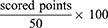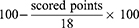Back to Journals » Journal of Pain Research » Volume 15
Comparison of Swiss versus Standard Acupuncture in Patients with Chronic Low Back Pain. A Study Protocol for a Randomized, Controlled, Single-Blind, Parallel Trial
Authors Pradhan SK , Angst F, Xu J, Gantenbein AR, Lehmann S, Sandor PS, Li Y, Furian M
Received 3 September 2022
Accepted for publication 29 November 2022
Published 22 December 2022 Volume 2022:15 Pages 4055—4064
DOI https://doi.org/10.2147/JPR.S388558
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Saroj K Pradhan,1– 3 Felix Angst,2 Jie Xu,1,3 Andreas R Gantenbein,4 Susanne Lehmann,2 Peter S Sandor,4 Yiming Li,1– 3 Michael Furian1
1Research Department, Swiss University of Traditional Chinese Medicine, Bad Zurzach, Switzerland; 2Research Department, Rehaklinik Bad Zurzach, Bad Zurzach, Switzerland; 3TCM Ming Dao, Bad Zurzach, Switzerland; 4Neurorehabilitation & Research Department, Rehaklinik Bad Zurzach, Bad Zurzach, Switzerland
Correspondence: Michael Furian, Swiss University of Traditional Chinese Medicine, Langwiesstrasse 7, Bad Zurzach, 5330, Switzerland, Tel +41 79 403 75 86, Email [email protected]
Introduction: Chronic low back pain (CLBP) cannot sufficiently be treated by pharmacological therapy and generates substantial health-care costs worldwide. Acupuncture, a cost-effective, safe and non-pharmacological therapy, has shown promising results in relieving acute low back pain; however, the optimal acupuncture therapy for CLBP remains controversial. This study will compare two acupuncture methods for pain relief in CLBP.
Methods and Analysis: This randomized, controlled, single-blind, parallel trial will be conducted in patients with clinically diagnosed CLBP with a disease duration ≥ 3 months and an average pain intensity of ≥ 4 points on an 11-point Pain Intensity Numerical Rating Scale (pain-NRS) on the previous 7 days. Patients will be randomized to 9-week acupuncture therapy using Jiu Gong Points (termed Swiss low back acupuncture, SLBA) or standard acupuncture (SA) therapy (weeks 1– 6: two sessions/week, weeks 7– 9: one session/week, 15 sessions/patient in total). Measurements will be conducted before the first session (T1), at the end of the 9-week therapy (T2) and after 3- and 6-month follow-up (T3 and T4). The primary hypothesis is that 9 weeks of SLBA will be superior in reducing the pain severity assessed by the pain-NRS compared to SA therapy for CLBP. Secondary outcomes will be derived from the Short-Form 36, Oswestry Disability Index, Multidimensional Pain Inventory questionnaire, Symptom Checklist-90 – Revised questionnaire and a daily pain diary. Assuming a minimal clinically important difference in the pain-NRS of 0.39 and an effect size of ≥ 0.6 between SLBA and SA, 80% power, 0.05 alpha level and 20% dropouts, a total of 55 patients/arm will be required. The primary outcome will be analyzed in the intention-to-treat population using chained linear regression models. Patients, outcome assessors and data analysts will be blinded to the treatment arm.
Trial Registration: Clinicaltrials.gov Identifier: NCT05232487.
Keywords: Chinese medicine, chronic disease, pain, Pain Intensity Numerical Rating Scale, Multidimensional Pain Inventory, Oswestry Disability Index, Randomized clinical trial
Background and Rationale
Low Back Pain
Low back pain (LBP) is a common, widespread and multifaceted syndrome, and represents one of the leading sources of years lived with disability.1 In Switzerland, patients with LBP generated mean annual costs of 467 million Euro in 2016 and 2017.2 Moreover, the quantity of prescriptions per patient was reported to be 5–6 per year, leading to pain medication costs of 4.7 million Euro. It is estimated that 20% of acute LBP can manifest as chronic low back pain (CLBP), defined as pain lasting longer than 3 months.3,4 CLBP not only negatively impacts the quality of life (QoL), functional status and working capability of those suffering, but also plays a pivotal role in the large annual health-care costs for LBP. Pharmacological therapies with at least a small magnitude of effect for pain relief in CLBP include non-steroidal anti-inflammatory drugs, opioids, Tramadol and antidepressants.3 In addition, non-pharmacological therapies with at least a small magnitude of effect for pain relief in CLBP include exercise, motor control exercises, yoga, mindfulness-based stress reduction, electromyography biofeedback, cognitive–behavioral therapy, multidisciplinary rehabilitation and acupuncture.5
CLBP in Traditional Chinese Medicine (TCM)
Huang Di Nei Jing 《黄帝内经》, written during the Warring States period (around 475–220 BC), translated verbatim as the Inner Canon of the Yellow Emperor, is an ancient and most eminent traditional Chinese medical book. The concept is derived from Taoist philosophy, and the essence of maintaining longevity is achieved by adopting the crucial path of the universe. Diseases are induced by disharmony between Yin and Yang, an imbalance of the five elements (wood, fire, earth, metal and water) on the Zang-Fu. The textbook is parted into Su Wen 《素问》 (Plain Questions) and Ling Shu 《灵枢》 (Spiritual Pivot), consisting of nine volumes and 81 chapters. In Chapter 41 of Su Wen (Acupuncture treatment of lumbago 刺腰痛), diagnosis and therapy with acupuncture for LBP were discussed.6 These early records from over 2000 years ago indicate that LBP was considered a medical problem at that time, and it persists as a large global health burden to the present day.
The pathogenesis of LBP according to TCM is due to cold and dampness, phlegm and dampness, Qi and blood stagnation, heat and dampness, kidney Qi deficiency, kidney Yin or Yang deficiency, or wind-cold dampness.7 According to Chapter 41 of Su Wen, disharmony in one or several of the following meridians (in Chinese Mai 脉) can cause LBP: Foot Tai Yang Mai (足太阳脉), Foot Shao Yang Mai (足少阳脉), Foot Yang Ming Mai (足阳明脉), Foot Shao Yin Mai (足少阴脉), Foot Jue Yin Mai (足厥阴脉), Jie Mai (解脉), Tong Yin Mai (同阴脉), Yang Wei Mai (阳维脉), Heng Luo Mai (横络脉), Hui Yin Mai (会阴脉), Fei Yang Mai (飞阳脉), Chang Yang Mai (昌阳脉) San Mai (散脉) and Rou Li Mai (肉里脉).8
Standard Acupuncture to Treat CLBP
TCM provides a broad spectrum of treatment modalities for CLBP, including Chinese materia medica, Tuina, Qi Gong, Tai Chi, dietary, life cultivation and standard acupuncture (SA). SA represents a unique technique utilized for centuries in China in managing LBP. On the basis of the TCM syndrome differential diagnosis, some acupuncture sites are additionally added or omitted (Jia Jian 加减) for the treatment. The elemental SA points are Wei Zhong (BL 40), Ji Zhong (GV 6), Yao Yang Guan (GV 3), Shen Shu (BL 23), Da Chang Shu (BL 25) and A-Shi Points.9 A systematic literature review published in 2017 concluded that acupuncture therapy to treat CLBP has low/moderate efficacy compared to no or sham acupuncture;5 however, in-depth information related to the acupuncture interventions was not provided. Despite evidence that acupuncture is able to relieve CLBP in the short term, it remains unknown which type of acupuncture is most beneficial for short- and long-term pain relief in patients with CLBP.
Swiss Low Back Acupuncture to Treat CLBP
In this context and during the last decades and centuries, different acupuncture methods evolved, among others, the acupuncture method newly termed “Swiss low back acupuncture” (SLBA). The SLBA originates from Jiu Gong Points (九宫穴), which is the abbreviation for “Nine Spinal Points” (脊椎九宫穴). The particular sites of Jiu Gong Points evolved from the eight trigrams and nine palaces square (Ba Gua Jiu Gong fang 八卦九宫方). The eight trigrams (Ba Gua 八卦) are a set of metaphysical and philosophical sigmas composed of three components of Yin and Yang, which are the essential concept of all-natural phenomena.10 The nine palaces (Jiu Gong, 九宫), an element of the eight trigrams, correspond to the nine regions in ancient China (Jiu Ye, 九野). They represent the four main (North=kidney; South=heart; East=liver; West=lung) and four secondary cardinal points, and the Earth as the center (Earth=spleen).11 In 825 patients with LBP due to lumbar herniated disc, it has been shown that an earlier version of SLBA around the most significant lesion of the lumbar vertebrae resulted in good improvements of pain.12 The uncontrolled study reported a cure of LBP in 65.2% of cases and an improvement in clinical symptoms of 32.4%. Owing to this positive report, hot needle acupuncture using Jiu Gong Points has further evolved in Switzerland and may be a promising tool to treat CLBP, since the optimal method of acupuncture against CLBP remains to be elucidated.13 However, the efficacy of SLBA has never been quantitatively compared to SA and a randomized clinical trial is warranted to provide the first robust evidence on its performance in CLBP.
Therefore, the purpose of this study is to conduct the first randomized clinical trial comparing SLBA and SA for pain relief in patients with CLBP.
Aims and Hypotheses
The aim of this study is to test the primary hypothesis that 9 weeks of SLBA will be superior in reducing the pain severity assessed by the Pain Intensity Numerical Rating Scale (pain-NRS) compared to SA therapy in patients with CLBP. Secondary hypotheses will be that the 9-week SLBA therapy will improve pain, QoL and well-being, assessed by the West Haven–Yale Multidimensional Pain Inventory questionnaire (MPI), Short-Form 36 (SF-36), Oswestry Disability Index (ODI) and Symptom Checklist-90 – Revised (SCL-90R) questionnaires, compared to SA therapy. In addition, we will test the hypotheses that improvements with SLBA therapy persist at follow-up periods of 3 and 6 months after completion of the 9-week acupuncture therapy.
Trial Design
This protocol follows the SPIRIT 2013 checklist (Supplement)25 and describes a randomized, controlled, single-blind, parallel trial, allocating patients with CLBP 1:1 to 9 weeks of either SLBA or SA therapy.
Methods
Study Setting
This clinical trial will be conducted at the TCM Ming Dao AG, Bad Zurzach, Switzerland, between 2023 and 2026. Patients will be recruited from surrounding rehabilitation clinics and in the local community by active advertisement with flyers and local press releases. Patients will be invited for a screening visit to assess their eligibility and, after successful inclusion and having obtained written consent, they will be randomized to 9 weeks of either SLBA or SA therapy. The study flowchart is illustrated in Figure 1. Assessments of the primary and secondary outcomes will be performed during the first visit for their acupuncture therapy (T1); after 9 weeks of treatment (T2), and after 3 (T3) and 6 months (T4) of follow-up after T2 (Table 1). This study has been approved by the Ethics Committee Northwest and Central Switzerland (ref: 2022–00354) and will be conducted according to the Declaration of Helsinki.
 |
Table 1 Assessment Schedule |
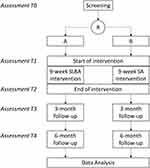 |
Figure 1 Study flowchart. Assessments T0–T4 are described in more detail in Table 1. Abbreviations: R, randomization; SLBA, Swiss low back acupuncture; SA, standard acupuncture. |
Eligibility Criteria
During the screening visit, patients will be assessed for their eligibility; therefore, the following inclusion criteria will be applied:
- Written informed consent;
- Female and male participants;
- Age between 18 and 75 years;
- Clinical diagnosis of CLBP with a disease duration of more than 3 months and an average pain intensity of minimally 4 points on the 11-point pain-NRS on the previous 7 days;4,14
- Sufficient knowledge of German to complete the questionnaires.
The following exclusion criteria will be applied:
- All participants who do not meet the above-mentioned inclusion criteria;
- History or known severe concomitant diseases (eg abdominal aortic aneurysm, heart disease, cancer, psychiatric disorders);
- Other causes of low back pain not related to the clinical diagnosis of CLBP, including inflammatory, malignant or autoimmune disease;
- Planned or previous back surgery within 6 months.
- Use of corticosteroids and/or other pain-relieving drugs that act through the central nervous system;
- Initiation of another therapy for CLBP within the last 4 weeks, eg physiotherapy;
- Planned termination of another therapy during the acupuncture therapy, eg physiotherapy, medication;
- Preceding acupuncture treatment for CLBP during the past 6 months;
- Pregnancy;
- Participation in another clinical trial.
Intervention
The SLBA and SA therapies will take place for a total duration of 9 weeks, with two acupuncture sessions/week from the 1st to 6th week and one session/week from the 7th to 9th week, resulting in 15 acupuncture sessions in total. One acupuncture session will last 50–60 minutes and will follow a written, standardized procedure. All participants will be placed in the prone position (if required with a pillow under the abdomen). For the acupuncture itself, only sterile, siliconized single-use needles, 0.25×40 mm, 0.25×25 mm and 0.30×75 mm, made of stainless surgical steel (manufactured by Maanshan Bond Medical Instruments Co. Ltd, Anhui, China), will be used. All acupuncture sessions will be conducted by four prespecified and professional acupuncturists trained in both the SLBA and SA therapy.
Standard Acupuncture
Table 2 contains a detailed description of the SA method. The SA method is described in the acupuncture textbook for TCM in China9 and all acupuncture points are selected based on TCM principles, international literature, and the opinion of national and international TCM experts. The following points have been selected: Ming Men (GV 4), Zhi Shi (BL 52), Tai Xi (KI 3) Yao Guan (GV 3), Guan Yuan Shu (BL 26), Ge Shu (BL 17), Ci Liao (BL 32), Wei Zhong (BL 40), Ji Zhong (GV 6), Yao Yang Guan (GV 3), Shen Shu (BL 23), Da Chang Shu (BL 25) and A-Shi Points. Acupuncture-related side effects will be noted.
 |
Table 2 Selection of Acupuncture Points for CLBP According to the Pathogenesis and the Affected Meridians |
Swiss Low Back Pain Acupuncture
The SLBA method is a further developed method from the Jiu Gong acupuncture technique, also known as the nine palaces points on the spine. The following acupuncture basic points will be used in every SLBA session (Table 2): LV3/LV5, Tai Xi (Kl 3), Fe Yang (BL 58). Additional acupuncture points will be used based on the origin of the CLBP, ie CLBP induced by cold and dampness: Tai Chong (LR 3), Guang Ming (GB 37), or CLBP induced by kidney insufficiency: Tai Xi (Kl 3) and Fe Yang (BL 58). Acupuncture-related side effects will be noted.
Outcomes
The primary outcome will be the change in the pain-NRS score following 9 weeks of SLBA compared to SA therapy. Secondary outcomes will be changes in the SF-36 components, ODI, SCL-90R and MPI pain severity after 9 weeks of SLBA compared to SA therapy; and between 3 and 6 months after SLBA and SA therapy.
Sample Size Estimation
Suzuki et al15 assessed the minimal clinically important difference (MCID) in the pain-NRS in CLBP patients with an initial pretreatment pain-NRS score of 4–10 points (identical to the current study). Following a multidisciplinary rehabilitation program, patients with “no improvement” reported a change in pain-NRS score of 0.61 points (≤4 initial pain-NRS ≤6) to 1.4 points (≤7 initial pain-NRS ≤10). In contrast, patients with “slight improvement” reported a change in pain-NRS score of 1.0 points (≤4 initial pain-NRS ≤6) to 1.79 points (≤7 initial pain-NRS ≤10). Based on these pain-NRS scores, the MCID in the pain-NRS can be estimated to be 0.39 points in both categories. Therefore, to detect an MCID between SLBA and SA of 0.39 points on the pain-NRS and assuming at least a moderate effect size of ≥0.6, power of 80%, two-sided alpha level of 0.05 and 20% dropouts, a total of 55 patients per treatment arm (110 patients in total) will be required.
Randomization and Blinding Procedure
Patients will be randomized using the web-based secuTrial® randomization tool and applying an allocation ratio of 1:1 to SLBA and SA therapy. Patients, outcome assessor and data analysts will be blinded to the intervention (SLBA or SA); however, acupuncturists performing the acupuncture sessions will not be blinded to the intervention. Blinding of patients will be achieved by not discussing intervention-related acupuncture points and other topics potentially revealing the nature of the applied acupuncture. In addition, standardized procedures, identical rooms and sessions will maintain blinding throughout the study. The success of patient blinding will be assessed by asking the patient about the type of received intervention at the end of the therapy.
Measurements and Assessments
Clinical Assessment
Patients will undergo a medical anamnesis and clinical assessment, recording age, occupation, gender, weight, height, heart rate, blood pressure, CLBP characterization, comorbidities and education. Previous therapies to improve CLBP will be assessed (eg physiotherapy, acupuncture).
Pain-NRS
The pain-NRS will be assessed at screening and before and after each acupuncture session. Patients will be asked to rate their current pain severity on an 11-point numerical rating scale from 0 (no pain) to 10 (maximal pain).17
Short-Form 36 Questionnaire
QoL will be assessed by the SF-36 questionnaire (version 2). Thirty-five of these questions can be categorized into eight dimensions of health, namely physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, emotional well-being, social functioning, pain and general health.18 Each dimension is transformed into a 0–100 scale on the assumption that each dimension carries equal weight. The lower the score, the higher the disability. One single item is additionally queried regarding the current state of health compared to the past year.19
Oswestry Disability Index
The ODI includes 10 items and assesses the functional status and QoL limitations of patients with CLBP. The questionnaire can be used to calculate the index (ODI), a measure for the respective functional limitations in various activities of daily living.16,20 Each item is scored from 0 to 5 and the total ODI score is calculated as:
where total scores of 0–20 represent “minimal disability”; 21–40 “moderate disability”; 41–60 “severe disability”; 61–80 “crippled”; and >80 points “bed-bound patients”.
Symptom Checklist-90 – Revised
The SCL-90R evaluates a broad range of psychological impairments and symptoms of psychopathology. The primary symptom dimensions assessed are anxiety, depression, hostility, paranoid ideation, phobic anxiety, psychoticism, somatization, obsessive–compulsive behavior and interpersonal sensitivity. In addition, a category of “additional items” is included to help the care provider assess other aspects of a patient’s symptomatology (eg item 19, “poor appetite”).16
For the present study, the categories anxiety (10 items), depression (13 items) and somatization (12 items) will be assessed. Each item will be scored on a five-point Likert scale ranging from 0 “not at all” to 4 “very strong”. For each category, the total score will be calculated by the sum of all items of the corresponding category, eg of the 10 items in the category anxiety; and the category score will be calculated by dividing the total score by the number of items.
West Haven–Yale Multidimensional Pain Inventory
The MPI is a reliable and valid self-report instrument that measures the impact of pain on the patient’s life, quality of social support and general activity.21,22 It has been previously shown that this condition-specific instrument, especially the three-item MPI pain severity domain, is highly responsive to changes in pain sensation during a 4-week rehabilitation program in patients with chronic pain.23 For this reason, patients will be asked to “Rate the level of your pain at the present moment” from 0 (no pain) to 6 (very intense pain); “On average, how severe was your pain in the last week?” from 0 (not at all severe) to 6 (extremely severe); and “How much suffering do you experience because of your pain?” from 0 (no suffering) to 6 (extreme suffering). The MPI pain severity score will be transformed into 0 (maximal pain) to 100 points (no pain) by the following equation:23
Pain Diary
Patients will be asked to complete a daily pain diary during the 9 weeks of acupuncture therapy (completed at the end of each day, before going to bed). In this diary, the patients will rate their perceived pain severity from 0 (none) to 5 (almost intolerable pain) during four predefined periods of the day (morning, afternoon, evening, night). Furthermore, drug intake related to pain relief will be documented.
Safety Evaluation
Incidence, severity and type of intervention-related side effects will be assessed and reported in numbers and proportions in the final manuscript.
Statistical Methods
The statistical analyses will be conducted by an independent statistician of the Rehabilitation Center Bad Zurzach, Switzerland, based on a prespecified data analysis plan. The primary outcome will be analyzed in the intention-to-treat population, with missing values imputed using chained linear regression models. Secondary outcomes will be analyzed in the per-protocol population receiving all scheduled therapy sessions. The change in pain-NRS (primary outcome) between SLBA and SA therapy will be analyzed using mixed linear regression analysis. Therefore, primary and secondary outcomes will be dependent variables, whereas visits (T1, T2, T3, T4), group assignment (SLBA, SA) and the interaction visits × group assignment will be fixed, and patients will be random effects. Exploratory subgroup analyses will be performed to examine whether baseline characteristics (gender, age, initial pain severity) or applied acupuncture point protocols influenced the pain relief achieved through acupuncture therapy. A two-sided p-value of 0.05 will represent statistical significance. In addition to statistical significance, the standardized mean difference (Cohen’s d) will be calculated and reported in order to assess the clinical relevance of the results.24 Statistical analysis will be performed using STATA and SPSS.
Because of the longitudinal nature of this study, annual interim analyses will be conducted by an independent statistician, blinded for group assignment. During this blinded analysis, the Peto approach will be applied, and the trial will be stopped owing to high efficiency or harm with SLBA (at a p<0.001 level) compared to SA on the primary outcome, the pain-NRS score. Moreover, a scientific committee blinded to the treatment groups will annually reassess and, if necessary, modify sample size estimation, recruitment procedures and finances. The scientific committee will also evaluate whether an additional control arm (waiting list or sham therapy) is feasible and necessary. This decision mainly depends on recruitment success during the first year. All amendments to the original protocol will be reported in the final manuscript.
Data Sharing Statement
Anonymized data underlying this study can be requested by qualified researchers providing an approved proposal.
Ethics and Dissemination
This study has been approved by the Ethics Committee Northwest and Central Switzerland (ref: 2022-00354) and will be conducted according to the Declaration of Helsinki. Participants will give informed consent to participate in the study before taking part. Results obtained from this randomized clinical trial will be published in peer-reviewed journals and presented at national and international conferences.
Data Monitoring Board
User administration and user training is performed by the Clinical Trial Unit of the Basel University Hospital according to predefined processes. An integrated audit trial system will maintain a record of initial entries and changes made; time and date of entry; and the user name of the person authorizing entry or change. Backup of secuTrial® study data is performed regularly according to the processes of the IT department of the University Hospital Basel. The CTU of the University Hospital Basel will be fulfilling monitoring duties, which will be independent from investigators and the sponsor.
Role of Sponsor
The sponsor of this trial is the Swiss University of Traditional Chinese Medicine, Bad Zurzach. The study sponsor and funders have no role in the decision of the study design; collection, management, analysis, and interpretation of data; writing of the report; or the decision to submit the report for publication.
Author Contributions
SKP and MF drafted the study protocol; All authors made significant contribution to the work reported (conception, study design and analysis), revised or critically reviewed the article, agreed to the journal of submission, reviewed and agreed to all versions of the article before submission, during revision, and final version, and agree to take responsibility and accountability for the content of the article.
Funding
This study receives funding from the International Academy of Traditional Medical Culture and Health Management (grant number: NA), TCM Ming Dao AG (grant number: NA), and the SWISS TCM UNI (grant number: NA), Bad Zurzach, Switzerland.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen S, Chen M, Wu X, et al. Global, regional and national burden of low back pain 1990–2019: a systematic analysis of the global burden of disease study 2019. J Orthop Translat. 2022;32:49–58.
2. Di Gangi S, Bagnoud C, Pichierri G, Rosemann T, Plate A. Characteristics and health care costs in patients with a diagnostic imaging for low back pain in Switzerland. Eur J Health Econ. 2022;23(5):823–835. doi:10.1007/s10198-021-01397-8
3. Chou R, Deyo R, Friedly J, et al. Systemic pharmacologic therapies for low back pain: a systematic review for an American college of physicians clinical practice guideline. Ann Intern Med. 2017;166(7):480–492. doi:10.7326/M16-2458
4. Hüllemann P, Keller T, Kabelitz M, et al. Clinical manifestation of acute, subacute, and chronic low back pain in different age groups: low back pain in 35,446 patients. Pain Pract. 2018;18(8):1011–1023. doi:10.1111/papr.12704
5. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American college of physicians clinical practice guideline. Ann Intern Med. 2017;166(7):493–505. doi:10.7326/M16-2459
6. Cannizzaro S. Lumbar pain treatment outline in Su Wen chapter 41. Tradit Med. 2020;1(1):2. doi:10.35702/Trad.10002
7. Gabriella H, Jian L. Low back pain-complex approach of treatment by different CAM modalities (acupuncture and other types of dry needling, “targeted RF noninvasive physiotherapy” for low back pain). Conf Pap Med. 2013;2013:326595. doi:10.1155/2013/326595
8. Liao SJ. Acupuncture for low back pain in huang di nei jing su wen. (Yellow emperor’s classic of internal medicine book of common questions). Acupunct Electrother Res. 1992;17(4):249–258. doi:10.3727/036012992816357666
9. Wang QC. Low back pain. In: The Teaching of Acupuncture and Moxibustion.
10. Zhang D. Sustainable schoolyard garden design. Interactions. 2008;21:20–29.
11. Zhu YZ. Brief Remark About the Awareness of Chinese Medicine. Beijing: People’s Medical Publishing House;; 2016.
12. Xu J, Tan BH. Clinical summary and research on the mechanism of 825 cases of lumbar intervertebral disc herniation treated with hot acupuncture at Jiu Gong point. Yunnan J Tradit Chin Med. 1999;20(2):1–3.
13. Pach D, Yang-Strobel X, Lüdtke R, et al. Standardized versus individualized acupuncture for chronic low back pain: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:125937. doi:10.1155/2013/125937
14. Zieger A, Kern A, Barth J, Witt CM. Do patients’ pre-treatment expectations about acupuncture effectiveness predict treatment outcome in patients with chronic low back pain? A secondary analysis of data from a randomised controlled clinical trial. PLoS One. 2022;17(5):e0268646. doi:10.1371/journal.pone.0268646
15. Suzuki H, Aono S, Inoue S, et al. Clinically significant changes in pain along the pain intensity numerical rating scale in patients with chronic low back pain. PLoS One. 2020;15(3):e0229228. doi:10.1371/journal.pone.0229228
16. Benz T, Lehmann S, Elfering A, Sandor PS, Angst F. Comprehensiveness and validity of a multidimensional assessment in patients with chronic low back pain: a prospective cohort study. BMC Musculoskelet Disord. 2021;22(1):291. doi:10.1186/s12891-021-04130-x
17. Ferraz MB, Quaresma MR, Aquino LR, Atra E, Tugwell P, Goldsmith CH. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J Rheumatol. 1990;17(8):1022–1024.
18. Ware JE
19. Angst F, Benz T, Lehmann S, et al. Extended overview of the longitudinal pain-depression association: a comparison of six cohorts treated for specific chronic pain conditions. J Affect Disord. 2020;273:508–516. doi:10.1016/j.jad.2020.05.044
20. Lee CP, Fu TS, Liu CY, Hung CI. Psychometric evaluation of the Oswestry disability index in patients with chronic low back pain: factor and Mokken analyses. Health Qual Life Outcomes. 2017;15(1):192. doi:10.1186/s12955-017-0768-8
21. Angst F, Lehmann S, Sandor PS, Benz T. Catastrophizing as a prognostic factor for pain and physical function in the multidisciplinary rehabilitation of fibromyalgia and low back pain. Eur J Pain. 2022;26(7):1569–1580. doi:10.1002/ejp.1983
22. Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain. 1985;23(4):345–356. doi:10.1016/0304-3959(85)90004-1
23. Angst F, Verra ML, Lehmann S, Aeschlimann A. Responsiveness of five condition-specific and generic outcome assessment instruments for chronic pain. BMC Med Res Methodol. 2008;8:26. doi:10.1186/1471-2288-8-26
24. Verra ML, Angst F, Brioschi R, et al. Effectiveness of subgroup-specific pain rehabilitation: a randomized controlled trial in patients with chronic back pain. Eur J Phys Rehabil Med. 2018;54(3):358–370. doi:10.23736/S1973-9087.17.04716-5
25. Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, Dickersin K, Hróbjartsson A, Schulz KF, Parulekar WR, Krleza-Jeric K, Laupacis A, Moher D, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013 Jan 8;346:e7586. doi:10.1136/bmj.e7586.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.