Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Construction of a Nomogram-Based Prediction Model for the Risk of Diabetic Kidney Disease in T2DM
Authors Wang X, Liu X, Zhao J, Chen M, Wang L
Received 3 October 2023
Accepted for publication 23 December 2023
Published 12 January 2024 Volume 2024:17 Pages 215—225
DOI https://doi.org/10.2147/DMSO.S442925
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Xian Wang,1 Xiaming Liu,1 Jun Zhao,1 Manyu Chen,1 Lidong Wang2
1Graduate School of Chengde Medical College, Chengde, Hebei, People’s Republic of China; 2Department of Endocrinology and Immunology, Chengde Central Hospital Affiliated to Chengde Medical College, Chengde, Hebei, People’s Republic of China
Correspondence: Lidong Wang, Department of Endocrinology and Immunology, Chengde Central Hospital Affiliated to Chengde Medical College, Chengde, Hebei, People’s Republic of China, Tel +86 13703142117, Email [email protected]
Introduction: To investigate the predictors of diabetic kidney disease (DKD) in type 2 diabetes mellitus (T2DM) patients and establish a nomogram model for predicting the risk of DKD.
Methods: The clinical data of T2DM patients, admitted to the Endocrinology Department of Chengde Central Hospital from October 2019 to September 2020 and divided into a case group or a control group based on whether they had DKD, were collected. The predictive factors of DKD were screened by univariate and multivariate analysis, and a nomogram prediction model was constructed for the risk of DKD in T2DM. Bootstrapping was used for model validation, receiver operating characteristic (ROC) curve and GiViTI calibration curve were used for evaluating the discrimination and calibration of prediction model, and decision analysis curve (DCA) was used for evaluating the practicality of model.
Results: Predictors for DKD are diabetic retinopathy (DR), hypertension, history of gout, smoking history, using insulin, elevation of body mass index (BMI), triglyceride (TG), cystatin C (Cys-C), and reduction of 25 (OH) D. The nomogram prediction model based on the above nine predictors had good representativeness (Bootstrap method: precision: 0.866, Kappa: 0.334), differentiation [the area under curve (AUC) value: 0.868], and accuracy (GiViTI-corrected curved bands, P = 0.836); the DAC curve analysis showed that the prediction model, whose threshold probability was in the range of 0.10 to 0.70, had clinical practical value.
Conclusion: The risk of DKD in T2DM could be predicted accurately by DR, hypertension, history of gout, smoking history, using insulin, elevation of BMI, TG, Cys-C, and reduction of 25 (OH) D.
Keywords: type 2 diabetes mellitus, diabetic kidney disease, predictive model, 25-hydroxyvitamin D, diabetic retinopathy
Introduction
According to the latest epidemiological survey, the overall prevalence rate of type 2 diabetes mellitus (T2DM) in China, whose trend continued to increase had reached 14.92%, and the treatment rate and the standard rate of the diagnosed patients were less than 50%.1 T2DM can result in many complications, serious harm, high teratogenic and disabling rates, which seriously affects the life and prognosis of patients. The main manifestations of diabetic kidney disease (DKD), whose onset is insidious, are increased foam in urine in the early stage, and gradually hypertension, decreased urine volume, edema and other symptoms, and finally kidney failure.2 The prevalence rate of DKD in T2DM patients in China was as high as 21.8%, which has become the main cause of chronic kidney disease in China.3 However, few studies have focused on the risk of DKD in T2DM patients.
The purpose of this study was to investigate the predictors of DKD in T2DM and establish a prediction model based on the clinical data of T2DM patients, so as to identify high-risk patients with DKD early and reduce the risk of DKD.
Methods
Study Designs and Participants
This was a hospital-based, retrospective study. We selected all the patients with DKD in T2DM admitted to the Second Affiliated Hospital of Chengde Medical College from October 2019 to September 2020. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Chengde Medical College and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the patients. In total, 403 participants aged 23–81 years were included in the study. Of these patients, 59 (14.6%) were the patients with DKD, 212 (52.6%) were male, and 118 (29.3%) had diabetic retinopathy (DR). The inclusion criteria for patients with DKD were (1) T2DM4 and (2) age ≥ 18 years old. The exclusion criteria for the study were (1) other types of diabetes, (2) cancer, (3) infection, (4) severe liver dysfunction, (5) chronic renal insufficiency caused by other reasons, (6) the inaccuracy of urinary albumin/creatinine ratio (UACR), and (7) missing data. DKD, in the absence of signs or symptoms of other primary causes of kidney damage, is usually clinically diagnosed based on the presence of albuminuria defined as a urinary albumin-to-creatinine ratio ≥30 mg/g and/or reduced eGFR which was <90 mL/min/1.73m2. The clinical and demographic characteristics of the study participants are summarized in Table 1.
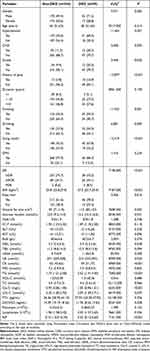 |
Table 1 Clinical and Demographic Characteristics of the Study Participants |
Candidate Variables
There were 37 variables classified into three categories in our study, as explanatory risk factors for the patients with DKD. The demographic characteristics included age, sex, history of smoking and drinking, and body mass index (BMI). The clinical information of the patients included duration of T2DM, anamnesis, using insulin after admission, diabetic microangiopathy, fatty liver, and visceral fat area. The history of hypertension disease, coronary heart disease (CHD), stroke, and gout were in the anamnesis. The diabetic microangiopathy contained DKD, diabetes peripheral neuropathy (DPN), and diabetic retinopathy (DR). In addition, 21 blood biochemical indicators which may be helpful in predicting the risk of DKD were included in the study. The complete blood count, liver function test, renal function test, blood fat, fasting plasma glucose (FPG), glucose random, hemoglobin A1c (HbA1c) levels, fasting C-peptide (FC-P), 25-hydroxyvitamin D (25(OH)D), parathyroid hormone (PTH), and atherogenic index of plasma (AIP) which is equal to lg (TG/HDL-C).5
The result of weight in kilograms divided by the square of height in meters is the BMI. The history of hypertension disease, CHD, stroke, and gout were definite according to the previous medical history. The presence of DR was diagnosed by two professional ophthalmologists by reading the result of wide-area fundus photography, optical coherence tomography, and fundus fluorescein angiography. The venous blood of the patients was collected at 5 am after an overnight fast of at least 8 h for biochemical tests, except for glucose randomly collected at admission.
Statistical Analysis
Statistical analyses were conducted using SPSS version 26.0 and R version 4.3.1 for Windows. In univariate analysis, the quantitative variables that met normal distribution and variance homogeneity test were expressed as mean ± SD and were compared by the Student’s t-tests. The other quantitative variables expressed as median [interquartile range (IQR), 25–75%] were compared using the Mann–Whitney U-test. Categorial variables compared by the Chi-square tests, continuity correction Chi-square tests, or the Mann–Whitney U-test were expressed as number and percentage. The predictive model was established through stepwise logical regression used to filter variables, which were statistical differences in univariate analysis. Evaluate the representativeness of the model through bootstrap (n = 1000), nomogram column graph visualized the model, Receiver Operating Characteristic (ROC) curve evaluated the predictive performance of the model, the GiViTI calibration curve compared the consistency between actual values and model predictions, and the Decision Curve Analysis (DCA) was for the practicality of the model. Bilateral P ≤ 0.05 was statistically significant.
Results
Clinical and Demographic Characteristics of the Patients
A total of 403 patients in T2DM with DKD met the inclusion criteria in the research. Table 1 summarizes the clinical and demographic characteristics of the patients. Fifty-nine cases (14.6%) of these were the patients with DKD, while the non-DKD group comprised 344 cases (85.4%). Univariate regression analysis showed that the rate of males, history of hypertension, stroke, gout, smoking, and drinking, DR, fatty liver, and using insulin in the DKD group was significantly higher than that in the non-DKD group (P < 0.05). The value of BMI, visceral fat area, UREA, uric acid (UA), creatinine (CR), triglyceride (TG), Cys-C, and AIP was also significantly higher in the DKD group (P < 0.05). In addition, the direct bilirubin (DBIL), 25(OH)D, and low-density lipoprotein cholesterol (LDL-C) were lower in the DKD group (P < 0.05).
Feature Selection
Taking DKD as the dependent variable, variables with statistically significant differences in univariate analysis were taken as independent variables, and the result of feature screening based on logistic regression analysis is shown in Table 2 which included the history of hypertension, gout, and smoking, using insulin, BMI, TG, Cys-C, 25(OH)D, and DR (0 = NDR, 1 = NPDR, 2 = PDR).
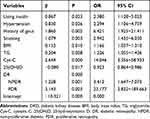 |
Table 2 Feature Selection for DKD |
The generalized variance inflation factor (GVIF) values of these were 1.047, 1.094, 1.074, 1.076, 1.054, 1.049, 1.031, 1.085, and 1.048, respectively, which did not support multicollinearity.6
Construction of a Nomogram-Based Prediction Model
Based on the results of feature selection, a nomogram-based prediction model of DKD in T2DM was constructed. Figure 1 shows a nomogram-based model consisting of scores, predictors, total scores, and occurrence risk. The scale of each line segment represents the value range corresponding to the predictor, and the length of the line segment is the value of the contribution of the predictor to the risk of DKD occurrence. The score at the top of the figure represents the corresponding score of each predictor under different values. The sum of score values of all predictors is calculated based on the score of each predictor, and the predicted value of DKD risk could be obtained on the Risk of DKD axis at the bottom.
Model Performance
The representativeness of the model was verified by bootstrapping. The accuracy was 0.866 and the Kappa value was 0.334, indicating that the representativeness of the model was acceptable.
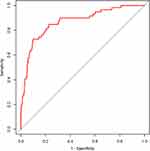
The area under the ROC curve shown in Figure 2 was 0.868 (95% CI: 0.814~0.921). The critical value of the model was 0.178 with the maximum Jorden index (0.642), when the sensitivity was 0.813 and the specificity was 0.828, indicating that the model had good prediction efficiency and differentiation. Figure 3 shows the GiViTI calibration curve of the model, whose 80% and 95% confidence intervals did not cross the 45° diagonal bisector. The P value of it was 0.836, indicating that the calibration curve of the model was close to the actual observation probability. Figure 4 shows the DCA of this model. When the prediction value of the model was between 0.10 and 0.70, it had clinical practical value for predicting the risk of T2DM progression to DKD.
 |
Figure 3 GiViTI calibration curve basing on the nomogram model predicting the risk of DKD in T2DM. Abbreviations: DKD, diabetic kidney disease; T2DM, type 2 diabetes mellitus. |
 |
Figure 4 DCA basing on the nomogram model predicting the risk of DKD in T2DM. Abbreviations: DCA, decision curve analysis; DKD, diabetic kidney disease; T2DM, type 2 diabetes mellitus. |
Discussion
In this study, history of hypertension, gout, and smoking, DR, using insulin, BMI, TG, Cys-C, and 25(OH)D were as the feature variables by stepwise Logistic regression to construct a nomogram-based model to predict the risk of DKD in T2DM patients. The model had good representativeness, the ability to predict, high consistency between actual values and predictions, and good practicality by bootstrapping ROC curve, GiViTI calibration curve, and DCA.
In this study, the history of hypertension disease was an independent risk factor for DKD in T2DM, which was consistent with previous research.7 Actively controlling blood pressure is considered the cornerstone of preventing and treating DKD and the guidelines recommend that the blood pressure control target for DKD is under 130/80mmHg, the preferred drug angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blocker (ARB) drugs.2 In recent years, it has been found that sodium-glucose cotransporter 2 inhibitor (SGLT2i), dipeptidyl peptidase IV inhibitor (DPP-4i), and glucagon-like peptide-1 receptor agonist (GLP-1RA) have not only hypoglycemic effects, but also antihypertensive effect, and have certain renal benefits for DM,8–10 which may be related to inhibiting inflammatory response and oxidative stress in T2DM.11,12 Therefore, the above hypoglycemic drugs should be taken as soon as possible to strengthen the protection of the kidney for T2DM with hypertension.
In this study, univariate analysis found that UA and the history of gout in DKD group were significantly higher than those in non-DKD group, and logistic analysis found that the latter was an independent risk factor for DKD, which was consistent with the conclusions of ROY.13 The possible reasons are related to hyperuricemia aggravating HOMA-IR, increasing local inflammation and oxidative stress, and aggravating atherosclerosis, leading to kidney injury and renal function decline.14–16 Therefore, the history of gout may have a certain predictive value for DKD, and clinicians should strengthen the management of blood uric acid in T2DM. For T2DM with a history of gout, attention should be paid not only to the uric acid but also to the changes in renal function and kidney structure.
DR and DKD, the main manifestations of diabetic microvascular complications, have common risk factors such as poor blood glucose control and hypertension, which often coexist in T2DM. Compared with DKD, which needs to be confirmed by kidney biopsy, DR can be diagnosed by fundus photography, so many clinicians often use DR to predict the presence of DKD. Sasso17 found that urinary albumin excretion rate (AER) may be associated with DR in T2DM with DKD, and this relationship was confirmed in patients with heavier DR. In this study, it was found that DR was correlated with DKD, which was consistent with the above conclusions. In addition, the probability of DKD in NPDR patients was 3.4 times that of NDR patients and the probability of DKD in PDR patients was 23.2 times that of NDR patients in this study. However, a large study18 in China found there were different risk factors in DR and DKD. The team18 believed that although DR and DKD have similar pathogenesis, there are still differences in some pathogenesis, so DR cannot be used to judge whether DKD is present. One of the reasons for the above differences may be the heterogeneity of inclusion criteria, exclusion criteria, and experimental design methods.
Animal study19 has shown that cigarette smoke worsens kidney damage in diabetic rats, and exposure to smoke for 4 weeks worsens DKD progression in diabetic mice. Han20 found that people who smoked, compared with non-smoking, showed a moderate decrease in glomerular filtration rate (eGFR), accompanied by tubular atrophy and renal interstitial fibrosis. Besides, blood glucose and lipid levels were blood glucose and lipid levels in the smoking.20 In this study, having a history of smoking was an independent risk factor for DKD in T2DM, and the probability of DKD in T2DM patients with a history of smoking was 3 times that of those without a history of smoking. On the one hand, the harmful components in tobacco may directly damage the intima of blood vessels, reduce the elasticity of blood vessels, promote the infiltration of inflammatory factors and white blood cells into the blood vessel wall, cause vascular constriction, exacerbate blood hypercoagulability, and lead to endothelial dysfunction, thus initiating or aggravating atherosclerosis and affecting the filtration function of the glomeruli.21 On the other hand, smoking may cause an increase in blood sugar and blood lipid, aggravate the damage of renal tubule and renal interstitial caused by high glucose and high lipid toxicity and oxidative stress, and further promote the occurrence of DKD.22 Therefore, health education and smoking cessation guidance for T2DM patients are of great significance for the prevention and treatment of DKD.
The use of insulin was a predictor of DKD in this study whose reason may be patients can manage their blood glucose through appropriate exercise, diet control, oral hypoglycemic drugs, and so on in the early stage of onset of T2DM or when the disease is relatively mild. Only when patients cannot reach the blood glucose standard through the above factors will clinicians recommend patients to use insulin to control blood glucose. Additionally, some patients with poor blood sugar control in the above way refuse to use insulin therapy for various reasons until the situation is relatively awful. Therefore, it is not difficult to imagine that patients treated with insulin are poor in β cell, more serious disease, and more diabetes complications, which result in the higher risk in DKD.
In this study, BMI was the independent risk factor for DKD in T2DM, which was consistent with the conclusions of previous studies.22–24 In the pathological mechanism, higher BMI may promote the occurrence of DKD through promoting HOMA-IR, chronic inflammatory response, endothelial dysfunction, fibrosis, and thrombosis.23–26 HOMA-IR, which has a complex relationship with DKD, usually promotes the occurrence of DKD in the early stage and is generally present in end-stage DKD through various mechanisms.27 There are many high-affinity insulin receptors in renal tubule cells and podocytes. When HOMA-IR is present, the glomerular insulin signal is impaired, causing renal epithelial cell dysfunction, proteinuria, and promoting renal fibrosis.28 Hence, HOMA-IR may be an important pathological component of DKD. Because higher BMI is often accompanied by HOMA-IR, patients with T2DM should keep BMI within an appropriate range to reduce the HOMA-IR, which may prevent the occurrence of DKD to a certain extent.26
Dyslipidemia, one of the main causes of kidney injury, plays an important role in the occurrence and development of DKD.29 Our study found that TG was one of the predictors for DKD, consistent with the conclusion of previous studies.30 The studies31,34 found that at least eight standard predictors, such as eGFR, urinary albumin, SCR, T1DM disease course, HbA1c, age, and BMI, effectively indicated that Cys-C, renal resistance index, and renal injury molecular-1 may be related to the risk of DKD, which confirmed the potential value of Cys-C in predicting the risk of developing DKD in patients with T1DM. The results of this study showed that patients with higher Cys-C had a significantly increased risk of DKD, and subsequent analyses found that Cys-C could be used to predict the occurrence of DKD. However, the specificity of Cys-C is poor, which is a shortcoming of Cys-C.
Renal fibrosis is one of the markers of progression of DKD, and epithelial-mesenchymal-transition (EMT) is a key mechanism of progression of diabetic renal fibrosis.35,36 In animal study, the lack of Vit D can promote the occurrence of EMT and lead to the rapid progression of DKD by enhancing the expression of transcriptional co-suppressor ZEB1/ZEB2 and down-regulating the expression of miR-200b gene, which could inhibit the transcription and translation of ZEB1/ZEB2.37 In this study, 25(OH)D was found to be a protective factor for the progression of T2DM to DKD, which was consistent with previous studies.38,39 The prevalence of DKD is still increasing despite the use of Vit D replacement therapies, based mainly on blood glucose, and blood pressure control to delay the progression of ESRD. However, it is indisputable that correcting Vit D can still play a role in delaying the development of DKD.40,41
We should acknowledge several limitations of this study. First, this was a single-center retrospective study, which inevitably had a certain selection bias. Second, some confounding factors not included in the study may not have been adequately addressed. Third, the sample size of this study was not large enough, and external verification was absent. In the future, multi-center prospective and large sample studies can be further conducted to verify our results and improve the robustness and extrapolation of the model’s prediction efficiency.
Conclusion
The prediction model constructed in this study had 9 predictors, including history of hypertension disease, gout, smoking, insulin use, BMI, DR, TG, Cys-C, and 25(OH)D, and had a good overall performance after the tests, bootstrapping, ROC curve, GiViTI calibration curve, and DCA.
What is more, the nomogram-based model can help clinicians to predict the risk of DKD in a non-invasive and accurate way, in order to facilitate early action, which may have significant benefits for patients.
Consent for Publication
Consent for publication was obtained from all the authors.
Acknowledgments
We would like to thank all participants because no significant study could have been conducted without them.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was not funded.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Guo L. Diabetes map of China. Beijing: National Center for Gerontology; 2022. Available from: https://class.medlive.cn/class/live/show/73315.
2. American Diabetes Association Professional Practice Committee, American Diabetes Association Professional Practice Committee. 11. chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S175–S184. doi:10.2337/dc22-S011
3. Zhang XX, Kong J, Yun K. Prevalence of diabetic nephropathy among patients with type 2 diabetes mellitus in China: a meta-analysis of observational studies. J Diabetes Res. 2020;2020:2315607. doi:10.1155/2020/2315607
4. ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46:S19–S40. doi:10.2337/dc23-S002
5. Qin Z, Zhou K, Li Y, et al. The atherogenic index of plasma plays an important role in predicting the prognosis of type 2 diabetic subjects undergoing percutaneous coronary intervention: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19:23. doi:10.1186/s12933-020-0989-8
6. Yue S, Li S, Huang X, et al. Construction and validation of a risk prediction model for acute kidney injury in patients suffering from septic shock. Dis Markers. 2022;2022:9367873.
7. Wang J, Wang Y, Li Y, et al. High normal urinary albumin-creatinine ratio is associated with hypertension, type 2 diabetes mellitus, HTN with T2DM, dyslipidemia, and cardiovascular diseases in the Chinese population: a report from the REACTION study. Front Endocrinol. 2022;13:864562. doi:10.3389/fendo.2022.864562
8. Triantafylidis LK, Hawley CE, Fagbote C, et al. A pilot study embedding clinical pharmacists within an interprofessional nephrology clinic for the initiation and monitoring of empagliflozin in diabetic kidney disease. J Pharm Pract. 2021;34(3):428–437. doi:10.1177/0897190019876499
9. Trakarnvanich T, Satirapoj B, Suraamornkul S, et al. Effect of Dipeptidyl Peptidase-4 (DPP-4) inhibition on biomarkers of kidney injury and vascular calcification in diabetic kidney disease: a randomized controlled trial. J Diabetes Res. 2021;2021:7382620. doi:10.1155/2021/7382620
10. Yamada S, Tanabe J, Ogura Y, et al. Renoprotective effect of GLP-1 receptor agonist, liraglutide, in early-phase diabetic kidney disease in spontaneously diabetic torii fatty rats. Clin Exp Nephrol. 2021;25:365–375.
11. Leng W, Wu M, Pan H, et al. The SGLT2 inhibitor dapagliflozin attenuates the activity of ROS-NLRP3 inflammasome axis in steatohepatitis with diabetes mellitus. Ann Transl Med. 2019;7(18):429. doi:10.21037/atm.2019.09.03
12. Liljedahl L, Pedersen MH, McGuire JN, et al. The impact of the glucagon-like peptide 1 receptor agonist liraglutide on the streptozotocin-induced diabetic mouse kidney proteome. Physiol Rep. 2019;7(4):e13994. doi:10.14814/phy2.13994
13. Roy S, Schweiker-Kahn O, Jafry B, et al. Risk factors and comorbidities associated with diabetic kidney disease. J Prim Care Community Health. 2021;12:21501327211048556. doi:10.1177/21501327211048556
14. Bahadoran Z, Mirmiran P, Kashfi K, et al. Hyperuricemia-induced endothelial insulin resistance: the nitric oxide connection. Pflugers Arch. 2022;474:83–98.
15. Liu S, Yuan Y, Zhou Y, et al. Phloretin attenuates hyperuricemia-induced endothelial dysfunction through co-inhibiting inflammation and GLUT9-mediated uric acid uptake. J Cell Mol Med. 2017;21:2553–2562. doi:10.1111/jcmm.13176
16. Zhao Z, Zhao Y, Zhang Y, et al. Gout-induced endothelial impairment: the role of SREBP2 transactivation of YAP. FASEB J. 2021;35:e21613. doi:10.1096/fj.202100337R
17. Sasso FC, Pafundi PC, Gelso A, et al. Relationship between albuminuric CKD and diabetic retinopathy in a real-world setting of type 2 diabetes: findings from no blind study. Nutr Metab Cardiovasc Dis. 2019;29:923–930. doi:10.1016/j.numecd.2019.05.065
18. Liu Z, Li X, Wang Y, et al. The concordance and discordance of diabetic kidney disease and retinopathy in patients with type 2 diabetes mellitus: a cross-sectional study of 26,809 patients from 5 primary hospitals in China. Front Endocrinol. 2023;14:1133290. doi:10.3389/fendo.2023.1133290
19. Jiang S, Quan DV, Sung JH, et al. Cigarette smoke inhalation aggravates diabetic kidney injury in rats. Toxicol Res. 2019;8(6):964–971. doi:10.1039/c9tx00201d
20. Han Q, Wang S, Zhang J, et al. The association between cigarette smoking and diabetic nephropathy in Chinese male patients. Acta Diabetol. 2018;55(11):1131–1141. doi:10.1007/s00592-018-1197-9
21. Talukder MA, Johnson WM, Varadharaj S, et al. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am J Physiol Heart Circulatory Physiol. 2011;300:H388–H396. doi:10.1152/ajpheart.00868.2010
22. Shigidi MM, Karrar WN. Risk factors associated with the development of diabetic kidney disease in Sudanese patients with type 2 diabetes mellitus: a case-control study. Diabetes Metab Syndr. 2021;15(6):102320. doi:10.1016/j.dsx.2021.102320
23. Jiang W, Wang J, Shen X, et al. Establishment and validation of a risk prediction model for early diabetic kidney disease based on a systematic review and meta-analysis of 20 cohorts. Diabetes Care. 2020;43(4):925–933. doi:10.2337/dc19-1897
24. Tu X, Luo N, Lv Y, et al. Prognostic evaluation model of diabetic nephropathy patients. Ann Palliat Med. 2021;10(6):6867–6872. doi:10.21037/apm-21-1454
25. Zhang W, Du J, Dong H, et al. Obesity metabolic phenotypes and unplanned readmission risk in diabetic kidney disease: an observational study from the nationwide readmission database. Arch Med Res. 2023;54(6):102840. doi:10.1016/j.arcmed.2023.102840
26. Shi S, Ni L, Tian Y, et al. Association of obesity indices with diabetic kidney disease and diabetic retinopathy in type 2 diabetes: a real-world study. J Diabetes Res. 2023;2023:3819830. doi:10.1155/2023/3819830
27. Ahlqvist E, Prasad RB, Groop L. Subtypes of type 2 diabetes determined from clinical parameters. Diabetes. 2020;69(10):2086–2093. doi:10.2337/dbi20-0001
28. Jawa A, Kcomt J, Fonseca VA. Diabetic nephropathy and retinopathy. Med Clin North Am. 2004;88(4):1001–36, xi. doi:10.1016/j.mcna.2004.04.012
29. Zhang B, Wan Y, Zhou X, et al. Characteristics of serum metabolites and gut microbiota in diabetic kidney disease. Front Pharmacol. 2022;13:872988. doi:10.3389/fphar.2022.872988
30. Gong L, Wang C, Ning G, et al. High concentrations of triglycerides are associated with diabetic kidney disease in new-onset type 2 diabetes in China: findings from the China cardiometabolic disease and cancer cohort (4c) study. Diabetes Obesity Metab. 2021;23:2551–2560. doi:10.1111/dom.14502
31. Tang C, Deng X, Qu J, et al. Fenofibrate attenuates renal tubular cell apoptosis by up-regulating MCAD in diabetic kidney disease. Drug Des Devel Ther. 2023;17:1503–1514. doi:10.2147/DDDT.S405266
32. Wang C, Wang L, Liang K, et al. Poor control of plasma triglycerides is associated with early decline of estimated glomerular filtration rates in new-onset type 2 diabetes in china: results from a 3-year follow-up study. J Diabetes Res. 2020;2020:3613041. doi:10.1155/2020/3613041
33. Nitta K, Hayashi T, Uchida K, et al. Serum cystatin C concentration as a marker of glomerular filtration rate in patients with various renal diseases. Intern Med. 2002;41:931–935. doi:10.2169/internalmedicine.41.931
34. Trutin I, Bajic Z, Turudic D, et al. Cystatin C, renal resistance index, and kidney injury molecule-1 are potential early predictors of diabetic kidney disease in children with type 1 diabetes. Front Pediatr. 2022;10:962048. doi:10.3389/fped.2022.962048
35. Srivastava SP, Hedayat AF, Kanasaki K, et al. microRNA crosstalk influences epithelial-to-mesenchymal, endothelial-to-mesenchymal, and macrophage-to-mesenchymal transitions in the kidney. Front Pharmacol. 2019;10:904. doi:10.3389/fphar.2019.00904
36. Srivastava SP, Koya D, Kanasaki K. MicroRNAs in kidney fibrosis and diabetic nephropathy: roles on EMT and EndMT. Biomed Res Int. 2013;2013:125469. doi:10.1155/2013/125469
37. Hua W, Ten Dijke P, Kostidis S, et al. TGFβ-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer. Cell Mol Life Sci. 2020;77(11):2103–2123. doi:10.1007/s00018-019-03398-6
38. Felício JS, de Rider Britto HA, Cortez PC, et al. Association between 25(OH)Vitamin D, HbA1c and albuminuria in diabetes mellitus: data from a population-based study (VIDAMAZON). Front Endocrinol. 2021;12:723502. doi:10.3389/fendo.2021.723502
39. Abasheva D, Dolcet-Negre MM, Fernández-Seara MA, et al. Association between circulating levels of 25-Hydroxyvitamin D(3) and Matrix Metalloproteinase-10 (MMP-10) in patients with type 2 diabetes. Nutrients. 2022;14(17):3484. doi:10.3390/nu14173484
40. Souza CS, Deluque AL, Oliveira BM, et al. Vitamin D deficiency contributes to the diabetic kidney disease progression via increase ZEB1/ZEB2 expressions. Nutr Diabetes. 2023;13(1):9. doi:10.1038/s41387-023-00238-2
41. Wang Y, Quan F, Cao Q, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2021;28:231–243. doi:10.1016/j.jare.2020.07.007
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.