Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
COPD in People with HIV: Epidemiology, Pathogenesis, Management, and Prevention Strategies
Authors Byanova KL, Abelman R , North CM, Christenson SA, Huang L
Received 29 June 2023
Accepted for publication 9 November 2023
Published 29 November 2023 Volume 2023:18 Pages 2795—2817
DOI https://doi.org/10.2147/COPD.S388142
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Katerina L Byanova,1,* Rebecca Abelman,2,* Crystal M North,3 Stephanie A Christenson,1 Laurence Huang1,2
1Division of Pulmonary, Critical Care, Allergy, and Sleep Medicine, Department of Medicine, University of California San Francisco, San Francisco, CA, USA; 2Division of HIV, Infectious Diseases, and Global Medicine, Department of Medicine, University of California San Francisco, San Francisco, CA, USA; 3Division of Pulmonary and Critical Care Medicine, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
*These authors contributed equally to this work
Correspondence: Laurence Huang, HIV, Infectious Diseases, and Global Medicine Division, Room 2807, UCSF Pride Hall, 2540 23rd Street, San Francisco, CA, 94110, USA, Tel +1 (415) 476-4082, Extension 406, Fax +1 (415) 476-6953, Email [email protected]
Abstract: Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disorder characterized by airflow limitation and persistent respiratory symptoms. People with HIV (PWH) are particularly vulnerable to COPD development; PWH have demonstrated both higher rates of COPD and an earlier and more rapid decline in lung function than their seronegative counterparts, even after accounting for differences in cigarette smoking. Factors contributing to this HIV-associated difference include chronic immune activation and inflammation, accelerated aging, a predilection for pulmonary infections, alterations in the lung microbiome, and the interplay between HIV and inhalational toxins. In this review, we discuss what is known about the epidemiology and pathobiology of COPD among PWH and outline screening, diagnostic, prevention, and treatment strategies.
Keywords: HIV, COPD, tuberculosis, air pollution, immune activation, smoking, pulmonary infections
Introduction
Chronic obstructive pulmonary disease (COPD) is a prevalent chronic respiratory condition that represents the third leading cause of death worldwide.1,2 According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 definition, COPD is a
Heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, expectoration, and/or exacerbations) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction.2
People with HIV (PWH) are particularly vulnerable to the development and progression of COPD, with both higher rates of COPD and an earlier and more rapid decline in lung function than in the general population, even after accounting for cigarette smoking and other known risk factors, such as intravenous drug use.3–7 The exact mechanisms that underlie HIV-associated COPD are incompletely known, but environmental exposures, heightened immune activation and systemic inflammation, accelerated aging, a predilection for the development of pneumonia, and alterations in the lung microbiome likely play important roles (Figure 1).8–11 The purpose of this review is to describe what is currently understood about the epidemiology and pathobiology of COPD among PWH, to indicate selected areas of active investigation, and to outline screening, diagnostic, prevention, and treatment strategies.
 |
Figure 1 Drivers of COPD in PWH. |
Epidemiology
Prevalence
As survival among PWH has improved with the use of antiretroviral therapy (ART), COPD has become an increasingly important comorbidity. PWH develop an earlier and more rapid decline in lung function, even after adjustment for traditional risk factors.3,5–7,12–15 A recent retrospective study evaluating comorbidities in PWH based on hospital discharge data found that COPD was the most common comorbidity across the 10-year study period and that COPD prevalence was higher among PWH than among those without HIV (23.5% versus 14.0%).16 Prevalence estimates of COPD among PWH have ranged from 3.4% to over 40% in prior studies; notably, most of these have been conducted in Europe and North America.17,18 Part of this heterogeneity is due to differences in COPD classification methods, such as self-report, International Classification of Diseases (ICD) diagnostic codes, use of CT scans, and spirometry.17,19 For example, a systematic review and meta-analysis by Bigna et al evaluating the global prevalence of COPD among PWH found that the prevalence varied from 5.6% to 10.6% depending on the diagnostic criteria used, with a higher prevalence when using spirometric criteria instead of self-report or ICD diagnostic codes.4
Geography
COPD in PWH occurs anywhere PWH reside. However, the risk factors for the development of COPD in PWH vary regionally due to differences in age, rates and duration of tobacco smoking, exposure to biomass fuels, and prevalence of tuberculosis, all of which have been implicated in COPD development.2,20–22 While the majority of studies on COPD in PWH have been conducted in the US and Europe, most PWH live in sub-Saharan Africa, where there is a high prevalence of both tuberculosis (TB) and exposure to biomass fuels, and where patients are typically younger and less likely to smoke tobacco. While earlier studies suggested that ART itself may be a risk factor for worsening lung function,23,24 Kunisaki et al conducted a multinational randomized controlled trial (RCT) in the modern ART era and did not find a difference in lung function based on timing of ART initiation.25
Biologic Sex
Biologic sex may also contribute to differences in COPD trajectories among PWH. In one study of longitudinal lung function changes in PWH, female sex was associated with distinct lung function trajectories, including baseline low diffusing capacity for carbon monoxide (DLco).26 In a study by McNeil et al of virally suppressed adults with HIV and their seronegative counterparts in Uganda, women with HIV demonstrated an accelerated FEV1 decline as compared to women without HIV, a finding that was not seen among men with and without HIV.27 Interestingly, in a large US-based cross-sectional analysis comparing women with and without HIV, women with HIV had a lower DLco than women without HIV, but there were no differences in spirometric outcomes by HIV status.28,29 In another study including the same cohort of women, baseline COPD prevalence was similar among men with and without HIV and women with and without HIV, but COPD incidence was higher among men with HIV when compared to men without HIV.30 In contrast, Abelman et al found in a post-pneumonia Ugandan cohort that women with HIV had over three-fold higher odds of COPD on spirometry compared to men with HIV, a sex-based difference not found in women and men without HIV.31 Further work is currently underway to investigate whether these reported HIV-associated sex-specific differences in COPD rates are driven by immunologic, hormonal, or environmental factors.
Risk Factors for COPD in PWH
There are many risk factors for the development of COPD in PWH including HIV itself,5,32 cigarette smoking and other inhalational exposures, air pollution, opportunistic infections and pneumonia, microbiome alterations,33,34 accelerated aging,35–38 and socioeconomic factors.39 This section focuses on the major drivers, such as smoking, as well as potential risk factors under investigation, such as chronic cytomegalovirus (CMV) coinfection.
Smoking
Smoking is the key risk factor for COPD in PWH. Smoking is more prevalent among PWH compared to their seronegative counterparts.40–42 However, studies of co-exposure to HIV and tobacco smoke suggest that PWH who smoke may also be more susceptible to smoking-induced lung damage than HIV-uninfected people who smoke. For example, Diaz et al found emphysema to be more prevalent among smokers with HIV as compared to smokers without HIV.43 Further, in a longitudinal multi-center cohort of 13,687 veterans with and without HIV, Crothers et al found that the prevalence and incidence of both COPD and lung cancer were higher among those with HIV compared to those without HIV despite similar levels of smoking.5 Importantly, among PWH on ART, smoking may reduce life expectancy more than HIV itself.44–46 While the pathophysiologic mechanism driving this HIV-associated difference is incompletely known, recent work suggests that, among PWH, tobacco smoke suppresses alveolar macrophage production of T-cell recruiting chemokines. This impairs the migration of cytotoxic T cells from the airway mucosa into the alveolar space, leading to localized airway mucosa inflammation and tissue destruction.47
Air Pollution
Air pollution – the leading environmental cause of death globally48 – is now the greatest threat to human health,49 and COPD is a leading cause of the nearly 7 million annual deaths attributed to air pollution.48,50 Air pollution results from a variety of human-related activities and natural events that include emissions from vehicles, factories, and power plants; traffic-related products; biomass fuel burning (ie, charcoal, firewood, animal dung, crop residues) for cooking and heating; dust storms; forest fires; and volcanic eruptions. The dominant pollution sources vary by region. Traffic- and industry-related sources drive exposure in high-income countries and urban settings, while biomass-related sources drive exposure in low- and middle-income countries and rural settings.51 Air pollution causes acute and chronic lung dysfunction, structural lung abnormalities, submaximal lung growth in childhood and adolescence, and augments lung disease risk in vulnerable populations.52–63 Even small acute increases in fine particulate matter (PM2.5) exposure worsen mortality,64 and there is no “safe” level of exposure.65 Biomass-associated COPD, compared to tobacco-associated COPD, is characterized by more small airways disease and fibrosis, less emphysema, higher DLco, and less airflow obstruction – in effect, a more fibrotic and less emphysematous phenotype.66–69 Exposure to biomass fuel smoke has also been associated with defective bacterial phagocytosis.70 In addition, PM2.5 exposure may also potentiate TB risk,21,71,72 which by itself is a risk factor for COPD and an important consideration in TB-endemic regions.
Similar to the influence of tobacco smoke, PWH may be more susceptible to air pollution-associated lung damage. For example, among PWH living in San Francisco, exposure to higher levels of outdoor air pollution was associated with increased susceptibility to Pneumocystis infection.73–75 Using ambulatory carbon monoxide (CO) sensors to measure personal air pollution exposure among 260 adults with and without HIV in rural Uganda, North et al found that exposure to short-term CO levels that exceed WHO air quality guidelines was associated with self-reported respiratory symptoms among PWH but not among HIV-uninfected comparators.76 Characterizing air pollution exposure among PWH and exploring the potentially outsized influence of air pollution exposure on lung health in this population is an area of ongoing investigation. As global smoking prevalence continues to decline and rapid industrialization and urbanization progresses, air pollution is poised to replace tobacco as the leading cause of chronic lung disease,77–79 and a multifaceted approach that also focuses on this often overlooked risk factor for lung disease among PWH is critical.
Opportunistic Infections and Pneumonia
PWH have historically had higher rates of pneumonia, and while incidence of bacterial pneumonia has decreased with the advent of ART,80,81 it remains common in this population.82–84 In the current era, PWH have similar rates of acute respiratory infections as people without HIV, but PWH experience more severe disease.85 Pneumonia has been associated with higher rates of COPD and lung function abnormalities in PWH.86–89 For example, Drummond et al conducted a US-based multi-center study evaluating spirometry in adults with and without HIV and found that participants with airflow obstruction were more likely to have a history of bacterial pneumonia and Pneumocystis jirovecii (PJP) infection.90 Specifically, PJP, an opportunistic infection that occurs in PWH with CD4 counts <200 cells/mm,3 elevated HIV RNA, and colonization by Pneumocystis have each been associated with higher risk of COPD among PWH.88,91,92 There are numerous contributors to the increased risk of pneumonia in PWH, including alterations in immunity, which lead to persistently elevated markers of immune activation and inflammation, as well as environmental and behavioral risk factors, and a higher prevalence of COPD, which is both a consequence of and a risk factor for pneumonia.9,93–96
Globally, tuberculosis is the leading infectious cause of death among PWH;97 PWH are 19 times more likely to develop TB disease than their seronegative counterparts.98,99 Pulmonary TB has been found to cause permanent scarring, bronchiectasis, pleural fibrosis, damage to small and large airways, as well as lung parenchymal damage, all of which may contribute to permanent lung function impairment.20,100 Whereas during the treatment phase of TB this impairment is typically restrictive, there is increasing evidence of a relationship between prior pulmonary TB infection and the subsequent development of obstruction and COPD.20,87 Rates differ significantly by the population under study, but pulmonary TB has been found to lead to airway obstruction in 18.4–86% of people in the general population.100 HIV is now recognized as a risk factor for post-TB lung disease, although the extent of this relationship is currently under study.87,100–104 There is some evidence to suggest that HIV may be associated with reduced severity of post-TB lung disease, but this is an area that merits further evaluation.100,105,106
Chronic CMV Infection
CMV is an important and omnipresent coinfection in HIV that has been associated with cardiovascular and cerebrovascular disease, other non-AIDS events, and increased mortality.107–112 Given the high rates of CMV antibody seropositivity among PWH, CMV IgG titers are commonly used as markers of CMV activity and have been shown to correlate with adverse outcomes.112,113 However, studies of CMV’s effect on lung function and COPD in PWH are limited. While chronic CMV infection in children with perinatally acquired HIV on ART has been associated with an abnormal FEV1,114 CMV’s association with COPD and other chronic lung diseases in adults with HIV has not been evaluated. Emerging data from the general population, however, suggest that chronic CMV infection is associated with COPD,115 and that higher CMV IgG titers are associated with COPD-related mortality.113 CMV is also associated with abnormal DLco in solid organ transplant recipients, although this has not been studied in PWH.116–118
There are several proposed mechanisms for CMV-mediated systemic immune effects, including persistent immune activation, endothelial dysfunction, and alterations in the gut microbiome.17,119–121 Similar biomarker activation patterns are noted in PWH with CMV and those with COPD. For example, sCD163, sCD14, and IL-6 are increased in both CMV IgG-positive PWH122–124 and PWH with lung function abnormalities, including both abnormal spirometry and abnormal DLco.10,121 These data suggest that there may be a shared mechanistic pathway between chronic CMV infection and chronic lung disease in PWH, but further work is needed to understand and characterize this relationship.
HIV-Specific Influences on COPD Pathogenesis
Several HIV-specific mechanisms may contribute to the increased incidence and accelerated development of COPD in PWH. Chronic HIV infection and the direct effects of HIV-related proteins on lung cells, altered lung and systemic immune responses (both immunosuppressive and pro-inflammatory), altered airway and gut microbial communities, impaired response to pathogens, and toxicity from antiretroviral therapies may all contribute to COPD pathogenesis in this population.23,24,125–132
HIV Infection
As the lung acts as a reservoir for HIV even after viral suppression, chronic HIV infection may directly contribute to COPD pathogenesis in various ways.132–134 Newly replicated viral particles released slowly over time bind to and interact with many cell types within the lung, which can lead to direct injury, oxidative stress, low-level chronic inflammation, and impaired response to pathogens.128,135 Although other cell types in the lung may be infected, alveolar macrophages are the best studied reservoir of HIV in the lung.132 HIV infection impairs macrophage phagocytic activity, thus hindering response to pathogens.127,132 HIV also skews the macrophage phenotype towards a pro-inflammatory and protease-producing phenotype through the release of a host of cytokines, chemokines, oxidants, and proteases, all of which contribute to COPD pathology. Cytokine and chemokine signaling in HIV-infected macrophages trigger a pro-inflammatory response including neutrophil and lymphocyte infiltration. Kaner et al found that alveolar macrophage expression of proteases such as matrix metalloproteinases 9 and 12 (MMP-9, MMP-12) is higher in PWH who smoke with emphysema than their seronegative counterparts.131 In murine models, MMPs degrade the extracellular matrix, directly contributing to emphysematous tissue destruction.136
Altered Adaptive Immune Responses
COPD development is not only mediated by HIV direct effects, but also by the altered cell-mediated adaptive immune responses in PWH, in particular, altered CD4+ T-cell responses. Numerous studies have shown a relationship between low CD4+ T cell counts and COPD or accelerated lung function decline, although conflicting data also exists.23,125,126,137 T cell exhaustion is typically seen in response to chronic antigen stimulation, such as chronic viral infection, and results in decreased functionality. In PWH, CD4+ T cells show signs of exhaustion even in the presence of ART, with an increased expression of programmed cell death protein-1 (PD-1), as well as impaired proliferative capacity.130,138,139 Furthermore, in PWH with COPD, airway mucosal CD4+ T cell numbers are depleted and poorly responsive to pathogens.130 These findings suggest that dysfunctional CD4+ T cell responses may uniquely contribute to COPD pathogenesis in PWH.
Activated and dysfunctional CD8+ T cells also appear to contribute to the disordered adaptive immune response in chronic HIV infection, and thus could contribute to COPD pathogenesis.138,139 PWH show persistent expansion of CD8+ T cells in blood and alveolar compartments, and the decreased CD4+/CD8+ ratio is associated with lung abnormalities even in PWH on ART.140,141 These expanded CD8+ T cell populations also show dysfunction, which is typically indicative of an accelerated aging or “immunosenescent” response. Like CD4+ T cells, CD8+ T cells display exhaustion markers, including PD-1, and a low proliferative capacity.138,139 The expanded population skews towards memory T cell and terminally differentiated CD8+ T cell populations unable to respond to new insults. Despite their impaired function, these exhausted T-cells produce a low-grade inflammatory response at mucosal surfaces, which is considered central to COPD pathology.
Changes to the Airway Epithelium
Alterations to the airway epithelium, the main barrier protecting the lungs from outside insults, such as cigarette smoke, air pollution, and inhaled toxins, can also play a major role in COPD pathogenesis. HIV has both direct and indirect effects on the airway epithelium, contributing to disordered barrier function, decreased mucociliary clearance, and generation of pro-inflammatory mediators. For example, HIV enters epithelial cells and disrupts cell–cell adhesion.129 HIV-associated proteins released from other infected cells disrupt epithelial tight junctions and induce oxidative stress.142 HIV and cigarette smoke synergistically disrupt mucociliary clearance, additively suppressing CFTR expression to decrease mucus hydration in cell culture models and inducing goblet cell metaplasia/hyperplasia to increase mucus production in simian models.143,144 Finally, when HIV binds specifically to basal cells, epithelial progenitor cells release proteases such as MMP-9 and pro-inflammatory mediators that induce migration and proliferation of macrophages and neutrophils.145
Changes in the Lung and Gut Microbiome
Lastly, shifts in both the lung and the gut microbiome can also contribute to chronic inflammatory responses in the lung and, hence, COPD pathogenesis. Data are conflicting on whether lung microbial communities differ in PWH based on 16S sequencing.146–148 However, subtle differences in the microbiome at the species or strain level or at a functional level cannot be discerned via these sequencing methods. It is plausible that at least a subset of PWH experience pathologic microbial alterations in the airways because of a more hospitable environment for pathogen growth. If present in PWH, microbiome perturbations could contribute to chronic airway inflammation. Furthermore, microbial translocation from a compromised gut mucosa, stimulating a chronic systemic inflammatory response, may contribute to lung disease in PWH as has been seen in asthma and pulmonary infections.149
Diagnosis and Clinical Findings of COPD in PWH
Screening and Diagnosis
COPD remains both underdiagnosed and misdiagnosed in people with HIV.150,151 While currently the US Preventative Services Task Force does not recommend screening for COPD in the general population,152 higher COPD prevalence among PWH raises the question whether screening should be done in this subpopulation. Currently, there are no screening and diagnostic criteria specific to PWH. While several studies have evaluated different screening approaches, no conclusive recommendations can be made regarding COPD screening and diagnosis in PWH at this time.150,153–156 For example, a group in Canada offered screening spirometry to all patients in an HIV clinic;156 notably, less than a third of the invited participants agreed to participate, and only 11% had airflow obstruction.
Recruitment and retention throughout the screening-to-diagnosis cascade have been major challenges in all studies. For example, a group in Italy implemented a three-step case-finding program, involving a 5-question screening questionnaire (which included questions about age, smoking history, cough and sputum production, shortness of breath, and exercise limitation), portable spirometry, and diagnostic spirometry.150 They found that 282 participants (19.6%) had a positive screening questionnaire, defined as having a positive answer to at least three questions, but only 33 participants ultimately completed diagnostic spirometry, of whom 22 met criteria for COPD. High participant dropout at each step of the screening process has been similarly reported elsewhere,153–155 even when the authors bypassed the screening spirometry and had a shorter questionnaire.155 Even within these limitations, COPD prevalence based on the screening outcomes has been consistently higher than the known COPD prevalence in each respective clinic,154 further underscoring the underappreciated burden of chronic lung disease in this population. Additional challenges with screening this high-risk population include lack of a high-performing, validated screening questionnaire in PWH and poor correlation between respiratory symptoms and obstruction on pulmonary function tests (PFTs).155 To our knowledge, qualitative studies focused on identifying patient, provider, or systems-level issues contributing to high dropout rates in screening studies among PWH have not been conducted. Having diagnostic spirometry available at the time of a positive screening questionnaire may help reduce high dropout rates.
Any PWH suspected of having COPD should undergo diagnostic testing with, at a minimum, portable spirometry and, in our opinion, full PFTs with pre- and post-bronchodilator spirometry, total lung capacity and lung volumes if spirometry is abnormal, and DLco measurement. Chest radiography demonstrates classic findings (Figure 2) mostly in individuals with advanced disease but is useful in ruling out alternative etiologies that also present with respiratory symptoms similar to those of COPD. Occasionally, additional testing such as chest computed tomography (CT) scans may be warranted to characterize the observed PFT abnormalities, and certain CT findings such as the presence of large bulla (Figure 3) may lead to consideration of additional therapies (eg, bullectomy).
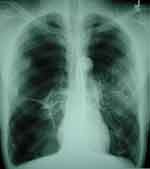 |
Figure 2 Chest radiograph from person with HIV and COPD demonstrating hyperinflation, flattened diaphragms, and bilateral bullous lung disease (Courtesy of Laurence Huang, MD). |
Longitudinal Lung Function Trajectories of COPD in PWH
While there is a paucity of data on the natural history of COPD in PWH, lung function declines faster in PWH compared to HIV-negative controls, even when HIV is well-controlled and smoking rates are comparable.6,7,157 Notably, findings from the Pittsburgh HIV Lung Cohort suggested that there may be distinct lung function trajectories among PWH, in which differences in the rate of decline are associated with specific symptoms and distinct profiles of elevated immune activation biomarkers.26 Importantly, this study did not exclusively enroll individuals with COPD. In the general population, COPD studies have shown that lung function decline accelerates as COPD severity increases,158 but whether similar trajectories are seen in PWH is an area currently under study. In a study evaluating factors associated with lung function decline among PWH by Li et al, the authors found that lung function decline occurred more rapidly in older individuals and those with GOLD stage 1 than those with GOLD stage 0 COPD.126 Taken together, these studies suggest that PWH with COPD may demonstrate distinct lung function trajectories when compared to their seronegative counterparts, although additional study is needed in this area.
Lung Function Trajectories in People with Perinatally Acquired HIV
While this review is focused on COPD in adults with HIV, the growing number of individuals with perinatally acquired HIV and their lung function trajectory should also be considered. Children and adolescents with HIV have a higher risk of pulmonary infections, including TB, and even with early ART initiation they remain more vulnerable to small airways dysfunction and risk of obstructive lung disease and other pulmonary abnormalities on spirometry and imaging.159–166 Even children who were exposed to but not infected with HIV remain at risk for abnormal lung function.167 Further, lung function in children seems to be affected by the timing of maternal ART initiation (pre-pregnancy versus during pregnancy).167 In addition, lung development and the ability to reach maximal lung function is impaired by HIV, repeat infections, smoking, pollution, and poverty, which in turn increases the risk for the development of chronic lung disease in adulthood.168,169 As this vulnerable population ages, we are likely to see an increased burden of chronic obstructive disease earlier in life. As most of our understanding of lung function trajectories in PWH with COPD comes from adult PWH from higher income settings, focused efforts for early screening, diagnosis, and management of this condition are needed in areas with high prevalence of adolescents and adults with perinatally acquired HIV.
Diffusing Capacity for Carbon Monoxide
Abnormal diffusing capacity for carbon monoxide is the most prevalent finding on PFTs in PWH, even when spirometry is normal.29,170 DLco impairment is non-specific and can be attributed to emphysema, fibrosis, pulmonary hypertension, or anemia. In PWH, it is also often associated with prior respiratory infections such as PJP, TB, or bacterial pneumonia, and the DLco abnormality may persist long after clinical and radiographic resolution of infection.89,126 Other risk factors for abnormal DLco include HIV infection, CD4 < 200 cells/mm,3 intravenous drug use, and hepatitis C infection.29,101,170–172
DLco abnormalities can predict the development, symptoms, and outcomes of COPD. Among people who smoke, DLco can become abnormal before spirometric criteria for COPD are met; DLco may also be a marker of early emphysema prior to the development of spirometric obstruction, small airways disease, or early vascular abnormalities.173–175 While there are additional and unique risk factors for abnormal DLco in PWH compared to the general population, perhaps suggestive of an HIV-specific lung function abnormality,10,176 it is also plausible that isolated DLco abnormalities may serve as a marker for early COPD in some patients. Among PWH, abnormal DLco, like abnormal FEV1, is an independent predictor of worse respiratory symptoms (such as dyspnea, cough, and mucus production),170 as well as a worse 6-minute walk test.177,178 Finally, abnormal DLco is an independent predictor of mortality in PWH with COPD.179,180
Imaging Findings in PWH with COPD
New techniques for quantitative imaging assessment have allowed in-depth characterization of imaging abnormalities in people with COPD. As current GOLD criteria define COPD based on chronic respiratory symptoms,2 chest imaging findings such as emphysema describe the structural abnormalities that drive this clinical entity. In the general population of people who smoke, studies have found that evidence of small airways disease and air trapping on imaging could predict COPD development and faster spirometry decline.181,182 Importantly, multiple imaging findings such as early interstitial lung abnormalities,183 pulmonary artery to aorta ratio >1,184 pulmonary arterial vascular pruning,185 progression186 and homogeneity of emphysema,187 airway wall thickness,188,189 and air trapping have all been associated with disease severity and adverse outcomes in COPD.181
Studies in PWH have shown a high prevalence of emphysema even in individuals without overt respiratory disease.190 In addition, Leung et al found that people with low DLco and a combination of centrilobular and paraseptal emphysema were more likely to have progression of emphysema,191 and significant emphysema burden was associated with increased mortality.192 Elevated TNFα and IL-1β, soluble CD14, nadir CD4, and low CD4/CD8 ratio are also independently associated with emphysema in PWH,140,193,194 although reports of a direct association of HIV with emphysema are contradictory.194,195 While the exact mechanisms are an area of active investigation, HIV-mediated chronic inflammation and immune dysregulation likely play an important role in emphysema formation.
Symptoms, Exacerbations, and Mortality
Compared to HIV-negative individuals, PWH with COPD have a higher respiratory symptom burden, worse quality of life, and an increased risk for COPD exacerbations.24,196–202 For example, PWH with emphysema have a worse chronic cough, increased mucus production, and decreased 6-minute walk distance compared to HIV-negative controls.198 In PWH who inject drugs, obstructive lung disease has been associated with more severe dyspnea than in their seronegative counterparts.203 In addition, PWH perform worse on six-minute walk testing.178 While COPD is associated with increased frailty in individuals with and without HIV, physical limitation scores are worse among PWH.204,205 Finally, COPD in PWH is not only often comorbid with cardiovascular disease, but also a risk factor for myocardial infarction206 and has been associated with increased mortality.180,192
Management of COPD in PWH
PWH have historically been excluded from large randomized controlled trials of COPD treatments. Therefore, there are very few HIV-specific data on COPD management, and instead general COPD guidelines for both chronic disease management and COPD exacerbations are applied to PWH.207 These management strategies include guideline-driven inhaler therapy, pulmonary rehabilitation, routine vaccinations, surgical or bronchoscopic lung volume reduction in qualifying patients, and management of other medical comorbidities.2 Here, we will focus on a few HIV-specific considerations.
Smoking Cessation
Given the high smoking prevalence among PWH and the excess morbidity and mortality associated with smoking in this population, smoking cessation remains a fundamental aspect of COPD care in PWH. Unfortunately, prescribing rates for smoking cessation therapies have been low for PWH with tobacco use disorder for many reasons, including competing clinical priorities, lack of time, low rates of provider training in smoking cessation interventions, and limited knowledge of nicotine replacement therapies and varenicline.208,209 In addition, PWH face additional challenges on the path to sustained smoking cessation that are due to HIV-related stigma, high rates of comorbid substance use, anxiety and depression, financial instability, lack of insurance, low level of education, and racial biases.210–213 Tailoring smoking cessation therapies to this population is an active area of research.209,214–226 Increased awareness among HIV care providers of the importance of smoking cessation, financial support for smoking cessation initiatives, and intervention studies inclusive of PWH are needed to identify the best ways to support smokers with HIV on their path to quitting.
Choice of Inhalers
Special attention should be paid in the treatment of COPD to PWH who are taking ritonavir or other boosted ART regimens. Ritonavir and cobicistat block the CYP3A4 isozyme and can increase the concentration of most corticosteroids. As a result, use of inhaled corticosteroids (ICS) in patients on these medications has been reported to cause Cushing’s syndrome.227–230 Beclomethasone is the ICS drug with the best side effect profile and can be used in PWH treated with ritonavir or cobicistat.230 In PWH who are receiving ritonavir or cobicistat, an added consequence is the inability to use any combination medication for COPD that includes an ICS as fluticasone- and budesonide-containing combination inhaler therapies are contraindicated and beclomethasone is only available as a single, standalone inhaler. Given the already elevated risk of pulmonary tuberculosis and other pneumonias in this population, additional caution should be applied when using ICS, as they can increase the risk of lung infections in this already vulnerable population.231,232
Modulation of Chronic Inflammation
While no HIV-specific COPD therapies exist, there is an interest in the role of modulating chronic inflammation to improve lung function and clinical outcomes. For example, in a small double-blind pilot clinical RCT of rosuvastatin taken daily for the management of COPD in PWH, Morris et al showed that after 24 weeks of daily rosuvastatin therapy, FEV1 stabilized and DLco improved significantly.233 Another trial studied the role of weekly azithromycin in HIV-related chronic lung disease, defined as an irreversible obstructive defect with minimal radiographic abnormalities, in children and adolescents.234 While the authors found no improvement in lung function parameters after 72 weeks of treatment, they noted an increased time to and fewer total exacerbations. Furthermore, data in the general population have shown benefit of using angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) in slowing down the progression of emphysema on chest CT in COPD, albeit with no effect on longitudinal lung function on spirometry.235 A randomized controlled trial by MacDonald et al measured pneumoprotein levels as a proxy for lung function decline in PWH with COPD randomized to placebo or losartan treatment, but did not see any significant changes in the pneumoprotein plasma concentrations after 12 months of follow-up.236 Finally, an NHLBI-funded multi-site randomized controlled trial evaluating the influence of twice daily doxycycline on change in DLco among PWH who smoke is currently underway.237 In sum, findings from prior studies suggest that targeting chronic inflammation has the potential to improve lung function of PWH with COPD, but currently there are no definitive data to support any single drug’s use.
Prevention of COPD in PWH
Smoking Cessation
Smoking is perhaps the single most important modifiable risk factor for COPD among PWH. Evidence suggests that PWH may metabolize nicotine more rapidly than HIV-uninfected smokers,238 which could have important implications for the effectiveness of smoking cessation interventions among this population. A growing body of literature is focused on identifying effective smoking cessation interventions among PWH; Table 1 summarizes the randomized controlled trials that have been conducted or have recently completed enrollment on smoking cessation in PWH.218,220,225,226,239–262 For example, O’Cleirigh et al found that among 41 PWH who smoke and reported motivation to quit, those who were randomized to receive cognitive behavioral therapy for smoking cessation and anxiety/depression treatment in addition to nicotine replacement therapy were more likely to quit smoking compared to those who received nicotine replacement therapy alone,225 highlighting the importance of focusing concomitantly on smoking cessation and mental health in this population. A Cochrane review summarizing 14 randomized controlled trials of smoking cessation interventions among PWH in the United States found that pairing behavioral interventions with medications may facilitate short-term abstinence in comparison to medications alone but did not appear to facilitate long-term abstinence.263 Further, a systematic review of smoking cessation interventions among PWH found that successful smoking cessation was most likely when the intervention included cellphone-based technology.264 Although long-term smoking cessation is the goal, any reduction in exposure to tobacco products is likely to have significant health impacts. Using a Monte Carlo microsimulation model, Reddy et al demonstrated that sustained smoking cessation among PWH could result in over 260,000 expected years of life gained.44 This per-person survival gain is more than the life expectancy gained with early ART initiation or improved ART adherence, and among the general population is more than the life expectancy gained by initiating statins for primary cardiovascular disease prevention or clopidogrel for secondary cardiovascular disease prevention. Therefore, encouraging and supporting smoking cessation must remain a priority in the care for PWH.
 |
Table 1 Summary of Randomized Controlled Trials of Smoking Cessation in People with HIV |
Air Pollution Mitigation
Interventions aimed at reducing personal air pollution exposure can be categorized into policy-level approaches (regional, national, international) and personal-level approaches. Overall, there is no level of air pollution exposure below which there are no negative health impacts. In fact, evidence suggests that the greatest gains in health per unit reduction in air pollution exposure may occur at the lowest end of the exposure spectrum.265 While attention is being paid to regional and national air quality guidelines, individuals with HIV can adopt behavioral changes that may reduce their personal exposure. Evidence to guide these decisions is still an area of active research. In 2019, Carlsten et al published a summary of 10 key approaches to reduce personal exposure to outdoor and indoor pollution sources, including: using close-fitting face masks when exposure is unavoidable; preferential use of active transport (walking or cycling) rather than motorized transport; choosing travel routes that minimize near-road air pollution exposure; optimizing driving style and vehicle settings when in polluted conditions; moderating outdoor physical activity when and where air pollution levels are high; monitoring air pollution levels to inform when individuals should act to minimize exposure; minimizing exposure to household air pollution by using clean fuels, optimizing household ventilation, and adopting efficient cookstoves where possible; and using portable indoor air cleaners.266 Unfortunately, the data supporting these strategies are not of high quality, which highlights the importance of future work focused on carefully designed studies leveraging implementation science methodology to characterize the feasibility, acceptability, and effectiveness of behavioral interventions focused on improving air pollution-associated lung disease.
Infection Prevention
As pulmonary infections, many of which are preventable, have been implicated in the development of COPD among PWH, infection prevention is important for mitigating COPD risk. First, early ART initiation is imperative, as many pulmonary infections such as PJP are opportunistic infections and develop in the setting of high HIV viral loads and low CD4 counts. Primary prophylaxis for PJP prevention is recommended in PWH with CD4 counts <200 cells/mm3 and considered in those with CD4% <14%.267 Given the high morbidity and mortality associated with pneumococcal infection in PWH, pneumococcal immunization has been recommended in all adults with HIV.268 Consistent with general population recommendations, PWH should also receive annual flu vaccination, as well as the full COVID-19 vaccination series. Given the increased risk of TB disease and its associated mortality among PWH, screening for TB is recommended for all PWH at the time of HIV diagnosis and once a CD4 count ≥200 cells/mm3.269 PWH should be tested annually only if they have a history of a negative test for latent TB infection and are at high-risk for repeated or ongoing exposure to people with active TB disease.269 Among PWH diagnosed with latent TB, TB preventive treatment reduces both mortality and progression to active TB and thus should be offered to all PWH with a positive TB screening test without evidence of active TB disease.269,270
Future Directions
Although progress has been made in understanding the underlying mechanisms of COPD among PWH, significant knowledge gaps remain. For example, there are many cross-sectional studies evaluating the prevalence of COPD among PWH but only limited data on the natural disease course of COPD in PWH and whether it differs from the general population. Additionally, while studies suggest that PWH demonstrate a higher risk of COPD and a higher symptom burden, there are no HIV-specific screening guidelines for COPD in PWH. Further research is also needed on the interplay between risk factors such as mode of HIV transmission, biologic sex, aging, CMV infection, air pollution, and TB, as well as a deeper understanding of the epidemiology, development, and progression of chronic lung disease in PWH. Management strategies designed specifically for PWH with COPD are also warranted. Lastly, while much progress has been made in understanding the mechanistic pathways that render PWH particularly vulnerable to developing COPD, we remain limited in our ability to counteract these pathways and prevent COPD development. These are only a few examples highlighting the multiple avenues for future research, all of which have the potential to substantially improve both our scientific understanding of COPD among PWH and our ability to effectively prevent and treat this deadly, irreversible condition.
Conclusions
COPD is highly prevalent among PWH. With an aging global population of PWH, high rates of cigarette smoking, and air pollution, COPD is a growing health challenge, and improved diagnosis and treatment of COPD in PWH will become increasingly important. Further research is needed to understand the underlying mechanisms driving COPD in PWH, as well as HIV-specific screening and treatment modalities.
Disclosure
Katerina L Byanova and Rebecca Abelman are co-first authors for this study. Dr. Byanova was supported by NIH F32 HL166065. Dr. Abelman was supported by NIH T32 AI060530 and K12 HL143961. Dr. North was supported by NIH K23 HL154863. Dr. Christenson was supported by NIH R01 HL143998, she also reports personal fees from AstraZeneca, Sanofi, Regeneron, GlaxoSmithKline, Amgen, MJH Holdings LLC: Physicians’ Education Resource, Glenmark Pharmaceuticals, and Axon Advisors, outside the submitted work. Dr. Huang was supported by NIH R01 HL128156, R01 HL128156-07S2, and R01 HL143998.
References
1. World Health Organization. The Top 10 Causes of Death; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
2. GOLD. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease; 2023.
3. Crothers K, Butt AA, Gibert CL, et al. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130(5):1326–1333. doi:10.1378/chest.130.5.1326
4. Bigna JJ, Kenne AM, Asangbeh SL, Sibetcheu AT. Prevalence of chronic obstructive pulmonary disease in the global population with HIV: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(2):e193–e202. doi:10.1016/S2214-109X(17)30451-5
5. Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388–395. doi:10.1164/rccm.201006-0836OC
6. Drummond MB, Merlo CA, Astemborski J, et al. The effect of HIV infection on longitudinal lung function decline among IDUs: a prospective cohort. AIDS. 2013;27(8):1303–1311. doi:10.1097/QAD.0b013e32835e395d
7. Thudium RF, Ronit A, Afzal S, et al. Faster lung function decline in people living with HIV despite adequate treatment: a longitudinal matched cohort study. Thorax. 2023;78:535–542.
8. Shenoy MK, Iwai S, Lin DL, et al. Immune response and mortality risk relate to distinct lung microbiomes in patients with HIV and pneumonia. Am J Respir Crit Care Med. 2017;195(1):104–114. doi:10.1164/rccm.201603-0523OC
9. Cribbs SK, Crothers K, Morris A. Pathogenesis of HIV-related lung disease: immunity, infection, and inflammation. Physiol Rev. 2020;100(2):603–632. doi:10.1152/physrev.00039.2018
10. Jan AK, Moore JV, Wang RJ, et al. Markers of inflammation and immune activation are associated with lung function in a multi-center cohort of persons with HIV. AIDS. 2021;35(7):1031–1040. doi:10.1097/QAD.0000000000002846
11. Jeon D, Chang EG, McGing M, et al. Pneumoproteins are associated with pulmonary function in HIV-infected persons. PLoS One. 2019;14(10):e0223263. doi:10.1371/journal.pone.0223263
12. Morris A, George MP, Crothers K, et al. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc. 2011;8(3):320–325. doi:10.1513/pats.201006-045WR
13. Madeddu G, Fois AG, Calia GM, et al. Chronic obstructive pulmonary disease: an emerging comorbidity in HIV-infected patients in the HAART era? Infection. 2013;41(2):347–353. doi:10.1007/s15010-012-0330-x
14. Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59(12):1787–1797. doi:10.1093/cid/ciu701
15. Petrache I, Diab K, Knox KS, et al. HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax. 2008;63(5):463–469. doi:10.1136/thx.2007.079111
16. Rowell-Cunsolo TL, Hu G, Bellerose M, Liu J. Trends in comorbidities among human immunodeficiency virus-infected hospital admissions in New York City from 2006–2016. Clin Infect Dis. 2021;73(7):e1957–e1963. doi:10.1093/cid/ciaa1760
17. Byanova K, Kunisaki KM, Vasquez J, Huang L. Chronic obstructive pulmonary disease in HIV. Expert Rev Respir Med. 2021;15(1):71–87. doi:10.1080/17476348.2021.1848556
18. Kunisaki KM. Recent advances in HIV-associated chronic lung disease clinical research. Curr Opin HIV AIDS. 2021;16(3):156–162. doi:10.1097/COH.0000000000000679
19. Leung JM. HIV and chronic lung disease. Curr Opin HIV AIDS. 2023;18(2):93–101. doi:10.1097/COH.0000000000000777
20. Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86(1):76–85. doi:10.1159/000350917
21. Kurmi OP, Sadhra CS, Ayres JG, Sadhra SS. Tuberculosis risk from exposure to solid fuel smoke: a systematic review and meta-analysis. J Epidemiol Community Health. 2014;68(12):1112–1118. doi:10.1136/jech-2014-204120
22. Lee KK, Bing R, Kiang J, et al. Adverse health effects associated with household air pollution: a systematic review, meta-analysis, and burden estimation study. Lancet Glob Health. 2020;8(11):e1427–e1434. doi:10.1016/S2214-109X(20)30343-0
23. Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182(6):790–796. doi:10.1164/rccm.200912-1858OC
24. George MP, Kannass M, Huang L, Sciurba FC, Morris A, Pai NP. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4(7):e6328. doi:10.1371/journal.pone.0006328
25. Kunisaki KM, Niewoehner DE, Collins G, et al. Pulmonary effects of immediate versus deferred antiretroviral therapy in HIV-positive individuals: a nested substudy within the multicentre, international, randomised, controlled strategic timing of antiretroviral treatment (START) trial. Lancet Respir Med. 2016;4(12):980–989. doi:10.1016/S2213-2600(16)30319-8
26. Konstantinidis I, Qin S, Fitzpatrick M, et al. Pulmonary function trajectories in people with HIV: analysis of the Pittsburgh HIV Lung Cohort. Ann Am Thorac Soc. 2022;9(12):2013–2020. doi:10.1513/AnnalsATS.202204-332OC
27. McNeill J, Okello S, Sentongo R, et al. Chronic HIV infection is associated with accelerated FEV1 decline among women but not among men: a longitudinal cohort study in Uganda. Ann Am Thorac Soc. 2022;19(10):1779–1783. doi:10.1513/AnnalsATS.202111-1275RL
28. Wang RJ, Nouraie M, Kunisaki KM, et al. Lung function in women with and without human immunodeficiency virus. Clin Infect Dis. 2023;76(3):e727–e735. doi:10.1093/cid/ciac391
29. Fitzpatrick ME, Gingo MR, Kessinger C, et al. HIV infection is associated with diffusing capacity impairment in women. J Acquir Immune Defic Syndr. 2013;64(3):284–288. doi:10.1097/QAI.0b013e3182a9213a
30. Gingo MR, Balasubramani GK, Rice TB, et al. Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulm Med. 2014;14(1):75. doi:10.1186/1471-2466-14-75
31. Abelman RA, Fitzpatrick J, Zawedde J, et al. Sex modifies the risk of HIV-associated obstructive lung disease in Ugandans post-pneumonia. AIDS. 2023;37(11):1683–1692. doi:10.1097/QAD.0000000000003626
32. Ronit A, Lundgren J, Afzal S, et al. Airflow limitation in people living with HIV and matched uninfected controls. Thorax. 2018;73(5):431–438. doi:10.1136/thoraxjnl-2017-211079
33. Yang L, Dunlap DG, Qin S, et al. Alterations in oral microbiota in HIV are related to decreased pulmonary function. Am J Respir Crit Care Med. 2020;201(4):445–457. doi:10.1164/rccm.201905-1016OC
34. Shipley TW, Kling HM, Morris A, et al. Persistent pneumocystis colonization leads to the development of chronic obstructive pulmonary disease in a nonhuman primate model of AIDS. J Infect Dis. 2010;202(2):302–312. doi:10.1086/653485
35. Hernandez Cordero AI, Yang CX, Obeidat M, et al. DNA methylation is associated with airflow obstruction in patients living with HIV. Thorax. 2021;76(5):448–455. doi:10.1136/thoraxjnl-2020-215866
36. Hernandez Cordero AI, Yang CX, Yang J, et al. Airway aging and methylation disruptions in HIV-associated chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2022;206(2):150–160. doi:10.1164/rccm.202106-1440OC
37. Liu JC, Leung JM, Ngan DA, et al. Absolute leukocyte telomere length in HIV-infected and uninfected individuals: evidence of accelerated cell senescence in HIV-associated chronic obstructive pulmonary disease. PLoS One. 2015;10(4):e0124426. doi:10.1371/journal.pone.0124426
38. Xu S, Vucic EA, Shaipanich T, et al. Decreased telomere length in the small airway epithelium suggests accelerated aging in the lungs of persons living with human immunodeficiency virus (HIV). Respir Res. 2018;19(1):117. doi:10.1186/s12931-018-0821-0
39. Crothers K. Chronic obstructive pulmonary disease in patients who have HIV infection. Clin Chest Med. 2007;28(3):575–587, vi. doi:10.1016/j.ccm.2007.06.004
40. Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–344. doi:10.7326/M14-0954
41. Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J, Siddiqi K. Tobacco use among people living with HIV: analysis of data from demographic and health surveys from 28 low-income and middle-income countries. Lancet Glob Health. 2017;5(6):e578–e592. doi:10.1016/S2214-109X(17)30170-5
42. Johnston PI, Wright SW, Orr M, et al. Worldwide relative smoking prevalence among people living with and without HIV. AIDS. 2021;35(6):957–970. doi:10.1097/QAD.0000000000002815
43. Diaz PT, King MA, Pacht ER, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132:369–372.
44. Reddy KP, Parker RA, Losina E, et al. Impact of cigarette smoking and smoking cessation on life expectancy among people with HIV: a US-based modeling study. J Infect Dis. 2016;214(11):1672–1681. doi:10.1093/infdis/jiw430
45. Helleberg M, May MT, Ingle SM, et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS. 2015;29(2):221–229. doi:10.1097/QAD.0000000000000540
46. Helleberg M, Afzal S, Kronborg G, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56(5):727–734. doi:10.1093/cid/cis933
47. Corleis B, Cho JL, Gates SJ, et al. Smoking and human immunodeficiency virus 1 infection promote retention of CD8(+) T cells in the airway mucosa. Am J Respir Cell Mol Biol. 2021;65(5):513–520. doi:10.1165/rcmb.2021-0168OC
48. Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet. 2017;389(10082):1907–1918. doi:10.1016/S0140-6736(17)30505-6
49. Campbell-Lendrum D, Prüss-Ustün A. Climate change, air pollution and noncommunicable diseases. Bull World Health Organ. 2019;97(2):160–161. doi:10.2471/BLT.18.224295
50. Health Effects Institute. State of Global Air 2020: A Special Report on Global Exposure to Air Pollution and Its Health Impacts. Boston, MA: Health Effects Institute; 2020.
51. Karagulian F, Belis CA, Dora CFC, et al. Contributions to cities’ ambient particulate matter (PM): a systematic review of local source contributions at global level. Atmos Environ. 2015;120:475–483. doi:10.1016/j.atmosenv.2015.08.087
52. Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–1067. doi:10.1056/NEJMoa040610
53. Rice MB, Ljungman PL, Wilker EH, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med. 2015;191(6):656–664. doi:10.1164/rccm.201410-1875OC
54. Rice MB, Li W, Schwartz J, et al. Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: the Framingham Heart Study. Thorax. 2019;74(11):1063–1069. doi:10.1136/thoraxjnl-2018-212877
55. Rice MB, Ljungman PL, Wilker EH, et al. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med. 2013;188(11):1351–1357. doi:10.1164/rccm.201308-1414OC
56. Sack C, Vedal S, Sheppard L, et al. Air pollution and subclinical interstitial lung disease: the multi-ethnic study of atherosclerosis (Mesa) air-lung study. Eur Respir J. 2017;50(6):1700559. doi:10.1183/13993003.00559-2017
57. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581–1592. doi:10.1016/S0140-6736(14)60617-6
58. Li J, Sun S, Tang R, et al. Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:3079–3091. doi:10.2147/COPD.S122282
59. Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med. 2004;169(7):816–821. doi:10.1164/rccm.200306-779OC
60. Rhee J, Dominici F, Zanobetti A, et al. Impact of Long-Term Exposures to Ambient PM(2.5) and Ozone on ARDS Risk for Older Adults in the United States. Chest. 2019;156(1):71–79. doi:10.1016/j.chest.2019.03.017
61. Pope D, Diaz E, Smith-Sivertsen T, et al. Exposure to household air pollution from wood combustion and association with respiratory symptoms and lung function in nonsmoking women: results from the RESPIRE trial, Guatemala. Environ Health Perspect. 2015;123(4):285–292. doi:10.1289/ehp.1408200
62. Siddharthan T, Grigsby MR, Goodman D, et al. Association between household air pollution exposure and chronic obstructive pulmonary disease outcomes in 13 low- and middle-income country settings. Am J Respir Crit Care Med. 2018;197(5):611–620. doi:10.1164/rccm.201709-1861OC
63. Wang M, Aaron CP, Madrigano J, et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA. 2019;322(6):546–556. doi:10.1001/jama.2019.10255
64. Liu C, Chen R, Sera F, et al. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med. 2019;381(8):705–715. doi:10.1056/NEJMoa1817364
65. Cromar KR, Gladson LA, Ewart G. Trends in excess morbidity and mortality associated with air pollution above American thoracic society-recommended standards, 2008–2017. Ann Am Thorac Soc. 2019;16(7):836–845. doi:10.1513/AnnalsATS.201812-914OC
66. Ramirez-Venegas A, Sansores RH, Quintana-Carrillo RH, et al. FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am J Respir Crit Care Med. 2014;190(9):996–1002. doi:10.1164/rccm.201404-0720OC
67. González-García M, Maldonado Gomez D, Torres-Duque CA, et al. Tomographic and functional findings in severe COPD: comparison between the wood smoke-related and smoking-related disease. J Bras Pneumol. 2013;39(2):147–154. doi:10.1590/S1806-37132013000200005
68. Camp PG, Ramirez-Venegas A, Sansores RH, et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur Respir J. 2014;43(3):725–734. doi:10.1183/09031936.00206112
69. Rivera RM, Cosio MG, Ghezzo H, Salazar M, Perez-Padilla R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis. 2008;12(8):972–977.
70. Ghosh B, Gaike AH, Pyasi K, et al. Bacterial load and defective monocyte-derived macrophage bacterial phagocytosis in biomass smoke-related COPD. Eur Respir J. 2019;53(2):1702273. doi:10.1183/13993003.02273-2017
71. Sumpter C, Chandramohan D. Systematic review and meta-analysis of the associations between indoor air pollution and tuberculosis. Trop Med Int Health. 2013;18(1):101–108. doi:10.1111/tmi.12013
72. Rivas-Santiago CE, Sarkar S, Cantarella P, et al. Air pollution particulate matter alters antimycobacterial respiratory epithelium innate immunity. Infect Immun. 2015;83(6):2507–2517. doi:10.1128/IAI.03018-14
73. Blount RJ, Djawe K, Daly KR, et al. Ambient air pollution associated with suppressed serologic responses to Pneumocystis jirovecii in a prospective cohort of HIV-infected patients with Pneumocystis pneumonia. PLoS One. 2013;8(11):e80795. doi:10.1371/journal.pone.0080795
74. Djawe K, Levin L, Swartzman A, et al. Environmental risk factors for Pneumocystis pneumonia hospitalizations in HIV patients. Clin Infect Dis. 2013;56(1):74–81. doi:10.1093/cid/cis841
75. Blount RJ, Daly KR, Fong S, et al. Effects of clinical and environmental factors on bronchoalveolar antibody responses to Pneumocystis jirovecii: a prospective cohort study of HIV+ patients. PLoS One. 2017;12(7):e0180212. doi:10.1371/journal.pone.0180212
76. North CM, MacNaughton P, Lai PS, et al. Personal carbon monoxide exposure, respiratory symptoms, and the potentially modifying roles of sex and HIV infection in rural Uganda: a cohort study. Environ Health. 2019;18(1):73. doi:10.1186/s12940-019-0517-z
77. World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025. Geneva: World Health Organization; 2019.
78. Collaborators GBDT, Fullman N, Ng M. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the global burden of disease study 2015. Lancet. 2017;389(10082):1885–1906. doi:10.1016/S0140-6736(17)30819-X
79. Han L, Zhou W, Li W, Li L. Impact of urbanization level on urban air quality: a case of fine particles (PM(2.5)) in Chinese cities. Environ Pollut. 2014;194:163–170. doi:10.1016/j.envpol.2014.07.022
80. O’Connor J, Vjecha MJ, Phillips AN, et al. Effect of immediate initiation of antiretroviral therapy on risk of severe bacterial infections in HIV-positive people with CD4 cell counts of more than 500 cells per muL: secondary outcome results from a randomised controlled trial. Lancet HIV. 2017;4(3):e105–e112. doi:10.1016/S2352-3018(16)30216-8
81. Balakrishna S, Wolfensberger A, Kachalov V, et al. Decreasing Incidence and Determinants of Bacterial Pneumonia in People With HIV: the Swiss HIV Cohort Study. J Infect Dis. 2022;225(9):1592–1600. doi:10.1093/infdis/jiab573
82. Hull MW, Phillips P, Montaner JSG. Changing global epidemiology of pulmonary manifestations of HIV/AIDS. Chest. 2008;134(6):1287–1298. doi:10.1378/chest.08-0364
83. Sogaard OS, Lohse N, Gerstoft J, et al. Hospitalization for pneumonia among individuals with and without HIV infection, 1995–2007: a Danish population-based, nationwide cohort study. Clin Infect Dis. 2008;47(10):1345–1353. doi:10.1086/592692
84. Aston SJ, Ho A, Jary H, et al. Etiology and risk factors for mortality in an adult community-acquired pneumonia cohort in Malawi. Am J Respir Crit Care Med. 2019;200(3):359–369. doi:10.1164/rccm.201807-1333OC
85. Brown J, Pickett E, Smith C, et al. The effect of HIV status on the frequency and severity of acute respiratory illness. PLoS One. 2020;15(5):e0232977. doi:10.1371/journal.pone.0232977
86. Varkila MRJ, Vos AG, Barth RE, et al. The association between HIV infection and pulmonary function in a rural African population. PLoS One. 2019;14(1):e0210573. doi:10.1371/journal.pone.0210573
87. North CM, Allen JG, Okello S, et al. HIV infection, pulmonary tuberculosis and COPD in rural Uganda: a cross-sectional Study. Lung. 2018;196(1):49–57. doi:10.1007/s00408-017-0080-8
88. Morris A, Sciurba FC, Norris KA. Pneumocystis: a novel pathogen in chronic obstructive pulmonary disease? COPD. 2008;5(1):43–51. doi:10.1080/15412550701817656
89. Morris A, Huang L, Bacchetti P, et al. Permanent declines in pulmonary function following pneumonia in human immunodeficiency virus-infected persons. Am J Respir Crit Care Med. 2000;162(2):612–616. doi:10.1164/ajrccm.162.2.9912058
90. Drummond MB, Huang L, Diaz PT, et al. Factors associated with abnormal spirometry among HIV-infected individuals. AIDS. 2015;29(13):1691–1700. doi:10.1097/QAD.0000000000000750
91. Fitzpatrick ME, Tedrow JR, Hillenbrand ME, et al. Pneumocystis jirovecii colonization is associated with enhanced Th1 inflammatory gene expression in lungs of humans with chronic obstructive pulmonary disease. Microbiol Immunol. 2014;58(3):202–211. doi:10.1111/1348-0421.12135
92. Norris KA, Morris A, Patil S, Fernandes E. Pneumocystis colonization, airway inflammation, and pulmonary function decline in acquired immunodeficiency syndrome. Immunol Res. 2006;36(1–3):175–187. doi:10.1385/IR:36:1:175
93. Attia E, McGinnis K, Feemster LC, et al. Association of COPD with risk for pulmonary infections requiring hospitalization in HIV-infected veterans. J Acquir Immune Defic Syndr. 2015;70(3):280–288. doi:10.1097/QAI.0000000000000751
94. Alexandrova Y, Costiniuk CT, Jenabian MA. Pulmonary Immune Dysregulation and Viral Persistence During HIV Infection. Front Immunol. 2021;12:808722. doi:10.3389/fimmu.2021.808722
95. Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis. 2016;214(suppl 2):S44–S50. doi:10.1093/infdis/jiw275
96. De P, Farley A, Lindson N, Aveyard P. Systematic review and meta-analysis: influence of smoking cessation on incidence of pneumonia in HIV. BMC Med. 2013;15(11):1–12.
97. UNAIDS. UNAIDS Tuberculosis and HIV; 2022. Available from: https://www.unaids.org/en/resources/infographics/tuberculosis-and-hiv.
98. World Health Organization. Global Tuberculosis Report 2022. Geneva: World Health Organization; 2022.
99. Vasiliu A, Abelman R, Kherabi Y, Iswari Saktiawati AM, Kay A. Landscape of TB infection and prevention among people living with HIV. Pathogens. 2022;11(1552):1–14. doi:10.3390/pathogens11010001
100. Allwood BW, Byrne A, Meghji J, Rachow A, van der Zalm MM, Schoch OD. Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respiration. 2021;100(8):751–763. doi:10.1159/000512531
101. Samperiz G, Guerrero D, Lopez M, et al. Prevalence of and risk factors for pulmonary abnormalities in HIV-infected patients treated with antiretroviral therapy. HIV Med. 2014;15(6):321–329. doi:10.1111/hiv.12117
102. Ralph AP, Kenangalem E, Waramori G, et al. High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: under-recognised phenomena. PLoS One. 2013;8(11):e80302. doi:10.1371/journal.pone.0080302
103. Fiogbe AA, Agodokpessi G, Tessier JF, et al. Prevalence of lung function impairment in cured pulmonary tuberculosis patients in Cotonou, Benin. Int J Tuberc Lung Dis. 2019;23(2):195–202. doi:10.5588/ijtld.18.0234
104. Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55:32–38. doi:10.1136/thorax.55.1.32
105. Manji M, Shayo G, Mamuya S, Mpembeni R, Jusabani A, Mugusi F. Lung functions among patients with pulmonary tuberculosis in Dar es Salaam - a cross-sectional study. BMC Pulm Med. 2016;16(1):58. doi:10.1186/s12890-016-0213-5
106. Meghji J, Lesosky M, Joekes E, et al. Patient outcomes associated with post-tuberculosis lung damage in Malawi: a prospective cohort study. Thorax. 2020;75(3):269–278. doi:10.1136/thoraxjnl-2019-213808
107. Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–2283. doi:10.1097/QAD.0b013e3280108704
108. Cheng J, Ke Q, Jin Z, et al. Cytomegalovirus infection causes an increase of arterial blood pressure. PLoS Pathog. 2009;5(5):e1000427. doi:10.1371/journal.ppat.1000427
109. Levi LI, Sharma S, Schleiss MR, et al. Cytomegalovirus viremia and risk of disease progression and death in HIV-positive patients starting antiretroviral therapy. AIDS. 2022;36(9):1265–1272. doi:10.1097/QAD.0000000000003238
110. Lichtner M, Cicconi P, Vita S, et al. Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J Infect Dis. 2015;211(2):178–186. doi:10.1093/infdis/jiu417
111. Wang H, Peng G, Bai J, et al. Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): a meta-analysis of prospective studies up to 2016. J Am Heart Assoc. 2017;6(7). doi:10.1161/JAHA.116.005025
112. Hodowanec AC, Lurain NS, Krishnan S, Bosch RJ, Landay AL. Increased CMV IgG antibody titer is associated with Non-AIDS events among virologically suppressed HIV-positive persons. Pathog Immun. 2019;4(1):66–78. doi:10.20411/pai.v4i1.255
113. Nenna R, Zhai J, Packard SE, et al. High cytomegalovirus serology and subsequent COPD-related mortality: a longitudinal study. ERJ Open Res. 2020;6(2):00062–2020. doi:10.1183/23120541.00062-2020
114. Hameiri Bowen D, Sovershaeva E, Charlton B, et al. Cytomegalovirus-specific immunoglobulin G is associated with chronic lung disease in children and adolescents from sub-saharan Africa living with perinatal human immunodeficiency virus. Clin Infect Dis. 2021;73(1):e264–e266. doi:10.1093/cid/ciaa1757
115. Burkes R, Osterburg A, Hwalek T, Lach L, Panos RJ, Borchers MT. Cytomegalovirus seropositivity is associated with airflow limitation in a cohort of veterans with a high prevalence of smoking. Chronic Obstr Pulm Dis. 2021;8(4):441–449. doi:10.15326/jcopdf.2021.0221
116. van Son WJ, Tegzess AM, Hauw T, et al. Pulmonary dysfunction is common during a cytomegalovirus infection after renal transplantation even in asymptomatic patients. Possible relationship with complement activation. Am Rev Respir Dis. 1987;136(3):580–585. doi:10.1164/ajrccm/136.3.580
117. Wasilewska E, Kuziemski K, Niedoszytko M, et al. Impairment of lung diffusion capacity-a new consequence in the long-term childhood leukaemia survivors. Ann Hematol. 2019;98(9):2103–2110. doi:10.1007/s00277-019-03745-4
118. de Maar EF, Verschuuren EAM, Harmsen MC, The TH, van Son WJ. Pulmonary involvement during cytomegalovirus infection in immunosuppressed patients. Transpl Infect Dis. 2003;5(3):112–120. doi:10.1034/j.1399-3062.2003.00023.x
119. Ramendra R, Isnard S, Lin J, et al. CMV seropositivity is associated with increased microbial translocation in people living with HIV and uninfected controls. Clin Infect Dis. 2020;71(6):1438–1446. doi:10.1093/cid/ciz1001
120. Christensen-Quick A, Vanpouille C, Lisco A, Gianella S. Cytomegalovirus and HIV Persistence: pouring Gas on the Fire. AIDS Res Hum Retroviruses. 2017;33(S1):S23–S30. doi:10.1089/aid.2017.0145
121. Fitzpatrick ME, Nouraie M, Gingo MR, et al. Novel relationships of markers of monocyte activation and endothelial dysfunction with pulmonary dysfunction in HIV-infected persons. AIDS. 2016;30(9):1327–1339. doi:10.1097/QAD.0000000000001092
122. Lurain NS, Hanson BA, Hotton AL, Weber KM, Cohen MH, Landay AL. The association of human cytomegalovirus with biomarkers of inflammation and immune activation in HIV-1-infected women. AIDS Res Hum Retroviruses. 2016;32(2):134–143. doi:10.1089/aid.2015.0169
123. Hodowanec A, Williams B, Hanson B, et al. Soluble CD163 but not soluble CD14 is associated with cytomegalovirus immunoglobulin G antibody levels in virologically suppressed HIV+ individuals. J Acquir Immune Defic Syndr. 2015;70(5):e171–174. doi:10.1097/QAI.0000000000000841
124. Vita S, Lichtner M, Marchetti G, et al. Soluble CD163 in CMV-infected and CMV-uninfected subjects in virologically suppressive antiretroviral therapy in the ICONA cohort. J Acquir Immune Defic Syndr. 2017;74(3):347–352. doi:10.1097/QAI.0000000000001232
125. Risso K, Guillouet-de-Salvador F, Valerio L, et al. COPD in HIV-infected patients: CD4 cell count highly correlated. PLoS One. 2017;12(1):e0169359. doi:10.1371/journal.pone.0169359
126. Li Y, Nouraie SM, Kessinger C, et al. Factors associated with progression of lung function abnormalities in HIV-infected individuals. J Acquir Immune Defic Syndr. 2018;79(4):501–509. doi:10.1097/QAI.0000000000001840
127. Collini PJ, Bewley MA, Mohasin M, et al. HIV gp120 in the lungs of antiretroviral therapy-treated individuals impairs alveolar macrophage responses to pneumococci. Am J Respir Crit Care Med. 2018;197(12):1604–1615. doi:10.1164/rccm.201708-1755OC
128. Cota-Gomez A, Flores AC, Ling XF, Varella-Garcia M, Flores SC. HIV-1 Tat increases oxidant burden in the lungs of transgenic mice. Free Radic Biol Med. 2011;51(9):1697–1707. doi:10.1016/j.freeradbiomed.2011.07.023
129. Brune KA, Ferreira F, Mandke P, et al. HIV impairs lung epithelial integrity and enters the epithelium to promote chronic lung inflammation. PLoS One. 2016;11(3):e0149679. doi:10.1371/journal.pone.0149679
130. Popescu I, Drummond MB, Gama L, et al. Activation-induced cell death drives profound lung CD4(+) T-cell depletion in HIV-associated chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190(7):744–755. doi:10.1164/rccm.201407-1226OC
131. Kaner RJ, Santiago F, Crystal RG. Up-regulation of alveolar macrophage matrix metalloproteinases in HIV1(+) smokers with early emphysema. J Leukoc Biol. 2009;86(4):913–922. doi:10.1189/jlb.0408240
132. Cribbs SK, Lennox J, Caliendo AM, Brown LA, Guidot DM. Healthy HIV-1-infected individuals on highly active antiretroviral therapy harbor HIV-1 in their alveolar macrophages. AIDS Res Hum Retroviruses. 2015;31(1):64–70. doi:10.1089/aid.2014.0133
133. Lamers SL, Rose R, Maidji E, et al. HIV DNA is frequently present within pathologic tissues evaluated at autopsy from combined antiretroviral therapy-treated patients with undetectable viral loads. J Virol. 2016;90(20):8968–8983. doi:10.1128/JVI.00674-16
134. Costiniuk CT, Salahuddin S, Farnos O, et al. HIV persistence in mucosal CD4+ T cells within the lungs of adults receiving long-term suppressive antiretroviral therapy. AIDS. 2018;32(16):2279–2289. doi:10.1097/QAD.0000000000001962
135. Gundavarapu S, Mishra NC, Singh SP, et al. HIV gp120 induces mucus formation in human bronchial epithelial cells through CXCR4/alpha7-nicotinic acetylcholine receptors. PLoS One. 2013;8(10):e77160. doi:10.1371/journal.pone.0077160
136. Atkinson JJ, Lutey BA, Suzuki Y, et al. The role of matrix metalloproteinase-9 in cigarette smoke-induced emphysema. Am J Respir Crit Care Med. 2011;183(7):876–884. doi:10.1164/rccm.201005-0718OC
137. Drummond MB, Kirk GD, Astemborski J, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012;67(4):309–314. doi:10.1136/thoraxjnl-2011-200702
138. Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–1202. doi:10.1038/nm1482
139. Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi:10.1038/nature05115
140. Triplette M, Attia EF, Akgun KM, et al. A low peripheral blood CD4/CD8 ratio is associated with pulmonary emphysema in HIV. PLoS One. 2017;12(1):e0170857. doi:10.1371/journal.pone.0170857
141. Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10(5):e1004078. doi:10.1371/journal.ppat.1004078
142. Lassiter C, Fan X, Joshi PC, et al. HIV-1 transgene expression in rats causes oxidant stress and alveolar epithelial barrier dysfunction. AIDS Res Ther. 2009;6(1):1. doi:10.1186/1742-6405-6-1
143. Chinnapaiyan S, Dutta R, Bala J, et al. Cigarette smoke promotes HIV infection of primary bronchial epithelium and additively suppresses CFTR function. Sci Rep. 2018;8(1):7984. doi:10.1038/s41598-018-26095-z
144. Chand HS, Vazquez-Guillamet R, Royer C, et al. Cigarette smoke and HIV synergistically affect lung pathology in cynomolgus macaques. J Clin Invest. 2018;128(12):5428–5433. doi:10.1172/JCI121935
145. Chung NPY, Khan KMF, Kaner RJ, O’Beirne SL, Crystal RG. HIV induces airway basal progenitor cells to adopt an inflammatory phenotype. Sci Rep. 2021;11(1):3988. doi:10.1038/s41598-021-82143-1
146. Beck JM, Schloss PD, Venkataraman A, et al. Multicenter comparison of lung and oral microbiomes of HIV-infected and HIV-uninfected individuals. Am J Respir Crit Care Med. 2015;192(11):1335–1344. doi:10.1164/rccm.201501-0128OC
147. Segal LN, Alekseyenko AV, Clemente JC, et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome. 2013;1(1):19. doi:10.1186/2049-2618-1-19
148. Twigg HL, Knox KS, Zhou J, et al. Effect of advanced HIV Infection on the respiratory microbiome. Am J Respir Crit Care Med. 2016;194(2):226–235. doi:10.1164/rccm.201509-1875OC
149. Li SX, Armstrong A, Neff CP, Shaffer M, Lozupone CA, Palmer BE. Complexities of gut microbiome dysbiosis in the context of HIV infection and antiretroviral therapy. Clin Pharmacol Ther. 2016;99(6):600–611. doi:10.1002/cpt.363
150. Quiros-Roldan E, Pezzoli MC, Berlendis M, et al. A COPD case-finding program in a large cohort of HIV-infected persons. Respir Care. 2019;64(2):169–175. doi:10.4187/respcare.06247
151. Zifodya JS, Triplette M, Shahrir S, et al. A cross-sectional analysis of diagnosis and management of chronic obstructive pulmonary disease in people living with HIV: opportunities for improvement. Medicine (Baltimore). 2021;100(37):e27124. doi:10.1097/MD.0000000000027124
152. USPSTF. Final recommendation statement: chronic obstructive pulmonary disease: screening. US Preventive Services Task Force; 2022.
153. Shirley DK, Kaner RJ, Glesby MJ. Screening for Chronic Obstructive Pulmonary Disease (COPD) in an Urban HIV Clinic: a Pilot Study. AIDS Patient Care STDS. 2015;29(5):232–239. doi:10.1089/apc.2014.0265
154. Ghadaki B, Kronfli N, Vanniyasingam T, Haider S. Chronic obstructive pulmonary disease and HIV: are we appropriately screening? AIDS Care. 2016;28(10):1338–1343. doi:10.1080/09540121.2016.1189499
155. Lambert AA, Drummond MB, Kisalu A, et al. Implementation of a COPD screening questionnaire in an outpatient HIV clinic. COPD. 2016;13(6):767–772. doi:10.3109/15412555.2016.1161016
156. Costiniuk CT, Nitulescu R, Saneei Z, et al. Prevalence and predictors of airflow obstruction in an HIV tertiary care clinic in Montreal, Canada: a cross-sectional study. HIV Med. 2019;20(3):192–201. doi:10.1111/hiv.12699
157. Verboeket SO, Boyd A, Wit FW, et al. Changes in lung function among treated HIV-positive and HIV-negative individuals- analysis of the prospective AGEhIV cohort study. Lancet Healthy Longev. 2021;2(4):e202–211. doi:10.1016/S2666-7568(21)00033-7
158. Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95–99. doi:10.2147/COPD.S27480
159. Githinji LN, Gray DM, Hlengwa S, Myer L, Zar HJ. Lung function in South African adolescents infected perinatally with HIV and treated long-term with antiretroviral therapy. Ann Am Thorac Soc. 2017;14(5):722–729. doi:10.1513/AnnalsATS.201612-1018OC
160. Desai SR, Nair A, Rylance J, et al. Human immunodeficiency virus-associated chronic lung disease in children and adolescents in Zimbabwe: chest radiographic and high-resolution computed tomographic findings. Clin Infect Dis. 2018;66(2):274–281. doi:10.1093/cid/cix778
161. Barrera CA, du Plessis A-M, Otero HJ, et al. Quantitative CT analysis for bronchiolitis obliterans in perinatally HIV-infected adolescents—comparison with controls and lung function data. Eur Radiol. 2020;30(8):4358–4368. doi:10.1007/s00330-020-06789-7
162. du Plessis AM, Andronikou S, Machemedze T, et al. High-resolution computed tomography features of lung disease in perinatally HIV-infected adolescents on combined antiretroviral therapy. Pediatr Pulmonol. 2019;54(11):1765–1773. doi:10.1002/ppul.24450
163. Githinji LN, Gray DM, Zar HJ. Lung function in HIV-infected children and adolescents. Pneumonia. 2018;10(6):1–10. doi:10.1186/s41479-017-0045-y
164. Attia EF, Bhatraju PK, Triplette M, et al. Endothelial activation, innate immune activation, and inflammation are associated with postbronchodilator airflow limitation and obstruction among adolescents living with HIV. J Acquir Immune Defic Syndr. 2020;83(3):267–277. doi:10.1097/QAI.0000000000002255
165. Attia EF, Jacobson D, Yu W, et al. Immune imbalance and activation are associated with lower lung function in youth with perinatally acquired HIV. J Allergy Clin Immunol. 2020;145(5):1473–1476. doi:10.1016/j.jaci.2019.12.890
166. Attia EF, Maleche-Obimbo E, West TE, et al. Adolescent age is an independent risk factor for abnormal spirometry among people living with HIV in Kenya. AIDS. 2018;32(10):1353–1359. doi:10.1097/QAD.0000000000001815
167. Gray DM, Wedderburn CJ, MacGinty RP, et al. Impact of HIV and antiretroviral drug exposure on lung growth and function over 2 years in an African Birth Cohort. AIDS. 2020;34(4):549–558. doi:10.1097/QAD.0000000000002444
168. Voraphani N, Stern DA, Zhai J, et al. The role of growth and nutrition in the early origins of spirometric restriction in adult life: a longitudinal, multicohort, population-based study. Lancet Respir Med. 2022;10(1):59–71. doi:10.1016/S2213-2600(21)00355-6
169. Rylance S, Masekela R, Banda NPK, Mortimer K. Determinants of lung health across the life course in sub-Saharan Africa. Int J Tuberc Lung Dis. 2020;24(9):892–901. doi:10.5588/ijtld.20.0083
170. Crothers K, McGinnis K, Kleerup E, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr. 2013;64(3):271–278. doi:10.1097/QAI.0b013e3182a9215a
171. Raju S, Astemborski J, Drummond MB, et al. HIV is associated with impaired pulmonary diffusing capacity independent of emphysema. J Acquir Immune Defic Syndr. 2022;89(1):64–68. doi:10.1097/QAI.0000000000002818
172. Simonetti JA, Gingo MR, Kingsley L, et al. Pulmonary function in HIV-Infected recreational drug users in the era of anti-retroviral therapy. J AIDS Clin Res. 2014;5(11):365. doi:10.4172/2155-6113.1000365
173. Kirby M, Owrangi A, Svenningsen S, et al. On the role of abnormal DL(CO) in ex-smokers without airflow limitation: symptoms, exercise capacity and hyperpolarised helium-3 MRI. Thorax. 2013;68(8):752–759. doi:10.1136/thoraxjnl-2012-203108
174. Garcia-Rio F, Miravitlles M, Soriano JB, et al. Prevalence of reduced lung diffusing capacity and CT scan findings in smokers without airflow limitation: a population-based study. BMJ Open Respir Res. 2023;10(1):e001468. doi:10.1136/bmjresp-2022-001468
175. Criner RN, Hatt CR, Galban CJ, et al. Relationship between diffusion capacity and small airway abnormality in COPDGene. Respir Res. 2019;20(1):269. doi:10.1186/s12931-019-1237-1
176. Byanova KL, Fitzpatrick J, Jan AK, et al. Isolated abnormal diffusing capacity for carbon monoxide (iso↓DLco) is associated with increased respiratory symptom burden in people with HIV infection. PLoS One. 2023;18(7):e0288803. doi:10.1371/journal.pone.0288803
177. Diaz AA, Pinto-Plata V, Hernandez C, et al. Emphysema and DLCO predict a clinically important difference for 6MWD decline in COPD. Respir Med. 2015;109(7):882–889. doi:10.1016/j.rmed.2015.04.009
178. Robertson TE, Nouraie M, Qin S, et al. HIV infection is an independent risk factor for decreased 6-minute walk test distance. PLoS One. 2019;14(4):e0212975. doi:10.1371/journal.pone.0212975
179. Chandra D, Gupta A, Fitzpatrick M, et al. Lung function, coronary artery disease, and mortality in HIV. Ann Am Thorac Soc. 2019;16(6):687–697. doi:10.1513/AnnalsATS.201807-460OC
180. Gingo MR, Nouraie M, Kessinger CJ, et al. Decreased lung function and all-cause mortality in HIV-infected individuals. Ann Am Thorac Soc. 2018;15(2):192–199. doi:10.1513/AnnalsATS.201606-492OC
181. Bhatt SP, Washko GR, Hoffman EA, et al. Imaging Advances in Chronic Obstructive Pulmonary Disease. Insights from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) Study. Am J Respir Crit Care Med. 2019;199(3):286–301. doi:10.1164/rccm.201807-1351SO
182. Arjomandi M, Zeng S, Barjaktarevic I, et al. Radiographic lung volumes predict progression to COPD in smokers with preserved spirometry in SPIROMICS. Eur Respir J. 2019;54(4):1802214. doi:10.1183/13993003.02214-2018
183. Ash SY, Harmouche R, Ross JC, et al. Interstitial features at chest CT enhance the deleterious effects of emphysema in the COPDGene cohort. Radiology. 2018;288(2):600–609. doi:10.1148/radiol.2018172688
184. LaFon DC, Bhatt SP, Labaki WW, et al. Pulmonary artery enlargement and mortality risk in moderate to severe COPD: results from COPDGene. Eur Respir J. 2020;55(2):1901812. doi:10.1183/13993003.01812-2019
185. Washko GR, Nardelli P, Ash SY, et al. Arterial vascular pruning, right ventricular size, and clinical outcomes in chronic obstructive pulmonary disease. A longitudinal observational study. Am J Respir Crit Care Med. 2019;200(4):454–461. doi:10.1164/rccm.201811-2063OC
186. Ash SY, San Jose Estepar R, Fain SB, et al. Relationship between emphysema progression at CT and mortality in ever-smokers: results from the COPDGene and ECLIPSE cohorts. Radiology. 2021;299(1):222–231. doi:10.1148/radiol.2021203531
187. Ju J, Li R, Gu S, et al. Impact of emphysema heterogeneity on pulmonary function. PLoS One. 2014;9(11):e113320. doi:10.1371/journal.pone.0113320
188. Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181(4):353–359. doi:10.1164/rccm.200907-1008OC
189. Grydeland TB, Thorsen E, Dirksen A, et al. Quantitative CT measures of emphysema and airway wall thickness are related to D(L)CO. Respir Med. 2011;105(3):343–351. doi:10.1016/j.rmed.2010.10.018
190. Leader JK, Crothers K, Huang L, et al. Risk factors associated with quantitative evidence of lung emphysema and fibrosis in an HIV-infected cohort. J Acquir Immune Defic Syndr. 2016;71(4):420–427. doi:10.1097/QAI.0000000000000894
191. Leung JM, Malagoli A, Santoro A, et al. Emphysema distribution and diffusion capacity predict emphysema progression in human immunodeficiency virus infection. PLoS One. 2016;11(11):e0167247. doi:10.1371/journal.pone.0167247
192. Triplette M, Justice A, Attia EF, et al. Markers of chronic obstructive pulmonary disease are associated with mortality in people living with HIV. AIDS. 2018;32(4):487–493. doi:10.1097/QAD.0000000000001701
193. Thudium RF, Ringheim H, Ronit A, et al. Independent associations of tumor necrosis factor-alpha and interleukin-1 beta with radiographic emphysema in people living with HIV. Front Immunol. 2021;12:668113. doi:10.3389/fimmu.2021.668113
194. Attia EF, Akgun KM, Wongtrakool C, et al. Increased risk of radiographic emphysema in HIV is associated with elevated soluble CD14 and nadir CD4. Chest. 2014;146(6):1543–1553. doi:10.1378/chest.14-0543
195. Ronit A, Kristensen T, Hoseth VS, et al. Computed tomography quantification of emphysema in people living with HIV and uninfected controls. Eur Respir J. 2018;52(1):1800296. doi:10.1183/13993003.00296-2018
196. Lambert AA, Kirk GD, Astemborski J, Mehta SH, Wise RA, Drummond MB. HIV infection is associated with increased risk for acute exacerbation of COPD. J Acquir Immune Defic Syndr. 2015;69(1):68–74. doi:10.1097/QAI.0000000000000552
197. Sims Sanyahumbi AE, Hosseinipour MC, Guffey D, et al. HIV-infected Children in Malawi have decreased performance on the 6-minute walk test with preserved cardiac mechanics regardless of antiretroviral treatment status. Pediatr Infect Dis J. 2017;36(7):659–664. doi:10.1097/INF.0000000000001540
198. Triplette M, Attia E, Akgun K, et al. The differential impact of emphysema on respiratory symptoms and 6-minute walk distance in HIV infection. J Acquir Immune Defic Syndr. 2017;74(1):e23–e29. doi:10.1097/QAI.0000000000001133
199. Brown J, Roy A, Harris R, et al. Respiratory symptoms in people living with HIV and the effect of antiretroviral therapy: a systematic review and meta-analysis. Thorax. 2017;72(4):355–366. doi:10.1136/thoraxjnl-2016-208657
200. Campo M, Oursler KK, Huang L, et al. Association of chronic cough and pulmonary function with 6-minute walk test performance in HIV infection. J Acquir Immune Defic Syndr. 2014;65(5):557–563. doi:10.1097/QAI.0000000000000086
201. Drummond MB, Kirk GD, Ricketts EP, et al. Cross sectional analysis of respiratory symptoms in an injection drug user cohort: the impact of obstructive lung disease and HIV. BMC Pulm Med. 2010;10(27):1–9. doi:10.1186/1471-2466-10-27
202. Depp TB, McGinnis KA, Kraemer K, et al. Risk factors associated with acute exacerbation of chronic obstructive pulmonary disease in HIV-infected and uninfected patients. AIDS. 2016;30(3):455–463.
203. Drummond MB, Kirk GD, McCormack MC, et al. HIV and COPD: impact of risk behaviors and diseases on quality of life. Qual Life Res. 2010;19(9):1295–1302. doi:10.1007/s11136-010-9701-x
204. Akgun KM, Tate JP, Oursler KK, et al. Association of chronic obstructive pulmonary disease with frailty measurements in HIV-infected and uninfected Veterans. AIDS. 2016;30(14):2185–2193. doi:10.1097/QAD.0000000000001162
205. Lorenz DR, Mukerji SS, Misra V, et al. Predictors of transition to frailty in middle-aged and older people with HIV: a prospective cohort study. J Acquir Immune Defic Syndr. 2021;88(5):518–527. doi:10.1097/QAI.0000000000002810
206. Crothers K, Nance RM, Whitney BM, et al. COPD and the risk for myocardial infarction by type in people with HIV. AIDS. 2023;37(5):745–752. doi:10.1097/QAD.0000000000003465
207. Agusti A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Am J Respir Crit Care Med. 2023;207(7):819–837. doi:10.1164/rccm.202301-0106PP
208. Bold KW, Deng Y, Dziura J, et al. Practices, attitudes, and confidence related to tobacco treatment interventions in HIV clinics: a multisite cross-sectional survey. Transl Behav Med. 2022;12(6):726–733. doi:10.1093/tbm/ibac022
209. Foster MG, Toll BA, Ware E, Eckard AR, Sterba KR, Rojewski AM. Optimizing the implementation of tobacco treatment for people with HIV: a pilot study. Int J Environ Res Public Health. 2022;19(19):12896. doi:10.3390/ijerph191912896
210. Agterberg S, Weinberger AH, Stanton CA, Shuter J. Perceived racial/ethnic discrimination and cigarette smoking behaviors among a sample of people with HIV. J Behav Med. 2023;46(5):801–811. doi:10.1007/s10865-023-00401-1
211. Calvo-Sanchez M, Martinez E. How to address smoking cessation in HIV patients. HIV Med. 2015;16(4):201–210. doi:10.1111/hiv.12193
212. Cartujano-Barrera F, Lee D’Abundo M, Arana-Chicas E, et al. Barriers and facilitators of smoking cessation among latinos living with HIV: perspectives from key leaders of community-based organizations and clinics. Int J Environ Res Public Health. 2021;18(7):3437. doi:10.3390/ijerph18073437
213. Shirley DK, Kesari RK, Glesby MJ. Factors associated with smoking in HIV-infected patients and potential barriers to cessation. AIDS Patient Care STDS. 2013;27(11):604–612. doi:10.1089/apc.2013.0128
214. Cui Q, Robinson L, Elston D, et al. Safety and tolerability of varenicline tartrate (Champix((R))/Chantix((R))) for smoking cessation in HIV-infected subjects: a pilot open-label study. AIDS Patient Care STDS. 2012;26(1):12–19. doi:10.1089/apc.2011.0199
215. Elzi L, Spoerl D, Voggensperger J, et al. A smoking cessation programme in HIV-infected individuals: a pilot study. Antivir Ther. 2005;11:787–795.
216. Huber M, Ledergerber B, Sauter R, et al. Outcome of smoking cessation counselling of HIV-positive persons by HIV care physicians. HIV Med. 2012;13(7):387–397. doi:10.1111/j.1468-1293.2011.00984.x
217. Kierstead EC, Harvey E, Sanchez D, et al. A pilot randomized controlled trial of a tailored smoking cessation program for people living with HIV in the Washington, D.C. metropolitan area. BMC Res Notes. 2021;14(2):1–7. doi:10.1186/s13104-020-05417-3
218. Kim SS, Darwish S, Lee SA, Sprague C, DeMarco RF. A randomized controlled pilot trial of a smoking cessation intervention for US women living with HIV: telephone-based video call vs voice call. Int J Womens Health. 2018;10:545–555. doi:10.2147/IJWH.S172669
219. Kim SS, DeMarco RF. The Intersectionality of HIV-related stigma and tobacco smoking stigma with depressive and anxiety symptoms among women living with HIV in the United States: a cross-sectional study. J Assoc Nurses AIDS Care. 2022;33(5):523–533. doi:10.1097/JNC.0000000000000323
220. Kim SS, Lee SA, Mejia J, Cooley ME, Demarco RF. Pilot randomized controlled trial of a digital storytelling intervention for smoking cessation in women living with HIV. Ann Behav Med. 2020;54(6):447–454. doi:10.1093/abm/kaz062
221. Labbe AK, Wilner JG, Coleman JN, et al. A qualitative study of the feasibility and acceptability of a smoking cessation program for people living with HIV and emotional dysregulation. AIDS Care. 2019;31(5):609–615. doi:10.1080/09540121.2018.1533225
222. Lam JO, Levine-Hall T, Hood N, et al. Smoking and cessation treatment among persons with and without HIV in a U.S. integrated health system. Drug Alcohol Depend. 2020;213:108128. doi:10.1016/j.drugalcdep.2020.108128
223. Ledgerwood DM, Yskes R. Smoking cessation for people living with HIV/AIDS: a literature review and synthesis. Nicotine Tob Res. 2016;18(12):2177–2184. doi:10.1093/ntr/ntw126
224. Mann-Jackson L, Choi D, Sutfin EL, et al. A qualitative systematic review of cigarette smoking cessation interventions for persons living with HIV. J Cancer Educ. 2019;34(6):1045–1058. doi:10.1007/s13187-019-01525-2
225. O’Cleirigh C, Zvolensky MJ, Smits JAJ, et al. Integrated treatment for smoking cessation, anxiety, and depressed mood in people living with HIV: a randomized controlled trial. J Acquir Immune Defic Syndr. 2018;79(2):261–268. doi:10.1097/QAI.0000000000001787
226. Shuter J, Morales DA, Considine-Dunn SE, An LC, Stanton CA. Feasibility and preliminary efficacy of a web-based smoking cessation intervention for HIV-infected smokers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2014;67(1):59–66. doi:10.1097/QAI.0000000000000226
227. Soldatos G, Sztal-Mazer S, Woolley I, Stockigt J. Exogenous glucocorticoid excess as a result of ritonavir-fluticasone interaction. Intern Med J. 2005;35(1):67–68. doi:10.1111/j.1445-5994.2004.00723.x
228. Foisy MM, Yakiwchuk EM, Chiu I, Singh AE. Adrenal suppression and Cushing’s syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Med. 2008;9(6):389–396. doi:10.1111/j.1468-1293.2008.00579.x
229. Kedem E, Shahar E, Hassoun G, Pollack S. Iatrogenic Cushing’s syndrome due to coadministration of ritonavir and inhaled budesonide in an asthmatic human immunodeficiency virus infected patient. J Asthma. 2010;47(7):830–831. doi:10.3109/02770903.2010.485666
230. Saberi P, Phengrasamy T, Nguyen DP. Inhaled corticosteroid use in HIV-positive individuals taking protease inhibitors: a review of pharmacokinetics, case reports and clinical management. HIV Med. 2013;14(9):519–529. doi:10.1111/hiv.12039
231. Brassard P, Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and risk of tuberculosis in patients with respiratory diseases. Am J Respir Crit Care Med. 2011;183(5):675–678. doi:10.1164/rccm.201007-1099OC
232. Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641–647. doi:10.1183/09031936.00193908
233. Morris A, Fitzpatrick M, Bertolet M, et al. Use of rosuvastatin in HIV-associated chronic obstructive pulmonary disease. AIDS. 2017;31(4):539–544. doi:10.1097/QAD.0000000000001365
234. Ferrand RA, McHugh G, Rehman AM, et al. Effect of once-weekly azithromycin vs placebo in children with HIV-associated chronic lung disease: the BREATHE randomized clinical trial. JAMA Netw Open. 2020;3(12):e2028484. doi:10.1001/jamanetworkopen.2020.28484
235. Parikh MA, Aaron CP, Hoffman EA, et al. Angiotensin-converting inhibitors and angiotensin II receptor blockers and longitudinal change in percent emphysema on computed tomography. the multi-ethnic study of atherosclerosis lung study. Ann Am Thorac Soc. 2017;14(5):649–658. doi:10.1513/AnnalsATS.201604-317OC
236. MacDonald DM, Collins G, Wendt CH, et al. Short communication: a pilot study of the effects of losartan versus placebo on pneumoproteins in HIV: a secondary analysis of a randomized double blind study. AIDS Res Hum Retroviruses. 2022;38(2):127–130. doi:10.1089/aid.2020.0285
237. Doxycycline for emphysema in people living with HIV (The DEPTH Trial). Weill Medical College of Cornell University; 2023. Available from: https://beta.clinicaltrials.gov/study/NCT05382208?distance=50&cond=HIV&term=copd%20doxycycline&rank=2.
238. Ashare RL, Thompson M, Leone F, et al. Differences in the rate of nicotine metabolism among smokers with and without HIV. AIDS. 2019;33(6):1083–1088. doi:10.1097/QAD.0000000000002127
239. Stanton CA, Papandonatos GD, Shuter J, et al. Outcomes of a tailored intervention for cigarette smoking cessation among latinos living with HIV/AIDS. Nicotine Tob Res. 2015;17(8):975–982. doi:10.1093/ntr/ntv014
240. Tseng TY, Krebs P, Schoenthaler A, et al. Combining text messaging and telephone counseling to increase varenicline adherence and smoking abstinence among cigarette smokers living with HIV: a randomized controlled study. AIDS Behav. 2017;21(7):1964–1974. doi:10.1007/s10461-016-1538-z
241. Gritz ER, Danysh HE, Fletcher FE, et al. Long-term outcomes of a cell phone-delivered intervention for smokers living with HIV/AIDS. Clin Infect Dis. 2013;57(4):608–615. doi:10.1093/cid/cit349
242. Vidrine DJ, Arduino RC, Gritz ER. Impact of a cell phone intervention on mediating mechanisms of smoking cessation in individuals living with HIV/AIDS. Nicotine Tob Res. 2006;8 Suppl 1(1):S103–108. doi:10.1080/14622200601039451
243. Vidrine DJ, Arduino RC, Lazev AB, Gritz ER. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS. 2006;20(2):253–260. doi:10.1097/01.aids.0000198094.23691.58
244. Vidrine DJ, Marks RM, Arduino RC, Gritz ER. Efficacy of cell phone-delivered smoking cessation counseling for persons living with HIV/AIDS: 3-month outcomes. Nicotine Tob Res. 2012;14(1):106–110. doi:10.1093/ntr/ntr121
245. Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine replacement interventions for HIV positive smokers. AIDS Behav. 2009;13(3):545–554. doi:10.1007/s10461-007-9334-4
246. Lloyd-Richardson EE, Stanton CA, Papandonatos GD, et al. Motivation and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction. 2009;104(11):1891–1900. doi:10.1111/j.1360-0443.2009.02623.x
247. Moadel AB, Bernstein SL, Mermelstein RJ, Arnsten JH, Dolce EH, Shuter J. A randomized controlled trial of a tailored group smoking cessation intervention for HIV-infected smokers. J Acquir Immune Defic Syndr. 2012;61(2):208–215. doi:10.1097/QAI.0b013e3182645679
248. Cropsey KL, Hendricks PS, Jardin B, et al. A pilot study of screening, brief intervention, and referral for treatment (SBIRT) in non-treatment seeking smokers with HIV. Addict Behav. 2013;38(10):2541–2546. doi:10.1016/j.addbeh.2013.05.003
249. Cropsey KL, Jardin BF, Burkholder GA, Clark CB, Raper JL, Saag MS. An algorithm approach to determining smoking cessation treatment for persons living with HIV/AIDS: results of a pilot trial. J Acquir Immune Defic Syndr. 2015;69(3):291–298. doi:10.1097/QAI.0000000000000579
250. Humfleet GL, Hall SM, Delucchi KL, Dilley JW. A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings. Nicotine Tob Res. 2013;15(8):1436–1445. doi:10.1093/ntr/ntt005
251. Manuel JK, Lum PJ, Hengl NS, Sorensen JL. Smoking cessation interventions with female smokers living with HIV/AIDS: a randomized pilot study of motivational interviewing. AIDS Care. 2013;25(7):820–827. doi:10.1080/09540121.2012.733331
252. Pengpid S, Peltzer K, Puckpinyo A, et al. Screening and concurrent brief intervention of conjoint hazardous or harmful alcohol and tobacco use in hospital out-patients in Thailand: a randomized controlled trial. Subst Abuse Treat Prev Policy. 2015;10(1):22. doi:10.1186/s13011-015-0018-1
253. Mercie P, Arsandaux J, Katlama C, et al. Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 Inter-ACTIV): a randomised controlled phase 3 clinical trial. Lancet HIV. 2018;5(3):e126–e135. doi:10.1016/S2352-3018(18)30002-X
254. Mussulman LM, Faseru B, Fitzgerald S, Nazir N, Patel V, Richter KP. A randomized, controlled pilot study of warm handoff versus fax referral for hospital-initiated smoking cessation among people living with HIV/AIDS. Addict Behav. 2018;78:205–208. doi:10.1016/j.addbeh.2017.11.035
255. Ashare RL, Thompson M, Serrano K, et al. Placebo-controlled randomized clinical trial testing the efficacy and safety of varenicline for smokers with HIV. Drug Alcohol Depend. 2019;200:26–33. doi:10.1016/j.drugalcdep.2019.03.011
256. Ditre JW, LaRowe LR, Vanable PA, De Vita MJ, Zvolensky MJ. Computer-based personalized feedback intervention for cigarette smoking and prescription analgesic misuse among persons living with HIV (PLWH). Behav Res Ther. 2019;115:83–89. doi:10.1016/j.brat.2018.10.013
257. Gryaznov D, Chammartin F, Stoeckle M, et al. Smartphone app and carbon monoxide self-monitoring support for smoking cessation: a randomized controlled trial nested into the Swiss HIV cohort study. J Acquir Immune Defic Syndr. 2020;85(1):e8–e11. doi:10.1097/QAI.0000000000002396
258. Shuter J, Chander G, Graham AL, Kim RS, Stanton CA. Randomized trial of a web-based tobacco treatment and online community support for people with HIV attempting to quit smoking cigarettes. J Acquir Immune Defic Syndr. 2022;90(2):223–231. doi:10.1097/QAI.0000000000002936
259. Shuter J, Kim RS, An LC, Abroms LC. Feasibility of a smartphone-based tobacco treatment for HIV-infected smokers. Nicotine Tob Res. 2020;22(3):398–407. doi:10.1093/ntr/nty208
260. Stanton CA, Kumar PN, Moadel AB, et al. A multicenter randomized controlled trial of intensive group therapy for tobacco treatment in HIV-infected cigarette smokers. J Acquir Immune Defic Syndr. 2020;83(4):405–414. doi:10.1097/QAI.0000000000002271
261. Schnall R, Liu J, Alvarez G, et al. A smoking cessation mobile app for persons living with HIV: preliminary efficacy and feasibility study. JMIR Form Res. 2022;6(8):e28626. doi:10.2196/28626
262. Tindle HA, Freiberg MS, Cheng DM, et al. Effectiveness of varenicline and cytisine for alcohol use reduction among people with HIV and substance use: a randomized clinical trial. JAMA Netw Open. 2022;5(8):e2225129. doi:10.1001/jamanetworkopen.2022.25129
263. Pool ER, Dogar O, Lindsay RP, Weatherburn P, Siddiqi K. Interventions for tobacco use cessation in people living with HIV and AIDS. Cochrane Database Syst Rev. 2016;6:CD011120.
264. Moscou-Jackson G, Commodore-Mensah Y, Farley J, DiGiacomo M. Smoking-cessation interventions in people living with HIV infection: a systematic review. J Assoc Nurses AIDS Care. 2014;25(1):32–45. doi:10.1016/j.jana.2013.04.005
265. Pope CA, Cropper M, Coggins J, Cohen A. Health benefits of air pollution abatement policy: role of the shape of the concentration-response function. J Air Waste Manag Assoc. 2015;65(5):516–522. doi:10.1080/10962247.2014.993004
266. Carlsten C, Salvi S, Wong GWK, Chung KF. Personal strategies to minimise effects of air pollution on respiratory health: advice for providers, patients and the public. Eur Respir J. 2020;55(6):1902056. doi:10.1183/13993003.02056-2019
267. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV; 2019. Available from: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/pneumocystis-0.
268. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the advisory committee on immunization practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):109–117. doi:10.15585/mmwr.mm7104a1
269. World Health Organization. Evidence and research gaps identified during development of policy guidelines for tuberculosis; 2021; https://iris.who.int/handle/10665/350476.
270. Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;2010(1):Cd000171. doi:10.1002/14651858.CD000171.pub3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

