, which is consistent with the previous finding that SAA protein is rich in the proteomic analysis of the sera of patients hospitalized for severe AECOPD.18 Our study shows that SAA had a sensitivity of 76.81% and specificity of 88.74%, which is higher than the previously reported performance for CRP (sensitivity of 74.5% and specificity of 81%), echoing the conclusion that SAA is a favored biomarker compared to CRP.18 This result also underscores that AECOPD is strongly linked with systemic inflammation, since SAA, as an acute-phase protein, like CRP, is highly expressed by liver after induction by inflammatory mediators, including IL-1β, IL-6, and TNF-α, that prominently overexpress in AECOPD. SAA also directly binds to gram-negative bacteria19 and serves as an immune opsonin to clear pathogens, and as bacteria are thought of to play a role in up to 50% of exacerbations,20 the interaction between SAA and bacteria could contribute to SAA’s diagnostic role. Similarly, the close link between AECOPD and inflammation led us to investigate FIB and CC16 as biomarkers, due to their essential role in mediating inflammatory diseases.21 Consistently, the cutoff values used for SAA (20.44 ng/mL), FIB (4.07 g/L) and CC16 (9.98 mg/L) for AECOPD diagnosis were markedly higher than previously reported values for stable COPD (3.5–4.5 ng/mL for SAA, ~3.5 g/L for FIB,22 and 0.22 ng/mL for CC1621). In fact, SAA, FIB and CC16 have considerable discriminative values independently, showing that elevated levels of SAA, FIB, or CC16 strongly indicate a poor prognosis of AECOPD patients.
Notably, we demonstrated that the combination of SAA, FIB and CC16 led to a higher specificity in identifying AECOPD patients with a poor prognosis, which is of significant clinical value as biomarkers with a high specificity is in lack despite some traditional biomarkers, such as CRP, demonstrated a good detection sensitivity. The fact that the combination of the three biomarkers yielded better diagnostic specificity is in line with the merely moderate correlations between the three biomarkers, making a prediction based on all three biomarkers necessary. Our results not only verified that these are important biomarkers for diagnosing AECOPD, but also unveiled the strength of the combinatory tests for risk assessment of AECOPD patients. Further, it cannot be ruled out that by incorporating other metrics of the patient, such as demographics and comorbidity data, an even higher specificity could be achieved, whereby a more sophisticated approach, eg, machine learning,23 could play an important role.
Despite being the first study demonstrating the independent and combined diagnostic value of SAA, FIB and CC16 as biomarkers in AECOPD, there are several caveats to this initial study. First, despite Youden’s index24 as used to determine the cutoff values for each biomarker, an optimal cutoff value should be determined empirically based on further larger trials. In the future, SAA, FIB and CC16 may emerge as diagnostic tools particularly well suited for community-based, at-home, or early point-of-care AECOPD management due to the cost-effective and accessible nature of blood testing. One potential limitation is that our study is a single-center study and multiple-center study needs to be carried out and results should be validated prospectively. We also did not look into the presence of coinfection with virus and bacteria in our study, which could significantly worsen patient prognosis. In addition, differences in clinical guidelines may affect subject recruitment, which limits the translatability of our research. Further work is required before therapeutic implications and interpretative criteria can be established for these sensitive detection methods.
Conclusions
In conclusion, with the aim to address the heterogenous nature of AECOPD exacerbations, we have characterized SAA, FIB and CC16 as biomarkers for AECOPD risk assessment. We found that elevated SAA, FIB and CC16 levels are associated with poor prognosis of AECOPD patients and they have a good detection sensitivity and specificity when used individually or in combination. These biomarkers may have great clinical utility in identification, prognosis, and timely treatment planning of AECOPD. Future validation and utilization of these biomarkers will potentially expand the toolset for precision medicine of AECOPD.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Hoult G, Gillespie D, Wilkinson TMA, Thomas M, Francis NA. Biomarkers to guide the use of antibiotics for acute exacerbations of COPD (AECOPD): a systematic review and meta-analysis. BMC Pulm Med. 2022;22(1):194.
2. Ruvuna L, Sood A. Epidemiology of chronic obstructive pulmonary disease. Clin Chest Med. 2020;41(3):315–327. doi:10.1016/j.ccm.2020.05.002
3. Tung L-F, Shen S-Y, Shih -H-H, Chen Y-T, C-t Y, S-C H. Effect of high-flow nasal therapy during early pulmonary rehabilitation in patients with severe AECOPD: a randomized controlled study. Respir Res. 2020;21(1):1–11. doi:10.1186/s12931-020-1328-z
4. Oliveira A, Marques A. Understanding symptoms variability in outpatients with AECOPD. Pulmonology. 2018;24(6):357–360. doi:10.1016/j.pulmoe.2018.09.007
5. Yao C, Wang L, Shi F, et al. Optimized combination of circulating biomarkers as predictors of prognosis in AECOPD patients complicated with Heart Failure. Int J Med Sci. 2021;18(7):1592. doi:10.7150/ijms.52405
6. Jing Z, Chun C, Ning S, Hong Z, Bei H, Wan-Zhen Y. Systemic inflammatory marker CRP was better predictor of readmission for AECOPD than sputum inflammatory markers. Arch de Bronconeumología. 2016;523:138–144. doi:10.1016/j.arbres.2015.01.011
7. Yin J, Wang S, Qiu Y, et al. Screening for and combining serum intestinal barrier-related biomarkers to predict the disease severity of AECOPD. Ann Palliat Med. 2021;10(2):1548–1559. doi:10.21037/apm-20-1060
8. Dörtkardeş AB, Şahinduran Ş. Determination of serum amyloid A, haptoglobin and hepcidin levels in calves with endemic viral pneumonia. Ankara Üniver Veteriner Fakültesi Dergisi. 2020;67(2):127–131. doi:10.33988/auvfd.523958
9. Su W, Ju L, Hua Q, Hu J, Qian W. Values of combined C-reactive protein, procalcitonin and serum amyloid A in differential diagnosis of bacterial and non-bacterial community acquired pneumonia in children. Diagn Microbiol Infect Dis. 2022;105:115865. doi:10.1016/j.diagmicrobio.2022.115865
10. Li H, Xiang X, Ren H, et al. SAA is a biomarker to distinguish the severity and prognosis of Coronavirus Disease 2019 (COVID-19). J Infect. 2020.
11. Sorić Hosman I, Kos I, Lamot L. Serum amyloid A in inflammatory rheumatic diseases: a compendious review of a renowned biomarker. Front Immunol. 2021;11:631299. doi:10.3389/fimmu.2020.631299
12. Sun W, Cao Z, Ma Y, Wang J, Zhang L, Luo Z. Fibrinogen, a Promising Marker to Evaluate Severity and Prognosis of Acute Exacerbation of Chronic Obstructive Pulmonary Disease: a Retrospective Observational Study. Int J Chron Obstruct Pulmon Dis. 2022;17:1299. doi:10.2147/COPD.S361929
13. Li M, Wu Y, Han H, Yuan Y. Clinical significance of plasma D-dimer and fibrinogen detection in patients with AECOPD with hypoxemia. J Chine Phys. 2020;1464–1467.
14. Aydin C, Yıldız BP, Hattatoğlu DG. D-dimer/Fibrinogen ratio and recurrent exacerbations might have a potential impact to predict 90-day mortality in patients with COPD exacerbation. Malawi Med J. 2021;33(4):276–280. doi:10.4314/mmj.v33i4.8
15. He Y, Yu J, Zhu D, Gao J. Predictive Value of Serum Markers SFRP1 and CC16 in Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Evid Based Complement Alternat Med. 2022;2022:1–6. doi:10.1155/2022/6488935
16. Kropski JA, Fremont RD, Calfee CS, Ware LB. Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest. 2009;135(6):1440–1447. doi:10.1378/chest.08-2465
17. Ni W, Bao J, Yang D, et al. Potential of serum procalcitonin in predicting bacterial exacerbation and guiding antibiotic administration in severe COPD exacerbations: a systematic review and meta-analysis. Infect Dis. 2019;51(9):639–650. doi:10.1080/23744235.2019.1644456
18. Bozinovski S, Hutchinson A, Thompson M, et al. Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(3):269–278. doi:10.1164/rccm.200705-678OC
19. Shah C, Hari-Dass R, Raynes JG. Serum amyloid A is an innate immune opsonin for Gram-negative bacteria. Blood. 2006;108(5):1751–1757. doi:10.1182/blood-2005-11-011932
20. Basu S, Mukherjee S, Samanta A. Epidemiological study of bacterial microbiology in AECOPD patients of Kolkata, India. Asian J Pharm Clin Res. 2013;6(1):112–116.
21. Guo C, Sun X, Diao W, Shen N, He B. Correlation of clinical symptoms and sputum inflammatory markers with air pollutants in stable COPD patients in Beijing area. Int J Chron Obstruct Pulmon Dis. 2020;15:1507. doi:10.2147/COPD.S254129
22. Lin YH, Liao XN, Fan LL, Qu YJ, Cheng DY, Shi YH. Long-term treatment with budesonide/formoterol attenuates circulating CRP levels in chronic obstructive pulmonary disease patients of group D. PLoS One. 2017;12(8):e0183300. doi:10.1371/journal.pone.0183300
23. Cavailles A, Melloni B, Motola S, et al. Identification of patient profiles with high risk of hospital re-admissions for acute COPD exacerbations (AECOPD) in France using a machine learning model. Int J Chron Obstruct Pulmon Dis. 2020;15:949. doi:10.2147/COPD.S236787
24. Yuan M, Li P, Wu C. Semiparametric inference of the Youden index and the optimal cut‐off point under density ratio models. Canadian J Statistics. 2021;49(3):965–986. doi:10.1002/cjs.11600
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.