Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Comprehensive Genomic Profiling Identifies FAT1 as a Negative Regulator of EMT, CTCs, and Metastasis of Hepatocellular Carcinoma
Authors Huang ZL, Zhang PB, Zhang JT, Li F, Li TT, Huang XY
Received 28 November 2022
Accepted for publication 1 March 2023
Published 7 March 2023 Volume 2023:10 Pages 369—382
DOI https://doi.org/10.2147/JHC.S398573
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Imam Waked
Zi-Li Huang,1,2,* Ping-Bao Zhang,1,3,* Jun-Tao Zhang,4 Feng Li,5 Ting-Ting Li,6 Xiu-Yan Huang1
1Department of General Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China; 2Department of Radiology, Xuhui District Central Hospital of Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 3Department of Urology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 4Institute of Microsurgery on Extremities, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China; 5School of Materials of Science and Engineering, Shanghai Jiao Tong University, Shanghai, People’s Republic of China; 6Department of Infectious Disease, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiu-Yan Huang, Department of General Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 600 Yi Shan Road, Shanghai, People’s Republic of China, Tel/Fax +86-21-64701361, Email [email protected]
Background: FAT atypical cadherin 1 (FAT1) acts as a tumor suppressor or oncogene, which regulates cell adherence, proliferation, motility, and actin kinetics. FAT1 gene expression is closely related to hepatocarcinogenesis; however, the function and mechanism of FAT1 in hepatocellular carcinoma (HCC) remain unclear.
Methods: Here, we screened for the FAT1, which is intimately linked to the development and progression of HCC, both in circulating tumor cells (CTCs) and tumor tissues using next generation sequencing (NGS). Immunohistochemical staining was performed to detect FAT1 protein expression. To determine the impact of FAT1 on epithelial–mesenchymal transition (EMT), migration and invasion of HCC, an in vitro transwell assay and Western blot were performed. Moreover, Gene Set Enrichment Analysis was carried out to discover the underlying mechanism. Finally, animal experiments were conducted to confirm the effects of FAT1 on HCC metastasis and tumorigenicity.
Results: Our results showed that FAT1 expression was decreased in HCC tissues, while in vitro and in vivo, the FAT1 knockdown facilitated invasion, cell motility, colony formation, and proliferation. FAT1 knockdown also resulted in decreased expression of E-cadherin and markedly elevated expression of N-cadherin, vimentin, and snail. We also confirmed our hypothesis from the analysis of group differences in the CTC phenotype and lung metastasis in nude mice.
Conclusion: Our findings illustrated that FAT1 played a negative regulatory role in the HCC EMT and metastasis, providing further evidence for the role played by FAT1 in the formation and progression of HCC.
Keywords: hepatocellular carcinoma, FAT1, epithelial-mesenchymal transition, circulating tumor cells, metastasis
Introduction
Liver cancer ranks sixth in relation to new cancer cases and fourth in the most prevalent cause of death worldwide.1 According to reports, only 12% of patients with liver cancer in China survive for 5 years.2 Approximately 80% of patients with hepatocellular carcinoma (HCC) have a history of chronic hepatitis B virus or hepatitis C virus infection.3 Patients with advanced HCC have a high rate of recurrence and metastasis, together with the limited benefit of conventional treatment and a poor prognosis.4
The epithelial mesenchymal transition (EMT) is required for metastasis in various cancers.5,6 The prognosis of patients with cancer is impacted by the phenotypic shifting of cells, which promotes drug resistance and tumor recurrence.7
Circulating tumor cells (CTCs) play a critical role in establishing the spread and relapse of HCC.8 CellSearch® (Menarini-Silicon Biosystems) is the first FDA-approved device for automated CTC detection and involves using anti-epithelial cell adhesion molecule (EpCAM) antibodies.9 Despite the excellent CTC isolation effectiveness of CellSearch®, in epithelial malignant tumors, certain EMT-phenotype CTCs are very aggressive and can evade the positive enrichment methods for epithelial markers (such as EpCAM and CK).10 HCC CTCs express both epithelial markers, including EpCAM11 and CK19,12 and mesenchymal markers, including vimentin.13 Here, we optimized the CTC isolation method by selecting combinations of antibodies against various surface markers on HCC CTCs to prevent their escape during the capture process. The quantity of CTCs identified in patients with HCC is significantly associated with their TNM stage.14 Therefore, we enrolled ten patients with advanced HCC to participate in this study. Previous reports have demonstrated that the FAT1 protein inhibits the development of malignancy by regulating the EMT process in tumor cells where the FAT1 gene functions as a tumor suppressor gene;15–17 however, Zhu et al demonstrated that FAT1 and POU2F1 mediate the growth and metastasis of HCC cells.18 Meng et al suggested that, in conjunction with GPC3, the expression of genes associated with EMT is co-regulated by FAT1 in HCC cells, and thus promotes HCC cell metastasis.19 This study indicated that the effect of FAT1 on cancer progression may be cell type or microenvironment specific. It is likely that HCC cells in hypoxia in synergy with GPC3 can promote cell metastasis and progression. However, the role and mechanism of FAT1 action on HCC CTCs for distal metastasis of CTCs in the blood microenvironment remains to be clarified. Therefore, we interrogated CTCs which obtained from HCC patients and then found that FAT1 played a negative regulatory role in metastasis of HCC CTCs. In addition, we found that FAT1 expression was higher in tumor tissues in 6 patients, however, the remaining patients had lower FAT1 expression in the tumor tissues when compared with the adjacent non-tumor tissues. HCC cells under hypoxic conditions promoted tumor migration and growth through upregulation of snail and vimentin and downregulation of E-Cadherin by the combined action of FAT1 and GPC3.19 FAT1 also promoted the anti-apoptotic ability of HCC and thus also promoted the progression of HCC.20 On the other hand, knockdown of FAT1 also led to rapid loss of basal cell polarity as well as adhesion and tight junctions, thus promoting tumor progression.21 In this study, we captured CTCs from HCC patients using our modified CTC capture method,22 and extracted HCC tissues and CTC DNAs for next generation sequencing (NGS). We then combined the sequencing data from tissue and CTC to screen the FAT1 gene of interest, and cultured the HCC cell lines in a simulated blood environment and collected the treated tumor cells with magnetic beads. We found that the migration and invasion ability of the treated tumor cells were enhanced after knocking down FAT1, suggesting that interfering with FAT1 may promote metastasis of HCC CTCs.
Materials and Methods
Patient Tissues and Peripheral Blood Samples Collection
Ten patients were diagnosed with HCC at the Shanghai Sixth People’s Hospital. This study was conducted in accordance with the guidelines of the Declaration of Helsinki. All patients provided signed informed consent before sample collection. Peripheral blood was collected in 10-mL EDTA vacutainers before surgery, 7.5 mL of blood per case was centrifuged at 2500 rpm for 10 min within 24 h of collection, and the plasma layer was separated and transferred to EP tubes. After surgery, patient paired tumor and non-tumor tissues were also made into paraffin-embedded tissue samples. This study was approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (No. 2022–020; China Clinical Trial Registration Center - Registration No. ChiCTR2200055847), and the patients signed the written consent after receiving oral and written information.
Cell Culture
The cell lines of Hep3B and MHCC97H were purchased from the Shanghai Cell Bank of Chinese Academy of Sciences. Hep3B and MHCC97H cells were cultured in Dulbecco modified Eagle medium (DMEM, Gibco, USA) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) in a humidified incubator containing 5% CO2/95% air at 37°C. After magnetic separation, the cells were added to simulated blood23 cultured for 72 h, during which 20 ng/mL of TNF-α was added, and finally the cells were collected for subsequent in vitro and in vivo experiments.
Western Blot
Protease inhibitors (Roche, Switzerland) and lysis buffer (RIPA, Beyotime, Shanghai, China) were used to lyse whole cells on ice. The lysate was centrifuged for 15 min at 12,000 g and 4°C, and the di-octanoic acid (Sigma) technique was used to determine the content of the supernatant. For Western blot, 50 µg of protein was separated by electrophoresis on NUPAGE 10%–12% Transferring Bis-Tris gels (Invitrogen, CA, USA) before transferring to polyvinylidene difluoride (PVDF, 0.45 µm) membranes with a continuous current of 350 mA for 70/120 min. After being blocked for 2 h in a TBST buffer that included 5% skim milk powder and Tris-buffered saline, the membranes were incubated with the following specific antibodies at 4°C overnight: p-ERK1/2, ERK1/2, E-cadherin, N-cadherin, vimentin, and snail (Abcam, Cambridge, UK). Horseradish peroxidase-conjugated secondary antibodies (Sigma) were used to examine the blots, and a LAS4000 device was used to detect chemiluminescence (Fuji). Beta (β)-actin (Proteintech, Group, Wuhan, China) was applied as a loading control. The assays were conducted in triplicate.
Plasmid Constructs and Transfection
We used two lentiviral vector sequences (5′-GCAGCTGGAGAATATGATATT-3′, 5′-GCAACCGGCTCTCTCTATACT-3′) and a control sequence (5′-TTCTCCGAACGTGTCACGTTTC-3′) named FAT1sh1, FAT1sh2, and FAT1NC, respectively. The shRNAs were implanted into the vector pGLV-FAT1-Puro and co-transfected into Hep3B and MHCC97H cells. FAT1-overexpressed cell clones were generated by stably transfecting Hep3B cells with the lentivirus expression vector. Next, 2 µg/mL of puromycin was used to select stable cell lines over 2 weeks (Invitrogen). Hep3B and MHCC97H cells were cultured in DMEM (10% FBS) and placed in 4-well glass bottom culture plates with 1.5×104 cells/well. After 24 h, cells were transfected with plasmids (1 µg per well) using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA; 2 µL per well) in accordance with the manufacturer’s instructions. The new medium was applied after 4 h, and cells were cultured for 36 h before being exposed to 1.5 µg/mL of cytochrome B (Sigma, St. Louis, MO, USA) for 12 h.
Transwell Migration and Invasion Assays
During the Transwell migration experiment, the upper chamber of the Transwell was loaded with 2×105 cells in 300 μL serum-free DMEM, and the bottom chamber was loaded with 500 μL of serum-free medium. The cells were fixed with 4% formalin solution, stained with Gentian Violet, and incubated for 4 h at 37°C. Non-migrating cells were retained at the top layer and removed by wiping them with a cotton swab. Ten randomly selected microscopic regions were used to count the average number of cells that passed through the filter. Transwell chambers (Corning) were also used for in vitro invasion tests. Briefly, the cells were coagulated for 1 h at 37°C before covering the Transwell system with 300 μL of serum-free DMEM and cells (2 × 105). Next, 500 μL of the serum-free medium was added to the lower layer and cultured for 24 h at 37°C. Crystal violet was used to stain fixed cells, which were then counted in ten randomly selected microscopic regions.
Animal Experiments
This study was approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (No. 2022–020), all animal experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and regulations of the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. For in vivo experiments, 5×105 FAT1 knockdown stable Hep3B or Hep3BNC cells and FAT1-WT overexpressing MHCC97H or MHCC97HNC cells were administered subcutaneously into the right armpit of male BALB/c nude mice. The tumor size was measured every 7 days using calipers, and the tumor volume was calculated according to the following formula: volume = 1/2 × (width2 × length). The tumors were removed and measured after the mice were euthanized and sacrificed after 5 weeks.
RNA Isolation and Real-Time Quantitative PCR (qRT–PCR)
Cells cultured in the growth medium for 48 h were serum-starved for 24 h before total RNA extraction using TRIzol Reagent (Invitrogen) following the manufacturer’s instructions. The PrimeScriptTM RT reagent kit (Toyobo, Osaka) was used to reverse-transcribe 1 g of total RNA into cDNA after determining the RNA concentration. Subsequently, cDNA was used for qRT–PCR using the SYBR-Green PCR Master Mix (Takara, Osaka). qRT–PCR was performed on Hep3B, Hep3BNC, MHCC97H, MHCC97HNC, and the matching FAT1-depleted cells to determine alterations in the relevant genes. GAPDH was used as a control.
RNA Sequencing
RNA sequencing was performed on Hep3B, Hep3BNC and corresponding FAT1-depleted cells to monitor changes in their downstream genes. Huzhou Lieyuan Medical Laboratory Co. Ltd. handled the extraction of total RNA and sequence analysis. GSEA was used to evaluate the different genes in comparison with FAT1 knockdown and normal cells.
Hematoxylin and Eosin (HE) Staining
HE staining was used to confirm the presence of lung metastatic nodules in paraffin blocks made from 10% buffered formalin-fixed tumor and pulmonary lung tissue samples.
Immunohistochemistry (IHC)
Serial sections of the FFPE specimens were generated at a thickness of 5 μm. Antigen repair was performed in 0.01 M citrate buffer (pH 6.0) in a pressure cooker for 30 min, then 3% hydrogen peroxide for 5 min of pretreatment. After fixing with 10% buffered formalin, the tissue slices were incubated for 24 h at 4°C with anti-FAT1 (Sigma, 1:300 dilution), E-cadherin (CST, USA), Ki-67 (CST, USA), N-cadherin (Maixing, China), Snail (CST, USA), p-ERK1/2 (CST, USA), Vimentin (CST, USA). Sections were washed with phosphate buffered saline (PBS) before being exposed to the secondary antibody for 20 min at 37°C. The levels of FAT1 protein expression were evaluated with Aperio Cytoplasma software by IHC.
CTC Assay
The plasma in EP tubes was placed on a magnetic separation rack and left for 15 min after being stirred seven times over a 28 min incubation period at 25°C, before aspirating the supernatant. The samples were washed twice with 2 mL PBS, before adding 10 μL DAPI, CK19-FITC, and CD45-PE staining solutions sequentially and incubating for 30 min at 25°C. Following incubation, the samples were distributed equally on anti-dislodging slides, dried naturally, and photographed and counted under a fluorescent-microscopic device. Captured by Ep-LMB and CK+/CD45−/DAPI+ were defined as epithelial CTC (ECTC), and captured by Vi-LMB and CK+/CD45−/DAPI+ were defined as mesenchymal CTC (MCTC). The MCTC ratio was defined as the proportion of MCTCs to all CTCs in each patient sample (7.5 mL).
Identification of Genetic Variants
Ten FFPE and CTC samples from patients with HCC were submitted to Huzhou Lieyuan Medical Laboratory Co. Ltd. A total of 610 genes were included in the assay. The NGS assay of FFPE and CTC samples was conducted following previously published protocols.24,25
Statistical Analysis
SPSS statistical software (version 22.0, IBM SPSS, USA) was used to analyze the data. Results are shown as mean ± SEM, and three independent tests were conducted. FAT1 protein levels were examined in tumors and matched non-tumors using the rank sum test. The data between the two groups were evaluated using an unpaired t-test, and differences from multiple groups were examined using one-way ANOVA. P-values < 0.05 were considered statistically significant.
Results
Identification of FAT1 from Genomic Analysis of CTC and Tissue DNAs
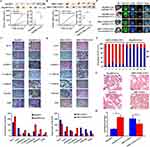
We optimized the CTC capture method using EpCAM-supplemented CTC enrichment and detection technology. CTCs and tumor tissues from patients with HCC were analyzed for genetic mutations, as shown in the workflow (Figure 1A). The mean age of 10 patients was 62 years (range, 46–79 years), and the number of patients at clinical stage IIIB and IVA was 8 (80%) and 2 (20%), respectively. We performed immunofluorescence analysis with anti-pan keratin antibodies to determine the number of CTCs in the blood of patients with HCC. CTCs were detected in all ten patients, with a median CTC count of 6 CTCs/mL (range: 4–9). Representative images of CTCs from patients are presented in Figure 1C. NGS genomic profiling of CTCs and tumor tissues from patients with HCC is shown in Figure 1B. Mutations were detected in all patients with HCC, with a median number of mutations detected in peripheral blood CTCs and HCC tissues of 7 (range: 1–29) and 6.5 (range: 2–19), respectively. CTC and histogenetic analyses showed that TP53 was the most commonly altered gene, followed by ALK, ARID1A, and FAT1. TP53 and ARID1A are tumor suppressor genes, while ALK is a proto-oncogene, all of which are associated with malignancy.26–28 Combinatorial analysis of the genomic mutation profiles obtained from CTC and tissue DNA showed some common genetic alterations, including FAT1 (Figure 1D and E). We next examined FAT1 protein expression in malignant and adjacent normal tissues of ten patients with HCC using IHC analysis. We found that FAT1 expression was higher in tumor tissues in 6 patients, however, the rest 4 patients had lower FAT1 expression in the tumor tissues when compared to the adjacent non-tumor tissues. Overall, the levels of FAT1 protein expression in cancer tissues were lower than those in adjacent normal tissues in this section (Figure 1F–G). Microarray datasets GSE36529 were downloaded from Gene Expression Omnibus (GEO) database. Reading expression profile data was conducted in R environment using GEOquery package.30 The GSE365 dataset contained 87 HCC tissue samples and 87 non-cancerous samples. By exploring this dataset, we found that FAT1 expression was higher in tumor tissues and metastases than normal tissues in some hepatitis B virus-positive HCC patients, while the remaining patients showed the opposite pattern of FAT1 expression, which was consistent with our above results. These results indicated that FAT1 expression showed a high degree of heterogeneity in the HCC population.
FAT1-Knockdown-Induced EMT Promotes HCC Cell Migration and Invasion
RT-qPCR and Western blot were used to evaluate the mRNA and protein concentrations of FAT1 in 2 HCC cell lines (Figure 2A), before clarifying the true effects of FAT1 knockdown and overexpression in these cells individually (Figure 2B and C). Knockdown of FAT1 enhanced cell division (Figure 2D) and the establishment of colonies (Figure 2E) in Hep3B and MHCC97H cell lines, while overexpression of FAT1 significantly slowed cell growth and colony formation (Figure 2F–G). Next, we evaluated the impact of FAT1 on the invasive cell motility of cultured cells. Interestingly, FAT1 knockdown markedly facilitated the colonization and mobility of Hep3B and MHCC97H cells. FAT1 knockdown in Hep3B and MHCC97H cells significantly enhanced tumor cell colonization and mobility, as assessed by the Transwell assay (Figure 3A) and wound healing migration assay (Figure 3B). However, we observed the opposite trend in exogenous FAT1-expressing cells (Figure 3C). We also measured the level of EMT markers in FAT1 knockdown cells (Figure 3D). E-cadherin mRNA expression was markedly reduced by FAT1 knockdown, which in turn caused N-cadherin, vimentin, and snail mRNA levels to increase. We further validated this trend with Western blotting (Figure 3E).
FAT1 Inhibits Proliferation Associated with the MAPK/ERK Signaling Pathway
We next conducted RNA sequencing of FAT1 knockdown Hep3B to identify alterations in their gene transcription levels. Differential genes (P<0.05) were enriched in processes by gene ontology analysis, including focal adhesion, regulation of immune response process, cell–substrate junction, and location (Figure 4A). Bioinformatics analysis of these genes indicated enrichment in cancer-related pathways (Figures 4B and S1). Notably, some genes in the tight junction signaling pathway (RAB13, etc.) associated with EMT showed varying levels of upregulation. We hypothesized that FAT1 regulates these signaling pathways to stimulate cell division and EMT. To validate this, the effects of FAT1 on MAPK/ERK signaling activity were examined. As anticipated, the amount of phosphorylated ERK1/2 (p-ERK1/2) was dramatically elevated by the suppression of FAT1. To block MEK1/2 activity in the knockdown group, we used the highly specific inhibitor U0126, then performed an MTT analysis and an EMT marker test to illustrate that FAT1 controls cell proliferation and EMT through the signal pathways. U0126 restricted the knockdown impact of FAT1, preventing continued cell proliferation (Figure 5A) and dysregulating the levels of EMT-associated proteins (Figure 5B). Additionally, U0126 inhibited the ability of Hep3BFAT1sh and MHCC97HFAT1sh cells to migrate and invade (Figure 5C).
Inhibitory Effects of FAT1 in vivo Confirm the Effects in vitro
To further demonstrate the tumor-suppressive effects of FAT1 in HCC, we injected 5×105 FAT1 silenced Hep3B or Hep3BNC cells, and 5×105 FAT1-WT overexpressing MHCC97H or MHCC97HNC cells into the right armpit of mice. Two weeks later, we saw that the FAT1-knockdown group markedly promoted tumor development compared to the control group (Figure 6A), whereas overexpression of FAT1-WT significantly inhibited tumor growth (Figure 6B). The strong growth-promoting impact of FAT1 knockdown and the inhibitory effects of FAT1-WT overexpression cells were further verified by IHC of Ki-67, providing evidence that FAT1 may impact tumorigenesis in HCC in vivo. Additionally, we measured the expression levels of relevant proteins in paraffin-embedded sections of nude mice tumor tissues by IHC. Compared to the in vitro study, similar expression patterns were seen in the in vivo experiments, indicating that FAT1 may control EMT during the development of HCC tumors; depletion of FAT1 induced EMT, whereas the overexpression of FAT1 in HCC enhanced the inhibition of EMT (Figure 6C and D). In comparison to the MHCC97HFAT1WT group, the MCTC ratio was greater in the Hep3BFAT1sh group (Figure 6E and F), and the number of lung metastases per section was markedly elevated in the Hep3BFAT1sh group (Figure 6G and H).
Discussion
CTCs are the keys to the mechanism of hematogenous metastasis of malignant tumors.31 In EMT, the replacement of the epithelial phenotype by a mesenchymal and migratory phenotype is a critical step in the spread of tumors.32 HCC cells with EMT phenotype are more prone to metastasis.33 Our modified CTC capture method used novel multi-labeled lipid magnetic nanoparticles to isolate HCC CTCs. Using combinatorial analysis of genomic mutation profiles obtained from HCC tissues and CTCs, we identified high-frequency mutations in FAT1 in ten patients with HCC. Based on previous studies,34 we hypothesized that FAT1 has a tight connection to HCC initialing, the EMT process of tumor cells, and distal metastasis of tumors.
The transmembrane protein FAT1 is important in tumors because it governs EMT, cell proliferation, and actin kinematics.35 Focusing on the relationship between FAT1 and spread of HCC cells, and in order to simulate the seeding of HCC CTCs in blood, we purposely cultured Hep3B and MHCC97H cells in a simulated blood environment and induced the cell EMT. Then, we further sorted out the tumor cells by magnetic beads for subsequent studies. Our results showed that FAT1 could influence HCC cell proliferation in vitro, and regulate the EMT process, thus inhibiting HCC invasion and metastasis, supporting earlier discoveries.36–38 However, our results differ from those of Fu et al39 although the same cell line was used, tumor cells in this study were treated and purified accordingly to simulate the blood environment. We have noted that previous studies on the tumorigenic role of NF-kB in HCC by Pikarsky et al40 and Maeda et al41 showed opposite results. Those findings indicated that the underlying mechanisms about the metastasis of HCC remain to be further explored.
Next, GSEA demonstrated that the MAPK/ERK, Wnt, and Notch signaling pathways were significantly altered after FAT1 knockdown. Cancer cells often exhibit persistent MAPK/ERK signaling pathway activation.42 Similarly, alteration in other signaling pathways is also believed to play a role in the development of tumors and the proliferation of cancer cells in various cancers.43 Notably, we detected alterations in the TIGHT JUNCTION signaling pathway, in which RAB13 could inhibit the expression of PKA, thus weakening the intercellular junctions and promoting EMT in tumors,44 while RhoA, under the regulation of CUX1, further affected the migration and invasion of tumor cells.45 The relationship among RAB13, RhoA, CUX1, and EMT in HCC remains to be explored. Consistent with previous reports, our results suggest that the effects of FAT1 on HCC cells involve the aberrant activation of multiple signaling pathways.46,47
To determine whether there is a link between FAT1 inactivation and critical signaling pathways, we discovered that HCC had low levels of FAT1 expression, which activated the MAPK/ERK pathway. Meanwhile, we observed that U0126 eliminated FAT1 knockdown effects to inhibit EMT. Moreover, the ratio of MCTC and the quantity of pulmonary metastases were dramatically increased in the FAT1-knockdown group compared with the FAT1-overexpressing group in vivo. Deregulation of FAT1 function promoted tumorigenesis in HCC, confirming our hypothesis. However, there are some limitations in our study. The number of enrolled cases was small and all participants were advanced HBV-associated HCC patients. We will continue to expand the sample size of clinical research and improve the in vivo experiments to further validate the results and methodology of the study.
Conclusions
In summary, we investigated the effects of FAT1 on EMT and metastasis of HCC from the perspective of CTCs for the first time. We used magnetic enrichment method to analyze and screen candidate genes, and applied a novel cell culture and purification strategy to conduct this study. Our research demonstrates a connection between the FAT1 tumor suppressor and the EMT process in HCC. Moreover, suppressing FAT1 facilitates the EMT process, which promoted the metastasis of HCC. These discoveries shed light on the processes underlying the onset and progression of HCC.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Informed Consent
This study was approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (No. 2022-020; China Clinical Trial Registration Center - Registration No. ChiCTR2200055847), all experiments were performed in accordance with the principles stated in the Declaration of Helsinki, and the patients signed the written consent after receiving oral and written information.
Acknowledgments
The authors acknowledge the contribution of all the investigators at all the participating study sites. The authors thank Huzhou Lieyuan Medical Laboratory Co., Ltd. for the help with CTCs and gene detection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the project funded by the central government to guide local scientific and technological development (YDZX20213100001001), and the Interdisciplinary Program of Shanghai Jiao Tong University (No. YG2017MS13).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
2. Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res. 2018;30(6):571–579. doi:10.21147/j.issn.1000-9604.2018.06.01
3. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273.e1. doi:10.1053/j.gastro.2011.12.061
4. Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene. 2006;25(27):3866–3884. doi:10.1038/sj.onc.1209550
5. Brabletz T. To differentiate or not--routes towards metastasis. Nat Rev Cancer. 2012;12(6):425–436. doi:10.1038/nrc3265
6. Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342(6159):1234850. doi:10.1126/science.1234850
7. Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3(8):e2888. doi:10.1371/journal.pone.0002888
8. Sun C, Liao W, Deng Z, et al. The diagnostic value of assays for circulating tumor cells in hepatocellular carcinoma. Medicine. 2017;96(29):e7513. doi:10.1097/MD.0000000000007513
9. Austin RG, Huang TJ, Wu M, Armstrong AJ, Zhang T. Clinical utility of non-EpCAM based circulating tumor cell assays. Adv Drug Deliv Rev. 2018;1(125):132–142. doi:10.1016/j.addr.2018.01.013
10. Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi:10.1038/nrc2620
11. Schulze K, Gasch C, Staufer K, et al. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer. 2013;133(9):2165–2171. doi:10.1002/ijc.28230
12. Sun YF, Wu L, Liu SP, et al. Dissecting spatial heterogeneity and the immune-evasion mechanism of CTCs by single-cell RNA-seq in hepatocellular carcinoma. Nat Commun. 2021;12(1):4091. doi:10.1038/s41467-021-24386-0
13. Liu YK, Hu BS, Li ZL, He X, Li Y, Lu LG. An improved strategy to detect the epithelial-mesenchymal transition process in circulating tumor cells in hepatocellular carcinoma patients. Hepatol Int. 2016;10(4):640–646. doi:10.1007/s12072-016-9732-7
14. Wang S, Zhang C, Wang G, et al. Aptamer-Mediated Transparent-Biocompatible Nanostructured Surfaces for Hepotocellular Circulating Tumor Cells Enrichment. Theranostics. 2016;6(11):1877–1886. doi:10.7150/thno.15284
15. Nakaya K, Yamagata HD, Arita N, et al. Identification of homozygous deletions of tumor suppressor gene FAT in oral cancer using CGH-array. Oncogene. 2007;26(36):5300–5308. doi:10.1038/sj.onc.1210330
16. Li M, Zhong Y, Wang M. Fat1 suppresses the tumor-initiating ability of nonsmall cell lung cancer cells by promoting Yes-associated protein 1 nuclear-cytoplasmic translocation. Environ Toxicol. 2021;36(11):2333–2341. doi:10.1002/tox.23347
17. Srivastava C, Irshad K, Dikshit B, et al. FAT1 modulates EMT and stemness genes expression in hypoxic glioblastoma. Int J Cancer. 2018;142(4):805–812. doi:10.1002/ijc.31092
18. Zhu HY, Cao GY, Wang SP, et al. POU2F1 promotes growth and metastasis of hepatocellular carcinoma through the FAT1 signaling pathway. Am J Cancer Res. 2017;7(8):1665–1679.
19. Meng P, Zhang YF, Zhang W, et al. Identification of the atypical cadherin FAT1 as a novel glypican-3 interacting protein in liver cancer cells. Sci Rep. 2021;11(1):40. doi:10.1038/s41598-020-79524-3
20. Valletta D, Czech B, Spruss T, et al. Regulation and function of the atypical cadherin FAT1 in hepatocellular carcinoma. Carcinogenesis. 2014;35(6):1407–1415. doi:10.1093/carcin/bgu054
21. Pastushenko I, Mauri F, Song Y, et al. Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature. 2021;589(7842):448–455 doi:10.1038/s41586-020-03046-1.
22. Huang XY, Li F, Li TT, et al. A clinically feasible circulating tumor cell sorting system for monitoring the progression of advanced hepatocellular carcinoma. J Nanobiotechnology. 2023;21(1):25. doi:10.1186/s12951-023-01783-9
23. Oyane A, Kim HM, Furuya T, Kokubo T, Miyazaki T, Nakamura T. Preparation and assessment of revised simulated body fluids. J Biomed Mater Res A. 2003;65(2):188–195. doi:10.1002/jbm.a.10482
24. Yi B, Wu T, Zhu N, et al. The clinical significance of CTC enrichment by GPC3-IML and its genetic analysis in hepatocellular carcinoma. J Nanobiotechnology. 2021;19(1):74. doi:10.1186/s12951-021-00818-3
25. Xue K, Luo B, Li X, Deng W, Zeng C, Zuo C. Consistency evaluation of lung adenocarcinoma tissue and circulating tumor cells related gene mutation detection based on multi-site immunomagnetic beads. J Biomater Appl. 2022;36(9):1700–1711. doi:10.1177/08853282211065861
26. Rebbani K, Marchio A, Ezzikouri S, et al. TP53 R72P polymorphism modulates DNA methylation in hepatocellular carcinoma. Mol Cancer. 2015;14:74. doi:10.1186/s12943-015-0340-2
27. He F, Li J, Xu J, et al. Decreased expression of ARID1A associates with poor prognosis and promotes metastases of hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:47. doi:10.1186/s13046-015-0164-3
28. Golding B, Luu A, Jones R, Viloria-Petit AM. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol Cancer. 2018;17(1):52. doi:10.1186/s12943-018-0810-4
29. Ye QH, Qin LX, Forgues M, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9(4):416–423. doi:10.1038/nm843
30. Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23(14):1846–1847. doi:10.1093/bioinformatics/btm254
31. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi:10.1126/science.1203543
32. Tanaka T, Goto K, Iino M. Sec8 modulates TGF-β induced EMT by controlling N-cadherin via regulation of Smad3/4. Cell Signal. 2017;29:115–126. doi:10.1016/j.cellsig.2016.10.007
33. Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65(4):798–808. doi:10.1016/j.jhep.2016.05.007
34. Martin OA, Anderson RL, Narayan K, MacManus MP. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nat Rev Clin Oncol. 2017;14(1):32–44. doi:10.1038/nrclinonc.2016.128
35. Peng Z, Gong Y, Liang X. Role of FAT1 in health and disease. Oncol Lett. 2021;21:398. doi:10.3892/ol.2021.12659
36. Katoh M. Function and cancer genomics of FAT family genes (review). Int J Oncol. 2012;41(6):1913–1918. doi:10.3892/ijo.2012.1669
37. Lee S, Stewart S, Nagtegaal I, et al. Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer. Cancer Res. 2012;72(17):4574–4586. doi:10.1158/0008-5472.CAN-12-0636
38. Jiang S, Zhu Y, Chen Z, et al. S100A14 inhibits cell growth and epithelial-mesenchymal transition (EMT) in prostate cancer through FAT1-mediated Hippo signaling pathway. Hum Cell. 2021;34(4):1215–1226. doi:10.1007/s13577-021-00538-8
39. Fu Y, Yang Z, Hu Z, et al. Preoperative serum ctDNA predicts early hepatocellular carcinoma recurrence and response to systemic therapies. Hepatol Int. 2022;16(4):868–878. doi:10.1007/s12072-022-10348-1
40. Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431(7007):461–466. doi:10.1038/nature02924
41. Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121(7):977–990. doi:10.1016/j.cell.2005.04.014
42. Yan P, Zhu H, Yin L, et al. Integrin αvβ6 promotes lung cancer proliferation and metastasis through upregulation of IL-8-mediated MAPK/ERK signaling. Transl Oncol. 2018;11(3):619–627. doi:10.1016/j.tranon.2018.02.013
43. Nusse R, Clevers H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi:10.1016/j.cell.2017.05.016
44. Köhler K, Louvard D, Zahraoui A. Rab13 regulates PKA signaling during tight junction assembly. J Cell Biol. 2004;165:175–180. doi:10.1083/jcb.200312118
45. Kedinger V, Nepveul A. The roles of CUX1 homeodomain proteins in the establishment of a transcriptional program required for cell migration and invasion. Cell Adh Migr. 2010;4:348–352. doi:10.4161/cam.4.3.11407
46. Perugorria MJ, Olaizola P, Labiano I, et al. Wnt-β-catenin signalling in liver development, health and disease. Nat Rev Gastroenterol Hepatol. 2019;16(2):121–136. doi:10.1038/s41575-018-0075-9
47. Okada T, Sinha S, Esposito I, et al. The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT by restraining Ras-MAPK signalling. Nat Cell Biol. 2015;17(1):81–94. doi:10.1038/ncb3082
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.