Back to Journals » International Journal of Nanomedicine » Volume 18
Controlled SPION-Exosomes Loaded with Quercetin Preserves Pancreatic Beta Cell Survival and Function in Type 2 Diabetes Mellitus
Authors Zhuang M, Rao L, Chen Y, Xiao S , Xia H, Yang J, Lv X, Qin D, Zhu C
Received 26 June 2023
Accepted for publication 23 September 2023
Published 12 October 2023 Volume 2023:18 Pages 5733—5748
DOI https://doi.org/10.2147/IJN.S422416
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Farooq A. Shiekh
Manjiao Zhuang,1,* Lei Rao,2,3,* Yadi Chen,1 Shangying Xiao,1 Haishan Xia,1 Jiangyong Yang,2 Xiaohua Lv,1 Dongyun Qin,1 Chunjie Zhu1
1Guangdong Provincial Key Laboratory of Research and Development of Natural Drugs, and School of Pharmacy, Guangdong Medical University, Dongguan, 523808, People’s Republic of China; 2Medical College, Shaoguan University, Shaoguan, 512026, People’s Republic of China; 3Department of Biomedicine, Chengdu Medical College, Chengdu, 610500, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Manjiao Zhuang, Guangdong Provincial Key Laboratory of Research and Development of Natural Drugs, and School of Pharmacy, Guangdong Medical University, Dongguan, 523808, People’s Republic of China, Email [email protected] Chunjie Zhu, School of Basic Medicine, Guangdong Medical University, Dongguan, 523808, People’s Republic of China, Email [email protected]
Introduction: Quercetin has an ideal therapeutic effect on islet function improvement in type 2 diabetes mellitus (T2DM). However, the therapeutic benefit of quercetin is hindered by its poor bioavailability and limited concentration in pancreatic islets. In this study, superparamagnetic iron oxide nanoparticle (SPION)-modified exosomes were prepared to load quercetin, hoping to endow quercetin with enhanced water solubility and active targeting capacity with the help of magnetic force (MF).
Methods: Transferrin-modified SPIONs (Tf-SPIONs) were synthesized by exploiting N-hydroxysuccinimidyl (NHS) conjugation chemistry, and quercetin-loaded exosomes (Qu-exosomes) were acquired by electroporation. Tf-SPION-modified quercetin-loaded exosomes (Qu-exosome-SPIONs) were generated by the self-assembly of transferrin (Tf) and the transferrin receptor (TfR). The solubility of quercetin was determined by high-performance liquid chromatography (HPLC) analysis. The pancreatic islet targeting capacity and insulin secretagogue and antiapoptotic activities of Qu-exosome-SPIONs/MF were evaluated both in vitro and in vivo.
Results: The Qu-exosome-SPIONs were well constructed and harvested by magnetic separation with a uniform size and shape in a diameter of approximately 86.2 nm. The water solubility of quercetin increased 1.97-fold when loaded into the SPION-modified exosomes. The application of SPIONs/MF endowed the Qu-exosomes with favorable targeting capacity. In vitro studies showed that Qu-exosome-SPIONs/MF more effectively inhibited or attenuated β cell apoptosis and promoted insulin secretion in response to elevated glucose (GLC) compared with quercetin or Qu-exosome-SPIONs. In vivo studies demonstrated that Qu-exosome-SPIONs/MF displayed an ideal pancreatic islet targeting capacity, thereby leading to the restoration of islet function.
Conclusion: The Qu-exosome-SPIONs/MF nano-delivery system significantly enhanced the quercetin concentration in pancreatic islets and thereby improved pancreatic islet protection.
Keywords: quercetin, exosome, SPION, solubility, targeted delivery, islet function
Introduction
T2DM is a chronic harmful disease and the third leading cause of death after cardiovascular disease and cancer.1 Pancreatic islet dysfunction and peripheral insulin resistance in T2DM may lead to hyperglycemia, which results in many complications, such as ketoacidosis, hypertension, and atherosclerosis.2,3 In addition, the high level of oxidative stress induced by hyperglycemia aggravates the damage to pancreatic β cells, which worsens T2DM.4 Thus, maintaining β cell number and function can effectively restore blood GLC regulation and is thus considered to be the main strategy for the treatment of T2DM.
Medicines that are used to promote insulin secretion for the clinical treatment of T2DM mainly include sulfonylureas, glinides, dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 receptor agonists.5 However, these medicines display significant side effects, such as limited efficacy and poor tolerability and exhibit significant mechanism-related side effects. More significantly, these medicines lack the ability to prevent pancreatic β cell apoptosis, and some even aggravate it.6 In recent decades, a series of clinical studies have confirmed that traditional Chinese medicines play an essential role in the treatment of T2DM.7 Quercetin, a flavonoid antioxidant, is a bioactive component of various Chinese traditional medicines with ideal medicinal value.8 Various studies have demonstrated that quercetin produces a protective effect on diabetes by preserving pancreatic islet morphology and β-cell numbers or promoting insulin secretion.9–11 Moreover, our previous study demonstrated that quercetin extracted from the flowers of the traditional Chinese medicine Edgeworthia gardneri displays ideal efficacy against pancreatic β-cell apoptosis and promotes insulin secretion both in vitro and in vivo.12 One study demonstrated that the oral bioavailability of quercetin is less than 17% in rats and 1% in humans, which has a great impact on its therapeutic effect.13 Moreover, quercetin has poor targeting properties and cannot accumulate in pathological tissues, which makes guaranteeing its efficacy difficult.
The emergence of nanotechnology has enabled the development of promising traditional T2DM medicine prospects, including improved bioavailability and controlled and targeted drug delivery and release.14 Materials such as inorganic nanoparticles, liposomes, micelles and microspheres, have been applied in an attempt to improve the therapeutic effect of quercetin.15–17 Exosomes have been considered to have low immunogenicity and have shown high tolerance when used as carriers of drugs to certain tissues.18,19 Additionally, the molecular constituents of exosomes are similar to those of the cells from which they are produced, which endows them with specific functions. A recent study reported that human mesenchymal stem cell (huMSC)-derived exosomes can alleviate T2DM by improving peripheral insulin resistance and reducing pancreatic β-cell destruction.20 Additionally, MSC-derived exosomes have been confirmed to be hyperproductive and hypoimmunogenic; therefore, huMSC-derived exosomes are a desirable vehicle to carry certain medicines for T2DM treatment.
SPIONs are ideal tools for targeted drug delivery due to their promising targeting properties, favorable biocompatibility, high stability, and low toxicity.21 SPIONs can be retained at the required area in the help of a sufficient density of external MF.22 Several studies have shown that exosomes modified by SPIONs display promising targeted drug delivery capacity under an external MF when treating tumors.22,23 Moreover, our previous study demonstrated that exosomes coupled with SPIONs could target pancreatic islet cells with the help of an external MF.24
Thus, the aim of our study is to construct a synergistic targeted delivery system by conjugating SPIONs to the surface of huMSC-derived exosomes loaded with quercetin. Carboxylated chitosan (CS) was applied to generated SPIONs with the expected size distribution. The COO− groups of carboxylated CS could react with the NH3+ groups of Tf to produce amido bonds in the presence of carbodiimide (EDC) and NHS sodium salt to produce Tf-SPIONs. Exosomes were harvested from huMSCs, and quercetin was loaded into the exosomes by electroporation. The membrane protein TfR is found in high abundance in exosomes; therefore, Tf-SPIONs and Qu-exosomes self-assembled to yield Qu-exosome-SPIONs. As a result, the huMSC-derived exosomes endowed quercetin with better water solubility. Moreover, the quercetin loaded in the exosomes showed pancreatic tissue targeting with the help of SPIONs/MF.
Materials and Methods
Experimental Reagent
Quercetin (≥ 95% pure), the reagents used for constructing Qu-exosome-SPIONs, including FeCl3·6H2O, FeCl2·4H2O, NH3·H2O, NHS and EDC, and Tween 80 were purchased from Sigma (St Louis, MO, USA). DMEM and fetal bovine serum were purchased from Gibco (Gaithersburg, MD, USA). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) was obtained from Merck (Darmstadt, Germany). Penicillin and streptomycin were procured from Invitrogen (CA, USA), and β-mercaptoethanol was purchased from Gibco (Gaithersburg, MD, USA). Streptozocin was obtained from Aladdin (Shanghai, China). The insulin antibody was purchased from Cell Signaling Technology (MA, USA).
Synthesis of the Qu-Exosome-SPIONs
CS-SPIONs were synthesized and harvested as described in our previous study with a carboxylated CS concentration of 0.08 mg/mL.25 Two hundred microliters of CS-SPIONs (1 mg/mL) were mixed with EDC and NHS at a molar ratio of 1:2:3 and incubated at 37 °C for 1 h (pH 5.5). To stop the reaction, 5 μL of 2-mercaptoethanol was added to the mixture. CS-SPIONs with an activated carboxylic group were harvested through magnetic separation and then resuspended in the same volume of phosphate buffered saline (PBS, pH 7.4). Thereafter, 10 μg of Tf was incubated with the activated CS-SPIONs for 12 h at 4 °C. Next, Tf-SPIONs were harvested through magnetic separation and resuspended in PBS after washing three times. The harvested Tf-SPIONs were stored at 4 °C.
HuMSC-derived exosomes were collected according to a previous study.20 Briefly, huMSCs (3 × 106 cells/well) were cultured in a 10 cm dish for 24 h, and then the cell culture supernatant was transferred to a dialysis bag and dialyzed against PBS for one day. Next, the dialyzed supernatant was centrifuged to remove the cells (200 × g for 5 min). The collected supernatant was subsequently centrifuged (12,000 × g for 45 min) and filtered through a 0.22 μm filter membrane to further reduce cell debris. The harvested supernatant packed with exosomes was then incubated with 200 μL of 0.5 mg/mL Tf-SPION for 4 h at 4 °C to obtain the exosome-SPIONs.
Then, 150 μL of a 1 mg/mL exosome solution and 50 μL of a 1 mg/mL quercetin solution were incubated in an electroporation cuvette. The mixture was electroporated at 350 V for 150 ms in an electroporator (Bio-Rad, USA).22 The mixture was then incubated at 37 °C for 30 min to guarantee complete pore closure. The resulting solution was centrifuged (4000 × g for 30 min) in a 100 kDa ultrafiltration tube to remove unloaded quercetin, and then the Qu-exosomes were harvested.
To obtain Qu-exosome-SPIONs, 2 mL of Qu-exosomes was first dialyzed against PBS overnight and then incubated with 200 μL of 0.5 mg/mL Tf-SPIONs for 8 h at 4 °C. Magnetic separation was used to remove the uncoupled Qu-exosomes. The harvested Qu-exosome-SPIONs were then washed with PBS and resuspended in PBS for storage. The concentration of the SPION solution was determined to be 1.87 μg, and the Tf concentration was 0.08 μg per micrograms of Qu-exosome-SPIONs.
Characterization of the Qu-Exosome-SPIONs
High-resolution transmission electron microscopy (TEM, JSM-7500F, JEOL Ltd., Japan) was used to characterize the surface morphology of the Qu-exosome-SPIONs. A Zetasizer Nano (Malvern Instruments, UK) was applied to assess the size distribution of the Qu-exosome-SPIONs that had been redispersed in distilled water.
Solubility and Stability of Qu-Exosome-SPIONs
The solubility of quercetin loaded into the exosomes was determined by HPLC. Lysis buffer containing Triton X-100 was applied to lyse the exosomes, and the quercetin concentration in the solution was detected by HPLC according to a previous study using a Waters Alliance 2695–2487 HPLC system with an Agilent C18 column (Waters, Milford, MA, USA).26
HPLC analysis of the quercetin extracted from the flowers of E. gardneri was conducted via gradient elution. The absorbance of the effluent was measured at 280 nm12. Briefly, quantification of the quercetin content was performed on a Waters Alliance 2695–2487 HPLC system equipped with an Agilent C18 column (Waters, USA). Chromatographic separation was performed with mobile phase A (H2O) and mobile phase B (acetonitrile containing 0.1% trifluoroacetic acid) with gradient elution at a flow rate of 1 mL/min in. The UV detector was set at a detection wavelength of 280 nm, and the data were analyzed by Empower.
Qu-exosome-SPIONs were mixed with serum and incubated at 37 °C for one week to evaluate their stability. The Qu-exosome-SPIONs were harvested from serum by magnetic separation and resuspended for the detection of size distribution at different time points by using a Zetasizer Nano. Additionally, the quercetin concentration was detected by HPLC.
Cell Culture of MIN-6 Cells
MIN-6 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Beijing, China). MIN-6 cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 µg/mL streptomycin, 1 mmol/L sodium pyruvate, 2 mmol/L L-glutamine, 10 mmol/L HEPES, and 50 mmol/L 2-mercaptoethanol. Cells were cultured in an incubator at 37 °C in an atmosphere of 5% CO2.27
Cellular Uptake of Qu-Exosome-SPIONs/MF
To track the cellular uptake of Qu-exosome-SPIONs, quercetin was labeled with Cy5.5. Briefly, MIN-6 cells were cultured in 6-well plates (4 × 105 cells/well) for approximately 24 h until they reached 70% cell confluence, Qu-exosome-SPIONs or Qu-exosome-SPIONs/MF (density: 1 T) were added to the cell culture medium for 1 h, and the free quercetin was removed by washing with PBS. Fluorescence microscopy (Carl Zeiss Meditec AG, Jena, Germany) was used to monitor the fluorescence intensity at 680/710 nm.
Antiapoptotic and Insulin Secretagogue Activities of Qu-Exosome-SPIONs/MF
To evaluate insulin secretion, MIN-6 cells were plated in 24-well plates (3 × 104 cells/well) for 24 h. Increasing concentrations of quercetin, Qu-exosome-SPIONs, and Qu-exosome-SPIONs/MF (MF density: 1 T) were incubated with the MIN-6 cells with 0 or 8.3 mmol/L GLC for 1 h. Subsequently, the culture medium was sampled to measure insulin levels. A mouse insulin ELISA kit was used to detect insulin levels according to the protocol.
To detect cell viability, MIN-6 cells were plated in 96-well plates (1× 104 cells/well) for approximately 24 h until they reached 70% confluence. Quercetin, Qu-exosome-SPIONs, and Qu-exosome-SPIONs/MF (MF density: 1 T) were incubated with the MIN-6 cells in 50 μmol/L H2O2 for 24 h and indicated concentrations of H2O2 were added at the beginning of the 1 h incubation period. Thereafter, the cells were washed with PBS twice and incubated with MTT (0.5 mg/mL) for another 4 h in the dark in a humidified atmosphere (5% CO2, 37 °C). Then, 100 μL of dimethyl sulfoxide was added to dissolve the precipitate after the cells were with PBS. The absorbance of the resulting product was measured at 492 nm with a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA). TUNEL staining was further employed to investigate the anti-apoptosis abilities of Qu-exosome-SPIONs/MF. MIN-6 cells were plated in 24-well plates (3 × 104 cells/well) for 24 h until they reached 70% confluence. Cells were fixed with 4% paraformaldehyde for 30 min and permeabilized with PBS containing 0.3% Triton X-100. Then, the cells were incubated with TUNEL reagent (Beyotime Biotechnology, Shanghai, China) at 37 °C for 1 h. Next, the cells were treated with DAPI (Beyotime Biotechnology, Shanghai, China) to stain the nuclei. After washing with PBS, images were recorded using a fluorescence microscope (Olympus-IX711, Japan). The percentage of TUNEL-positive cells was determined by counting 300 cells from each group.
In vivo Biodistribution of Qu-Exosome-SPIONs/MF
Kunming mice were intravenously administered NHS-CY5.5-labeled quercetin (5 mg/kg), Qu-exosome-SPIONs or Qu-exosome-SPIONs/MF (MF density: 1 T). In vivo imaging in mice was performed 10 or 30 min after intravenous injection by using noninvasive near-infrared fluorescence (NIRF). Whole-animal imaging was conducted using the IVIS Spectrum imaging system (Caliper Life Sciences, Boston, USA).
Acute Therapeutic Effect of Qu-Exosome-SPIONs/MF in T2DM Mice
All protocols of the animal study were conducted following the regulations of the Bioethics Committee of Guangdong Medical University (Approval NO. GDY2002062), and procedures were performed in accordance with the Chinese Laboratory animal-guideline for ethical review of animal welfare. High-fat diet/streptozocin (STZ)-induced T2DM model mice and their controls (C57BL/6J) were constructed and purchased from Guangdong Medical Experimental Center. Briefly, male C57BL/6J mice were fed a high-fat diet (HFD: 59% fat, 15% protein, and 20% carbohydrates) for 12 weeks; thereafter, the mice were intraperitoneally injected with a low dose of STZ (30 mg/kg) for 7 days to establish a T2DM mouse model.28 C57BL/6J mice in the normal control group received a standard diet for 12 weeks and were intraperitoneally injected with citrate–phosphate buffer. T2DM model mice were established with a random blood GLC level above 16.7 mmol/L.29
For the intraperitoneal GLC tolerance test (IPGTT), mice were fasted overnight and then intraperitoneally injected with 2 g/kg GLC 15 minutes after the administration of saline, quercetin (5 mg/kg), Qu-exosome-SPIONs, or Qu-exosome-SPIONs/MF. Blood GLC levels were monitored from the tip of the tail using a One Touch Ultra Meter (Johnson & Johnson, USA) at 0, 15, 30, 60, 90 and 120 min after GLC administration.30 After the experimental period, the animals were sacrificed by cervical dislocation. Mouse plasma insulin levels were simultaneously evaluated at corresponding time points using an ELISA kit (Thermo Fisher Scientific, UK).
Chronic Therapeutic Effect of Qu-Exosome-SPIONs/MF in T2DM Mice
T2DM mice were divided into five groups (n=6 per group). The mice in the normal control (C57BL/6J) group and mice in the T2DM model control group were administered 0.4 mL of saline intravenously. The T2DM mice in the quercetin group and Qu-exosome-SPIONs group were administered 5 mg/kg quercetin or Qu-exosome-SPIONs (5 mg of quercetin-equiv/kg) intravenously. The T2DM mice in the Qu-exosome-SPIONs/MF group were administered Qu-exosome-SPIONs (5 mg of quercetin/kg) intravenously, and a magnet was pasted on the surface of the pancreatic islets for 1 h after each injection. All the treatment were administered once daily. Mouse body weight and food intake were recorded throughout the whole experiment period. At the end of 8 weeks of therapy, homeostasis model assessment insulin resistance (HOMA-IR) value was calculated to evaluate IR, HOMA-IR = [fasting blood GLC (mmol/L) × fasting insulin (μIU/mL)]/22.5), and HOMA-β was calculated to evaluate β cell function, HOMA-β = 20 × fasting insulin (μIU/mL)/[(fasting blood GLC (mmol/L) - 3.5]).
Thereafter, the mice were sacrificed by cervical dislocation, and the pancreatic islets and livers were collected for hematoxylin and eosin (H&E) staining and determination of hepatic glycogen and hepatic triglyceride (TG), respectively. Blood was sampled by eyeball extirpating and centrifugated (5000 rmp, 10 min) at 4 °C after 45 minutes’ standing, and serum was harvested by collecting the supernatant. The glycosylated hemoglobin, serum alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels in the mice were also evaluated. Glycosylated hemoglobin was measured using a Mouse CML-AGE ELISA Kit (BMASSAY, Beijing, China). ALT and AST contents were measured using an automated analyzer (Mindray BS-240Vet, China). Hepatic glycogen and TG levels were detected by a Glycogen Content Assay Kit (Boxbio, Beijing, China) and a Triglyceride Quantification Assay Kit (Abcam, UK), respectively, following the protocols.
Histological analysis was performed to evaluate pancreatic islet morphology and function. Briefly, pancreatic tissues were collected and fixed in 4% paraformaldehyde overnight. For H&E staining, pancreatic tissues were embedded in paraffin and sectioned at a thickness of 5 μm. Microscopy was adopted to observe and photograph the stained sections (400×). Pancreatic sections were further immunostained for insulin (dilution 1: 200, Cell Signaling Technology, USA). The islet area was quantified using ImageJ software. An average of 20 islet areas was semi-quantified from each group.31
Statistical Analysis
The experimental data are expressed as the mean values ± standard errors of the means (SEMs). Statistical analyses were performed by one-way analysis of variance, unpaired two-tailed t tests or paired two-tailed t tests. Areas under the curve (AUCs) were calculated using the trapezoidal rule. A value of p < 0.05 was considered to indicate a statistically significant difference (*), and p < 0.01 indicated a highly significant difference (**). Experiments were performed in duplicate, and the number of independent experiments (n) is noted.
Results
Morphology and Characterization of Qu-Exosome-SPIONs
Tf-SPIONs and Qu-exosomes were generated previously to construct Qu-exosome-SPIONs. Qu-exosome-SPIONs were constructed by the self-assembly of Tf and TfR and harvested by magnetic separation. Qu-exosome-SPIONs were redispersed in solution and observed by TEM to be uniform and monodisperse with a diameter of approximately 85 nm (Figure 1A), which is in accordance with exosome morphology as reported by a previous study.19 Moreover, the TEM images showed that several black spots (red arrows in Figure 1A) were distributed on the surface of the exosomes, which indicated that SPIONs had been successfully conjugated. Figure 1B demonstrates that the size distribution of the Qu-exosome-SPIONs was 86.2 ± 6.5 nm, which was in accordance with the results from the TEM images. Thereafter, the size distribution of Qu-exosome-SPIONs in serum after one week was further investigated to determine the in vitro stability. Qu-exosome-SPIONs were collected by magnetic separation, and their average diameter remained unchanged; moreover, scarce aggregation appeared during the whole observation period (Figure 1C). The desirable stability of the Qu-exosome-SPIONs make it an ideal therapy for the treatment of T2DM. Additionally, the quercetin concentration was also detected after 7 days. Figure 1D demonstrates that the concentration of quercetin remained stable for up to 7 days, which was attributed to the protective barrier provided by the lipid bilayer of the exosomes.
Increasing the solubility of quercetin is essential to improve its therapeutic effect. Therefore, the solubility of quercetin was determined by the established HPLC method. As depicted in Figure 1E, the aqueous solubility of quercetin was greatly increased by the addition of ethanol and acetonitrile (10%) or an elevation in temperature (37 °C). Thus, the solubility of quercetin encapsulated in exosome-SPION was further investigated. The results demonstrate that quercetin loaded in exosome-SPIONs displayed higher water solubility (1.97-fold) than free quercetin, which may increase the in vivo efficiency of quercetin to produce a better therapeutic effect.
In vitro Targeting Property of Qu-Exosome-SPIONs/MF
Therapeutic agents with specific targeting properties can increase drug concentrations in the target area, thereby increasing cellular uptake. To evaluate cellular uptake, quercetin was labeled with Cy5.5. Figure 2 shows that SPION-decorated exosomes could significantly enhance the cellular uptake of quercetin with the help of an external MF, which may consequently improve its antiapoptotic and insulin secretagogue activities. This was because the external MF accelerated the gathering of Qu-exosome-SPIONs on the surface of MIN-6 cells, which in turn facilitated more quercetin entry into these cells.
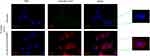 |
Figure 2 Targeting capacity of Qu-exosome-SPIONs in vitro. Quercetin was labeled with cy5.5 and the cells were washed before fluorescence microscopy observation. n=3. |
Antiapoptotic and Insulin Secretagogue Activities of Qu-Exosome-SPIONs/MF in MIN-6 Cells
Restoring β-cell functionality by promoting insulin secretion and maintaining β-cell numbers are two major strategies for treating T2DM. Therefore, the insulin secretagogue and antiapoptotic activities of quercetin, Qu-exosome-SPIONs, and Qu-exosome-SPIONs/MF were in 0 and 8.3 mmol/L GLC. Quercetin, Qu-exosome-SPIONs, and Qu-exosome-SPIONs/MF were unable to provoke insulin secretion in the 0 mmol/L GLC solution, which validated that quercetin relied on the presence of GLC to induce insulin secretion. Quercetin significantly elevated insulin secretion at concentrations above 5 μmol/L and provoked peak insulin secretion at a concentration of approximately 20 μmol/L in the presence of 8.3 mmol/L GLC (Figures 3A and S1). The insulin secretagogue capacity of Qu-exosome-SPIONs was similar to that of quercetin, while Qu-exosome-SPIONs/MF provoked more insulin secretion in the presence of 8.3 mmol/L GLC, which indicated that the active targeting property endowed by exosome-SPIONs/MF could notably improve the therapeutic effect of quercetin.
H2O2 was applied to construct a model of apoptosis in MIN-6 cells. The application of H2O2 (0, 10, 25, 50, 100 and 1000 μmol/L) decreased cell viability and insulin secretion in a concentration-dependent manner in 8.3 mmol/L GLC (Figure 3B). Incubation with H2O2 at a concentration of 50 μmol/L for 1 h caused approximately 50% cell apoptosis and inhibition of insulin secretion. Thus, the effects of quercetin, Qu-exosome-SPIONs, and Qu-exosome-SPIONs/MF on MIN-6 cell viability and insulin secretion in the presence of 50 μmol/L H2O2 were investigated. Figure 3C demonstrates that quercetin at a concentration of 10 μmol/L could significantly elevate MIN-6 cell viability and insulin secretion in 50 μmol/L H2O2. Qu-exosome-SPIONs/MF was found to more effectively prevent apoptosis and promote insulin secretion than quercetin or Qu-exosome-SPIONs. Additionally, a significant decrease in the proportion of TUNEL-positive cells in the Qu-exosome-SPIONs/MF treatment group proved that Qu-exosome-SPIONs/MF had the best anti-apoptosis activity (Figure 3D and E).
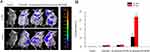
In vivo Targeting Property of Qu-Exosome-SPIONs/MF
To investigate the pancreatic islet targeting property of Qu-exosome-SPIONs/MF, whole-animal imaging was recorded 10 and 30 min after the intravenous administration of quercetin, Qu-exosome-SPIONs and Qu-exosome-SPIONs/MF by using NIRF imaging. As depicted in Figure 4A, weak fluorescence intensity was detected around the pancreatic islet 10 min after intravenous injection, after which the fluorescence intensity decreased and could no longer be detected after 30 min in mice injected with quercetin and Qu-exosome-SPIONs without MF. Higher fluorescence intensity was detected around the pancreatic islet at 10 min and was greatly enhanced by 30 min after the administration of Qu-exosome-SPIONs in the presence of a MF, which validated that Qu-exosome-SPIONs displayed desirable targeting with the help of an external MF. Moreover, the fluorescence intensity in the pancreatic islets was quantified. Compared with the quercetin group, the fluorescence intensity around the pancreatic islet was elevated approximately 11.5-fold at 10 min and 37.5-fold at 30 min in the Qu-exosome-SPIONs/MF group (Figure 4B). No differences in fluorescence intensity were observed between the quercetin and Qu-exosome-SPIONs without the application of MF groups at either 10 min or 30 min.
Acute Therapeutic Effect of Qu-Exosome-SPIONs/MF in vivo
The IPGTT was performed and the plasma insulin level was detected to assess the in vivo therapeutic effect of Qu-exosome-SPIONs/MF. Compared with normal mice, T2DM model mice displayed abnormal GLC tolerance along with GLC-dependent insulin secretion deficiency (Figure 5A and C). Intravenous administration of quercetin or Qu-exosome-SPIONs improved the impaired GLC tolerance in T2DM model mice, as validated by faster reduction in blood GLC. Moreover, Qu-exosome-SPIONs decreased the plasma GLC level more effectively than quercetin, which was attributed to the better stability and solubility of quercetin encapsulated within the exosomes. Intravenous administration of Qu-exosome-SPIONs accompanied by MF resulted in a better improvement in GLC tolerance than Qu-exosome-SPIONs without MF, indicating that the application of a MF clearly improved the pancreatic islet targeting capacity, thus exhibiting a better effect on blood GLC response. Accordingly, the plasma GLC AUC of Qu-exosome-SPIONs/MF was significantly smaller than that in the model, quercetin and Qu-exosome-SPIONs groups, which further confirmed that Qu-exosome-SPIONs/MF had the best hypoglycemic effect (Figure 5B). The observed change in plasma insulin level was in accord with the improvement in the plasma GLC level. Qu-exosome-SPIONs/MF exhibited the best therapeutic effect in terms of GLC-stimulated insulin secretion and was accompanied by the largest AUC, with Qu-exosome-SPIONs coming in second, and quercetin being last (Figure 5C and D).
Chronic Therapeutic Effect of Qu-Exosome-SPIONs/MF in vivo
To investigate the long-term therapeutic effects of Qu-exosome-SPIONs/MF, T2DM model mice were injected with quercetin, Qu-exosome-SPIONs and Qu-exosome-SPIONs/MF intravenously for 8 weeks. Weight gain is a typical symptom of T2DM; thus, the body weights of the T2DM mice were measured. The results showed that body weights of the mice in the quercetin and Qu-exosome-SPIONs treatment groups were significantly reduced compared with that in the T2DM model group mice. Further body weight reduction was observed in T2DM model mice treated with Qu-exosome-SPIONs/MF when compared with those treated with quercetin and Qu-exosome-SPIONs (Figure 6A). Moreover, treating T2DM mice with Qu-exosome-SPIONs significantly decreased food intake (control 2.24 ± 0.31 g/d, model 6.35 ± 0.84 g/d, quercetin 6.12 ± 0.75 g/d, Qu-exosome-SPIONs 5.87 ± 0.68 g/d, and Qu-exosome-SPIONs/MF 5.31 ± 0.70 g/d) compared with the model group (Figure 6B). These results indicated that the decrease in body weight in Qu-exosome-SPIONs/MF group may be caused by the diminished food intake.
The liver is highly involved in controlling blood GLC levels, and liver function disorders frequently occur in T2DM. As shown in Figure 6C and D, Qu-exosome-SPIONs/MF displayed the best therapeutic effects in reducing hepatic glycogen and hepatic TG in T2DM model mice compared with quercetin and Qu-exosome-SPIONs which indicated that Qu-exosome-SPIONs/MF displayed a preferable capacity in improving liver function. Moreover, treatment with Qu-exosome-SPIONs/MF significantly decreased plasma AST and ALT levels compared with the model group, which verified that Qu-exosome-SPIONs/MF had a protective effect on liver function (Table 1).
 |
Table 1 Effect of Qu-Exosome-SPIONs/MF on AST and ALT |
HOMA-IR and HOMA-β have been widely used as indices for quantifying insulin resistance and pancreatic β-cell function. As shown in Table 2, the HOMA-IR in the model group exhibited a significant increase compared with the control group, decreasing to 10.25 ± 1.18, 8.24 ± 1.12, and 4.23 ± 0.72 after intervention with quercetin, Qu-exosome-SPIONs and Qu-exosome-SPIONs/MF, respectively. Moreover, the HOMA-β results showed that treatment with Qu-exosome-SPIONs/MF greatly improved the HOMA-β index compared with the model group (86.67 ± 7.51 vs 43.36 ± 5.62). Glycosylated hemoglobin is the gold standard blood GLC evaluation and is commonly used in the clinic. Figure 6E demonstrates that daily administration of Qu-exosome-SPIONs/MF in T2DM model mice exhibited the best capacity to reduce glycosylated hemoglobin (reduced by 23%) compared with quercetin and Qu-exosome-SPIONs, which further verified that SPIONs/MF in combination with an exosome-based vehicle could significantly improve the therapeutic effect of quercetin when treating T2DM.
 |
Table 2 Effect of Qu-Exosome-SPIONs/MF on HOMA-IR and HOMA-β |
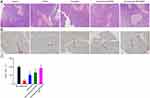
Thereafter, H&E staining was used for morphometric analysis of the islets in T2DM model mice treated with Qu-exosome-SPIONs/MF. The specimen revealed the pancreatic islet β cells in normal mice exhibited granulated cytoplasm and uniform nuclei. However, degranulated β cells with obvious vacuolation and dark, scant cytoplasm were observed in T2DM model mice. T2DM model mice treated with quercetin or Qu-exosome-SPIONs without MF exhibited varying degrees of improvement in terms of the histological structure of the damaged pancreatic islet β cells (Figure 7A). Treatment of Qu-exosome-SPIONs and an external MF notably restored the structure of the damaged islets and the extent of vacuolation, which further verified that the exosome-SPIONs/MF endowed quercetin with a better therapeutic effect to improve impaired pancreatic islet β cell function. Additionally, the greatest increases in the amount and area percentage of insulin-positive β cells (red arrows in Figure 7B) in the Qu-exosome-SPIONs/MF treatment group were detected compared with the model group (Figure 7C).
Discussion
Insulin resistance and exhaustion of the pancreas are two main characteristics of T2DM. The exhaustion of the pancreas is characterized by decreases in β cell functionality and mass, which result in the body producing increasingly less insulin and consequently leads to hyperglycemia.32 Therefore, restoring pancreatic β cell mass and function are the main strategies in the treatment of T2DM.
The application of insulin and insulin secretagogues can significantly improve insulin levels and restore impaired GLC regulation.5 However, endogenous insulin displays better kinetics in terms of decreasing gluconeogenesis than subcutaneously injected insulin. Additionally, insulin and insulin-secreting drugs in the sulfonylurea and glinide classes cannot respond precisely to increases in blood GLC levels, which may lead to hypoglycemia. Moreover, clinical insulin secretagogues are unable to restore pancreatic β cell mass, and some even aggravate pancreatic β cell loss, thus displaying limited therapeutic efficacy. Quercetin exhibits ideal antioxidant activity and anti-diabetic activity.33 Increasing evidences have shown that quercetin could elevate plasma insulin levels in T2DM model animals by maintaining β cell survival and promoting insulin release.9,11,34 Studies have demonstrated that pancreatic cell protection provided by quercetin involves decreasing the expression of iNOS, reducing the level of NO, and counteracting NF-Κb activation.35,36 The insulin secretagogue effect of quercetin involves the iPLC/PKC or cAMP/PKA signaling pathways.37,38 Moreover, our previous study demonstrated that the Ca2+/ERK1/2 signaling pathway was associated with quercetin-induced insulin secretion and that the mitochondria-associated apoptotic pathway was involved in the antiapoptotic effect of quercetin.12 However, the desirable therapeutic effect of quercetin is hindered by its poor bioavailability and subeffective concentration in pancreatic islets.39
In the present study, SPION-modified exosomes were administered to improve the therapeutic effect of quercetin in the treatment of T2DM. The morphology, size, and stability of the Qu-exosome-SPIONs were investigated in this study. The results showed that Qu-exosome-SPIONs were successfully constructed and harvested by MF with a uniform size and shape and a diameter of approximately 85 nm. Moreover, the SPIONs were observed to be clearly distributed on the surface of the exosomes with desirable stability.
SPIONs, the only clinically approved metal oxide nanoparticles, display immense potential in the targeted delivery of drugs.40 Their superparamagnetism endows SPIONs with the ability to target a specific tissue or organ by the application of an external MF; moreover, they have been shown to be associated with low toxicity and ideal biocompatibility in the human body. In vitro targeting experimental data showed that the cellular uptake of quercetin was significantly elevated when loaded into SPION-decorated exosomes in the presence of a MF. Moreover, the results of NIRF imaging demonstrated that the fluorescence intensity of the pancreatic islets increased approximately 11.5-fold after 10 min and 37.5-fold after 30 min in the Qu-exosome-SPION/MF group compared with those in the quercetin group, which verified that SPIONs/MF endowed quercetin with a desirable in vivo targeting capacity. The advancement of SPIONs is likely to meet the need for the targeted delivery of Qu-exosomes to the pancreas for T2DM treatment for clinical translation.
Poor solubility and vulnerability to changes in pH are the main obstacles to the clinical application of quercetin. Exosomes are ideal drug delivery vehicles compared with existing drug delivery systems. Due to the restrictions of the lipid bilayer, exosomes can provide a barrier to protect quercetin from degradation and thus overcome its pH instability. Sun et al demonstrated that curcumin encapsulated in exosomes was more stable and exhibited greater water solubility than free curcumin.41 The results here demonstrated that when encapsulated in exosomes, the aqueous solubility of quercetin was greatly increased (1.97-fold), and Qu-exosome-SPIONs showed desirable particle stability. Additionally, increasing evidence has demonstrated that exosomes are ideal delivery vehicles to overcome biological barriers.42 The blood–pancreas barrier that exists between pancreatic tissue and the blood is the major biological barrier for efficient drug delivery after systemic drug administration, which often leads to subeffective drug concentrations in the pancreatic islets and thus results in poor in vivo therapeutic efficacy. The application of SPION-decorated exosomes as vehicles to deliver quercetin to the pancreas in the presence of a MF was found to overcome the blood–pancreas barrier, thus achieving an effective therapeutic concentration of quercetin in the pancreatic islets and significantly improving β-cell functionality.
The therapeutic effects of Qu-exosome-SPIONs/MF on β cell functionality and mass were verified both in vitro and in vivo. Qu-exosome-SPIONs/MF displayed more effective in anti-apoptosis and insulin secretagogue activities than quercetin and Qu-exosome-SPIONs in MIN-6 cells in the presence of 50 μmol/L H2O2. Notably, this enhanced cell viability in turn resulted in a much better improvement in insulin secretion, which was attributed to the dual therapeutic functions of quercetin (promoting insulin secretion and resisting cell apoptosis) when treating the pancreas. Both Vessal et al and Coskun et al demonstrated that intraperitoneal injection of 15 mg/kg quercetin in STZ-induced diabetic rats could significantly elevate β cell numbers and serum insulin.9,43 Thus, according to the body surface area-based equivalent and its bioavailability after intraperitoneal injection, intravenous injection of 16 mg/kg quercetin in STZ-induced mice may be effective. The in vivo experimental results showed that intravenous injection of 5 mg/kg Qu-exosome-SPIONs/MF exhibited a better improvement in the IPGTT and demonstrated a significant plasma insulin enhancement compared with the quercetin treatment group. Additionally, the results of the chronic T2DM experiment demonstrated that administration of 5 mg/kg Qu-exosome-SPIONs/MF clearly improved T2DM symptoms, including body weight, food intake, hepatic TGs and glycogen, HOMA-IR, HOMA-β, and glycosylated hemoglobin. Thus, it can be concluded that the exosome-SPION delivery system can significantly increase the concentration of quercetin at the treatment site and decrease the needed dosage, cost of treatment and adverse effects on the body. Based on the body surface area-based equivalent, 0.55 mg of Qu-exosome-SPIONs (0.55 mg of quercetin equiv/kg) may be effective in humans. The results of H&E staining and insulin immunohistochemistry further confirmed that Qu-exosome-SPIONs/MF could significantly resist β cell apoptosis and improve β-cell numbers, thereby increasing insulin secretion in T2DM mice.
Conclusion
In this study, quercetin was loaded into SPION-modified exosomes to improve pancreatic islet functionality both in vitro and in vivo. The lipid bilayer of the exosomes provided quercetin with better stability and higher solubility (1.97-fold), and the specific targeting property of SPION/MF endowed the Qu-exosomes with ideal targeting capacity as depicted in scheme 1. Poor water solubility and a subeffective concentration in the target area frequently occur and are two major challenges in pharmaceutical development. Our study provides a favorable strategy to improve the solubility of poorly soluble therapeutics as well as their concentration in the targeted area which is valuable for the application T2DM therapeutics.
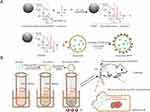 |
Scheme 1 Schematic illustration for (A) the synthesis of Qu-exosome-SPION and (B) how Qu-exosome-SPION/MF exhibit their antidiabetic activity in vivo. |
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CS, chitosan; EDC, carbodiimide; GLC, glucose; HPLC, high-performance liquid chromatography; huMSC, human mesenchymal stem cell; HOMA-IR, homeostasis model assessment insulin resistance; HFD, high-fat diet; IPGTT, intraperitoneal glucose tolerance test; MF, magnetic force; NHS, N-hydroxysuccinimidyl; NIRF, noninvasive near-infrared fluorescence; PBS, phosphate buffered saline; STZ, streptozocin; SPION, superparamagnetic iron oxide nanoparticle; T2DM, type 2 diabetes mellitus; TEM, transmission electron microscope; Tf, Transferrin; TfR, transferrin receptors; TG, triglyceride; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT).
Acknowledgments
This work was supported by Sichuan Science and Technology Program (number 2022NSFSC0627), Shaoguan Science and Technology Program (number 230330188036420), Shaoguan Health Bureau Program (number Y23134), School Foundation Program from Medical college of Shaoguan university (number EKY202201), Talent introduction Program from Medical college of Shaoguan university (number 9900064701), Doctoral Start-up Foundation of Guangdong Medical University (number GDMUB2020002), GuangDong Basic and Applied Basic Research Foundation (number 2020A1515110862 and 2021B1515140060), 100 Youth Research Project funding program of Guangdong Medical University (number GDMUD2022005), Discipline Construction Project of Guangdong Medical University (number 4SG23002G and 4SG23024G), Youth Fund of the National Natural Science Foundation of China (number 82204435). Medical Research Foundation of Guangdong Province (number A2022430).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrin Metab Clin of North America. 2021;50(3):337–355. doi:10.1016/j.ecl.2021.05.013
2. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus -- present and future perspectives. Nat Rev Endocrinol. 2011;8(4):228–236. doi:10.1038/nrendo.2011.183
3. Defronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 2000;133(1):73–74. doi:10.7326/0003-4819-133-1-200007040-00016
4. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
5. Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 diabetes mellitus: a review of multi-target drugs. Molecules. 2020;25(8). doi:10.3390/molecules25081987
6. Gloyn AL, Drucker DJ. Precision medicine in the management of type 2 diabetes. Lancet Diabetes Endo. 2018;6(11):891–900. doi:10.1016/S2213-8587(18)30052-4
7. Wang Y, Dai Z, Wang Q, et al. Clinical application of traditional Chinese medicine therapy for type 2 diabetes mellitus: an evidence map. Evid-Based Compl Alt: eCAM. 2022;2022:2755332. doi:10.1155/2022/2755332
8. Ghorbani A, Rashidi R, Shafiee-Nick R. Flavonoids for preserving pancreatic beta cell survival and function: a mechanistic review. Biomed Pharmacother. 2019;111:947–957. doi:10.1016/j.biopha.2018.12.127
9. Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res. 2005;51(2):117–123. doi:10.1016/j.phrs.2004.06.002
10. Li D, Jiang C, Mei G, et al. Quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes. Nutrients. 2020;12(10):2954. doi:10.3390/nu12102954
11. Maciel RM, Costa MM, Martins DB, et al. Antioxidant and anti-inflammatory effects of quercetin in functional and morphological alterations in streptozotocin-induced diabetic rats. Res Vet Sci. 2013;95(2):389–397. doi:10.1016/j.rvsc.2013.04.028
12. Zhuang M, Qiu H, Li P, Hu L, Wang Y, Rao L. Islet protection and amelioration of type 2 diabetes mellitus by treatment with quercetin from the flowers of Edgeworthia gardneri. Drug Drug Des Dev Ther. 2018;12:955–966. doi:10.2147/dddt.s153898
13. Khaled KA, El-Sayed YM, Al-Hadiya BM. Disposition of the flavonoid quercetin in rats after single intravenous and oral doses. Drug Dev Ind Pharm. 2001;29(4):397–403. doi:10.1081/ddc-120018375
14. Farokhzad OC, Robert L. Impact of nanotechnology on drug delivery. Acs Nano. 2009;3(1):16–20. doi:10.1021/nn900002m
15. Giannouli M, Karagkiozaki V, Pappa F, Moutsios I, Gravalidis C, Logothetidis S. Fabrication of quercetin-loaded PLGA nanoparticles via electrohydrodynamic atomization for cardiovascular disease. Mater Today: Proceedings. 2018;5:15998–16005. doi:10.1016/j.matpr.2018.05.044
16. Seong JS, Yun ME, Park SN. Surfactant-stable and pH-sensitive liposomes coated with N-succinyl-chitosan and chitooligosaccharide for delivery of quercetin. Carbohyd Polym. 2018;181:659–667. doi:10.1016/j.carbpol.2017.11.098
17. Debnath K, Jana NR, Jana NR. Quercetin encapsulated polymer nanoparticle for inhibiting intracellular polyglutamine aggregation. Acs Appl Bio Mater. 2019;2(12):5298–5305. doi:10.1021/acsabm.9b00518
18. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;15:6917–6934. doi:10.2147/ijn.s264498
19. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9(1). doi:10.1186/s13578-019-0282-2
20. Sun Y, Shi H, Yin S, et al. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12(8):7613–7628. doi:10.1021/acsnano.7b07643
21. Wei H, Hu Y, Wang J, Gao X, Qian X, Tang M. Superparamagnetic iron oxide nanoparticles: cytotoxicity, metabolism, and cellular behavior in biomedicine applications. Int J Nanomedicine. 2021;16:6097–6113. doi:10.2147/ijn.s321984
22. Qi H, Liu C, Long L, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. Acs Nano. 2016;10(3):3323–3333. doi:10.1021/acsnano.5b06939
23. Jia G, Han Y, An Y, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316. doi:10.1016/j.biomaterials.2018.06.029
24. Zhuang M, Du D, Pu L, et al. SPION-decorated exosome delivered BAY 55-9837 targeting the pancreas through magnetism to improve the blood GLC response. Small. 2019;15(52):1903135. doi:10.1002/smll.201903135
25. Zhuang MJ, Chen XL, Du D, et al. SPION decorated exosome delivery of TNF-alpha to cancer cell membranes through magnetism. Nanoscale. 2020;12(1):173–188. doi:10.1039/c9nr05865f
26. Hekmatirad S, Moloudizargari M, Moghadamnia AA, et al. Inhibition of exosome release sensitizes U937 cells to pegylated liposomal doxorubicin. Front Immunol. 2021;12:692654. doi:10.3389/fimmu.2021.692654
27. Perugini V, Best M, Kumar S, et al. Carboxybetaine-modified succinylated chitosan-based beads encourage pancreatic β-cells (Min-6) to form islet-like spheroids under in vitro conditions. JJ Mater Sci-Mater M. 2017;29(1):15. doi:10.1007/s10856-017-6018-0
28. Zhang C, Deng J, Liu D, et al. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a pparα/pparγ coactivator-1α pathway. Br J Pharmacol. 2018;175(22):4218–4228. doi:10.1111/bph.14482
29. Cheng Y, Yu X, Zhang J, et al. Pancreatic kallikrein protects against diabetic retinopathy in KK Cg-A(y)/J and high-fat diet/streptozotocin-induced mouse models of type 2 diabetes. Diabetologia. 2019;62(6):1074–1086. doi:10.1007/s00125-019-4838-9
30. Small L, Ehrlich A, Iversen J, et al. Comparative analysis of oral and intraperitoneal glucose tolerance tests in mice. Mol Metab. 2022;57:101440. doi:10.1016/j.molmet.2022.101440
31. Kawada-Horitani E, Kita S, Okita T, et al. Human adipose-derived mesenchymal stem cells prevent type 1 diabetes induced by immune checkpoint blockade. Diabetologia. 2022;65(7):1185–1197. doi:10.1007/s00125-022-05708-3
32. Ryan K. Selective disruption of Tcf7l2 in the pancreatic β cell impairs secretory function and lowers β cell mass. Hum Mol Genet. 2014;24(5):1390–1399. doi:10.1093/hmg/ddu553
33. Oh P. Preparation of quercetin esters and their antioxidant activity. J Agr Food Chem. 2019;67(38):10653–10659. doi:10.1021/acs.jafc.9b04154
34. Li JM, Wang W, Fan CY, et al. Quercetin preserves β -cell mass and function in fructose-induced hyperinsulinemia through modulating pancreatic akt/foxo1 activation. Evid-Based Compl Alt: eCAM. 2013;2013:303902. doi:10.1155/2013/303902
35. Carrasco-Pozo C, Tan KN, Reyes-Farias M, et al. The deleterious effect of cholesterol and protection by quercetin on mitochondrial bioenergetics of pancreatic β-cells, glycemic control and inflammation: in vitro and in vivo studies. Redox Biol. 2016;9:229–243. doi:10.1016/j.redox.2016.08.007
36. Kim E-K, Kwon K-B, Song M-Y, et al. Flavonoids protect against cytokine-induced pancreatic β-cell damage through suppression of nuclear factor κb activation. Pancreas. 2007;35(4):e1–9. doi:10.1097/mpa.0b013e31811ed0d2
37. Zhang Y, Liu D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur J Pharmacol. 2011;670(1):325–332. doi:10.1016/j.ejphar.2011.08.011
38. Kappel VD, Frederico MJS, Postal BG, Mendes CP, Cazarolli LH, Silva FRMB. The role of calcium in intracellular pathways of rutin in rat pancreatic islets: potential insulin secretagogue effect. Eur J Pharmacol. 2013;702(1):264–268. doi:10.1016/j.ejphar.2013.01.055
39. Lv X, Liu T, Ma H, et al. Preparation of essential oil-based microemulsions for improving the solubility, ph stability, photostability, and skin permeation of quercetin. AAPS PharmSciTech. 2017;18(8):3097–3104. doi:10.1208/s12249-017-0798-x
40. Zhi D, Yang T, Yang J, Fu S, Zhang S. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater. 2020;102:13–34. doi:10.1016/j.actbio.2019.11.027
41. Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi:10.1038/mt.2010.105
42. Shao J, Zaro J, Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int J Nanomedicine. 2020;15:9355–9371. doi:10.2147/ijn.s281890
43. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135(3):357–364. doi:10.1016/s1532-0456(03)00140-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.