Back to Journals » OncoTargets and Therapy » Volume 14
Correlation Between Thymoma and Soluble Interleukin-2 Receptor Expression in a Patient with Good Syndrome
Authors Kitano H, Yamaguchi F , Atarashi K, Hiraiwa M, Shiratori Y, Onozaki S, Shikama Y
Received 7 July 2021
Accepted for publication 24 September 2021
Published 12 October 2021 Volume 2021:14 Pages 5045—5049
DOI https://doi.org/10.2147/OTT.S326193
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Haruka Kitano, Fumihiro Yamaguchi, Kenji Atarashi, Mina Hiraiwa, Yo Shiratori, Shota Onozaki, Yusuke Shikama
Department of Respiratory Medicine, Showa University Fujigaoka Hospital, Yokohama, Kanagawa, 227-8501, Japan
Correspondence: Fumihiro Yamaguchi
Department of Respiratory Medicine, Showa University Fujigaoka Hospital, 1-30 Fujigaoka, Aoba-ku, Yokohama, Kanagawa, 227-8501, Japan
Tel +81-45-973-2848
Email [email protected]
Abstract: Good syndrome is a rare condition characterized by the presence of thymoma in combination with adult-onset hypogammaglobulinemia. Immunological features of Good syndrome include various immunodeficiencies accompanied with hypogammaglobulinemia. In patients with thymoma, paraneoplastic syndromes including hypogammaglobulinemia worsen the prognosis. We herein describe a patient with advanced-stage type A thymoma who was effectively treated with chemotherapy and exhibited a parallel decrease in the serum level of soluble interleukin-2 receptor (sIL-2R), which depends on cellular immunity. The present case suggests the efficacy of sIL-2R as a potential prognostic biomarker in a subset of patients with Good syndrome.
Keywords: thymoma, hypogammaglobulinemia, Good syndrome, soluble interleukin-2 receptors
Introduction
Thymoma, a rare thoracic neoplasm that originates from thymic epithelial cells, is a typically slow-growing tumor and spreads by local extension, and extrathoracic lesions are not common.1 Staging and histological subtype are the most important prognostic factors, and paraneoplastic syndromes, including hypogammaglobulinemia, worsen prognosis.2,3 There are currently no prognostic biomarkers that have been validated for thymoma. Adult-onset hypogammaglobulinemia occurs in 2–5% of patients with thymoma.4 Good syndrome, which was originally described as thymoma accompanied with hypogammaglobulinemia, is currently defined as thymoma with any type of unclassified immunodeficiency beyond hypogammaglobulinemia, including an abnormal lymphocyte count.2,5 Here, we describe a case of thymoma with systemic metastasis in a patient with Good syndrome and report the efficacy of soluble interleukin-2 receptor (sIL-2R) level as a potential biomarker for Good syndrome.
Case Report
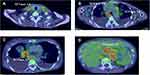
A 50-year-old Japanese woman presented with productive cough, dyspnea on effort, and diarrhea. She had no history of compromised immunity. Chest radiograph revealed widening of the mediastinum and bilateral pleural effusion. Positron emission tomography combined with computed tomography (CT) revealed fluorine-18 fluorodeoxyglucose uptake within a mass in anterior mediastinum and lymph nodes throughout the body (Figure 1). High-resolution CT showed bronchiectasis in addition to multiple nodules consistent with lung metastases. CT-guided needle biopsy was performed to obtain tissue samples from the anterior mediastinal tumor. Hematoxylin/eosin staining of the collected specimens revealed a fascicular pattern composed primarily of spindle-shaped or oval epithelial cells. Immunohistochemical examination demonstrated that the sample was positive for cytokeratin AE1/AE3 and p40 (Figure 2), consistent with thymic epithelial tumor. Concurrent serum tests revealed an sIL-2R level of 2774 U/mL and hypogammaglobulinemia. Bone marrow biopsy demonstrated normal cells. Atypical epithelial cells were detected in the pleural effusion, consistent with thymic dissemination. Therefore, the patient was diagnosed with advanced type A thymoma (Masaoka stage IVb) and Good syndrome.
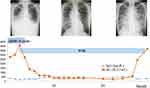
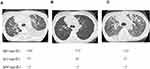
The patient received four cycles of the ADOC regimen, which comprised 40 mg/m2 doxorubicin and 50 mg/m2 cisplatin on day 1, 0.6 mg/m2 vincristine on day 3, and 700 mg/m2 cyclophosphamide on day 4. In addition, intravenous immunoglobulin was administered every four weeks. The size of the primary thymoma, systemic lymph node metastases, parenchymal lung metastases, and bilateral pleural effusion diminished in parallel to a decline in sIL-2R levels to the normal range (Figures 3 and 4). The patient achieved partial response with the ADOC chemotherapy according to the Response Evaluation Criteria in Solid Tumors version 1.1.6 Following a symptom-free period of 26 months, the patient died suddenly from pneumonia and autopsy revealed thymoma recurrence. Of note, the sIL-2R level was elevated in parallel to the clinical exacerbation (Figure 3).
Discussion
The present patient was diagnosed with type A thymoma based on the mediastinal tumor biopsy results and classified as stage IVb according to the Masaoka’s criteria7 because of lymphogenous and hematogenous metastases. Complete surgical resection with or without chemotherapy is recommended for stage I–IVa, but not for stage IVb, thymoma.8 Cisplatin plus anthracycline is the most commonly used first-line chemotherapy for advanced thymoma.9,10 Thymectomy, which is reported to achieve a favorable effect on associated conditions such as myasthenia gravis and pure red blood cell aplasia, does not reverse hypogammaglobulinemia.2,11 In addition, myasthenia gravis and pure red blood cell aplasia respond to corticosteroid therapy in some cases, suggesting an autoimmune pathogenesis in thymoma. Corticosteroid treatment, in contrast, does not correct the immunodeficiency,2 consistent with the findings in the present patient who was treated with chemotherapy. In the present case, bronchiectasis was scattered in bilateral upper lobes, suggesting recurrent respiratory tract infection. Hypogammaglobulinemia is associated with mortality in patients with thymoma,2,3 and immunoglobulin replacement has been recognized as an effective approach to reduce the incidence of infections.12 Intravenous immunoglobulin should therefore be considered particularly for patients with Good syndrome and repeated infections.5
There are currently no validated biomarkers that can predict the prognosis of patients with thymoma. In the present case, the sIL-2R levels were elevated at the time of diagnosis and returned to within normal limits after chemotherapy. However, the sIL-2R levels ultimately increased with the recurrence of thymoma, and the patient died. Thus, the changes in sIL-2R levels followed the patient’s clinical course. IL-2R comprises three noncovalently associated proteins, IL-2Rα, IL-2Rβ, and IL-2Rγc.13 Of the three chains, only IL-2Rα is unique to IL-2R. Although IL-2Rβ and IL-2Rγc are expressed in all lymphocytes including those that are dormant, IL-2Rα is detected only after lymphocyte activation. T lymphocyte activation by antigens and costimulators leads to the expression of the IL-2Rα chain. Thus, chronic T lymphocyte stimulation leads to IL-2Rα secretion and increased levels of IL-2Rα shed in serum, namely sIL-2R, is considered as a clinical marker of strong antigenic stimulation, such as that may occur in hematopoietic malignancies.14–16 Increased serum sIL-2R is also considered as an index of immune-related adverse events caused by immune checkpoint inhibitors.17 Multiple studies reported immunological diversity in Good syndrome.2,5,18–20 In addition to hypogammaglobulinemia, other immunological features of Good syndrome include reduction in peripheral B cell count, CD4+ T lymphopenia, inverted CD4/CD8 ratio, and reduced T lymphocyte proliferative response to mitogen stimulation. T lymphocytes might escape normal maturation and produce insufficient levels of sIL-2R in some patients with Good syndrome, whereas the current patient did not exhibit any bone marrow abnormalities, indicating an intact cellular immunity and sufficient sIL-2R production. In summary, the current case suggests that sIL-2R might be a useful prognostic biomarker in a subset of patients with Good syndrome.
Ethical Approval
Official approval for the study was obtained in advance from the Ethics Committee at Showa University (approved number 269).
Patient Consent for Publication
The patient provided written informed consent.
Acknowledgment
The authors are grateful to Mari Nakamoto, Saori Kawamura, Miku Kosuge, Hidekazu Cho, Shohei Shimizu, Akira Fujishima, Ayaka Mase, Yuki Osakabe, Toshitaka Funaki, Daisuke Inoue, Yohei Yamazaki, Hidetsugu Tateno, Takuya Yokoe for their assistance in the interpretation of the results and critical review of the manuscript.
Author Contributions
All authors examined and cared for the patient. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in connection with this paper, and received no payment or services from a third party in relation to this study.
References
1. Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg. 1996;112:376–384. doi:10.1016/S0022-5223(96)70265-9
2. Kelesidis T, Yang O. Good’s syndrome remains a mystery after 55 years: a systematic review of the scientific evidence. Clin Immunol. 2010;135:347–363. doi:10.1016/j.clim.2010.01.006
3. Jansen A, van Deuren M, Miller J, et al. Prognosis of Good syndrome: mortality and morbidity of thymoma associated immunodeficiency in perspective. Clin Immunol. 2016;171:12–17. doi:10.1016/j.clim.2016.07.025
4. Scorsetti M, Leo F, Trama A, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol. 2016;99:332–350. doi:10.1016/j.critrevonc.2016.01.012
5. Kelleher P, Misbah SA. What is Good’s syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol. 2003;56:12–16. doi:10.1136/jcp.56.1.12
6. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guide- line (version 1.1). Eur J Cancer. 2009;45:228–247. doi:10.1016/j.ejca.2008.10.026
7. Masaoka A. Staging system of thymoma. J Thorac Oncol. 2010;5:S304–S312. doi:10.1097/JTO.0b013e3181f20c05
8. Falkson CB, Bezjak A, Darling G, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol. 2009;4:911–919. doi:10.1097/JTO.0b013e3181a4b8e0
9. Berghmans T, Durieux V, Holbrechts S, et al. Systemic treatments for thymoma and thymic carcinoma: a systematic review. Lung Cancer. 2018;126:25–31. doi:10.1016/j.lungcan.2018.10.018
10. Venuta F, Rendina EA, Anile M, de Giacomo T, Vitolo D, Coloni GF. Thymoma and thymic carcinoma. Gen Thorac Cardiovasc Surg. 2012;60:1–12. doi:10.1007/s11748-011-0814-0
11. Narahari NK, Gongati PK, Uppin SG, Kapoor A, Kakarla B, Tella RD. A 66-year-old man with mediastinal mass and dyspnea. Chest. 2016;150:e109–15. doi:10.1016/j.chest.2016.07.002
12. Tarr PE, Sneller MC, Mechanic LJ, et al. Infections in patients with immunodeficiency with thymoma (Good syndrome). Report of 5 cases and review of the literature. Medicine. 2001;80:123–133. doi:10.1097/00005792-200103000-00005
13. Witkowska AM. On the role of sIL-2R measurements in rheumatoid arthritis and cancers. Mediators Inflamm. 2005;14:121–130. doi:10.1155/MI.2005.121
14. Chrobák L. Clinical significance of soluble interleukin-2 receptor. Acta Med. 1996;39:3–6.
15. Kusano Y, Yokoyama M, Terui Y, et al. High pretreatment level of soluble interleukin-2 receptor is a robust prognostic factor in patients with follicular lymphoma treated with R-CHOP-like therapy. Blood Cancer J. 2017;7:e614. doi:10.1038/bcj.2017.96
16. Murakami J, Arita K, Wada A, et al. Serum soluble interleukin-2 receptor levels for screening for malignant lymphomas and differential diagnosis from other conditions. Mol Clin Oncol. 2019;11:474–482. doi:10.3892/mco.2019.1922
17. Takai R, Funakoshi Y, Suto H, et al. Serum soluble interleukin-2 receptor as a potential biomarker for immune-related adverse events. Anticancer Res. 2021;41:1021–1026. doi:10.21873/anticanres.14857
18. Arnold SJ, Hodgson T, Misbah SA, Patel SY, Cooper SM, Venning VA. Three difficult cases: the challenge of autoimmunity, immunodeficiency and recurrent infections in patients with Good syndrome. Br J Dermatol. 2015;172:774–777. doi:10.1111/bjd.13293
19. Martinez B, Browne SK. Good syndrome, bad problem. Front Oncol. 2014;4:307. doi:10.3389/fonc.2014.00307
20. Kawamura T, Naito T, Kobayashi H, et al. Acquired immunodeficiency associated with thymoma: a case report. BMC Cancer. 2019;19:762. doi:10.1186/s12885-019-5980-y
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.