Back to Journals » Journal of Inflammation Research » Volume 16
Comparison of Neoadjuvant Immunotherapy Plus Chemotherapy versus Neoadjuvant Chemoradiotherapy for Patients with Esophageal Squamous Cell Carcinoma: A Propensity Score Matching Study
Authors Zhao J , Hao S, Tian J, Li Y, Han D
Received 27 June 2023
Accepted for publication 1 August 2023
Published 8 August 2023 Volume 2023:16 Pages 3351—3363
DOI https://doi.org/10.2147/JIR.S424454
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Junfeng Zhao,1,2,* Shaoyu Hao,3,4,* Jing Tian,5 Ying Li,6 Dan Han1,2
1Department of Radiation Oncology, Shandong University Cancer Center, Jinan, Shandong, People’s Republic of China; 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University, and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China; 3Department of Thoracic Surgery, Shandong University Cancer Center, Jinan, Shandong, People’s Republic of China; 4Department of Thoracic Surgery, Shandong Cancer Hospital and Institute, Shandong First Medical University, and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China; 5Department of Radiation Oncology, Jinan Zhangqiu District People’s Hospital, Jinan, Shandong, People’s Republic of China; 6Department of Medical Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University, and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dan Han, Department of Radiation Oncology, Shandong University Cancer Center, Shandong Cancer Hospital and Institute, Shandong First Medical University, and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China, Tel +86-17862893373, Email [email protected]
Purpose: This study compares the efficacy and safety of neoadjuvant immunotherapy combined with chemotherapy (NICT) and neoadjuvant chemoradiotherapy (NCRT) combined with radical esophagectomy in patients with resectable, locally advanced esophageal squamous cell cancer (ESCC).
Patients and Methods: Patients with locally advanced ESCC treated with NICT or NCRT combined with esophagectomy between March 2016 and May 2022 were retrospectively analyzed and propensity score matched (PSM) in a 1:2 ratio to balance potential bias.
Results: After PSM, 110 patients who received NCRT and 55 patients who received NICT were selected for the final analysis. The probability of tumor regression grade 0 and the rate of pathological complete remission (pCR) were significantly higher in the NCRT group than in the NICT group (57.3% vs 32.7%, P=0.003 and 48.2% vs 29.1%, P=0.030, respectively). The incidence of postoperative complications in the NCRT group was not significantly different from that in the NICT group (P=0.082). Patients in the NCRT group had significantly better disease-free survival (DFS) and overall survival (OS) than those in the NICT group (12-month DFS rate: 94.3% vs 81.8%, P=0.006; 12-month OS rate: 100.0% vs 95.4%, P=0.032). However, the results of the 24-month follow-up showed that there was also a statistically significant difference in DFS between the two groups. Patients with postoperative pCR had a longer DFS (P< 0.001).
Conclusion: Short-term follow-up results show that NCRT has a significantly better pathologic response and prognosis than NICT in the treatment of patients with locally advanced ESCC. NCRT and NICT have similar safety profiles.
Keywords: esophageal squamous cell carcinoma, neoadjuvant, chemoradiotherapy, immunotherapy, immune checkpoint inhibitor, esophagectomy
Introduction
Esophageal cancer (EC) is the predominant malignant tumor of the digestive system. According to the latest global cancer data, EC has the seventh-highest incidence and sixth-highest fatality rate.1 Surgery alone does not significantly benefit patients with locally advanced esophageal squamous cell cancer (ESCC), with a 5-year overall survival (OS) of 14%, and a combination of surgery combined with chemotherapy, immunotherapy, and radiotherapy is the mainstay of treatment.2 Neoadjuvant therapy combined with surgery has become the standard treatment for patients with locally advanced EC. Based on the CROSS and NEOCRTEC5010 studies, neoadjuvant chemoradiotherapy (NCRT) has become the current standard of care for locally advanced operable EC.3,4 In the era of immunotherapy, the rise of immune checkpoint inhibitors (ICIs) has offered a new treatment modality for multiple tumors.5–8 ESCC exhibits relatively high levels of immune-related biomarkers (eg, high tumor mutation burden expression and programmed cell death-ligand 1 overexpression) compared to esophageal adenocarcinoma, indicating the potential sensitivity of ESCC to ICIs.9,10 Preclinical data show that ICI treatment as a preoperative neoadjuvant therapy has more advantages than postoperative adjuvant therapy because neoadjuvant immunotherapy (IT) can more effectively cause a systemic antitumor immune response by killing tumor cells and releasing antigens before tumor resection, and damage to T-cell function is also reduced.11 Some studies, including those on TD-NICED study, have demonstrated the efficacy of neoadjuvant immunotherapy combined with chemotherapy (NICT).12 In a Phase II study of tislelizumab in combination with chemotherapy (NCT03469557), ESCC showed objective response rates, and disease control rates of 46.7% and 80%, respectively.13 Keystone −001 study showed that pembrolizumab combined with chemotherapy as neoadjuvant therapy for resectable ESCC had high major pathologic response (MPR) rates, pathologic complete remission (pCR) rates, and R0 resection rates with acceptable tolerability.14 A pilot study including 16 patients with locally advanced ESCC investigated the clinical value and tolerance of neoadjuvant camrelizumab plus paclitaxel and carboplatin, and indicated that NICT exhibits good efficacy and acceptable tolerance.15 Both NICT and NCRT are major clinical neoadjuvant treatment options, and numerous studies have compared various neoadjuvant treatment options to determine the best neoadjuvant treatment; however, no study has directly compared NICT with NCRT. Therefore, this study aimed to compare the efficacy and safety of NICT with NCRT combined with radical esophagectomy for the treatment of patients with locally advanced ESCC after propensity score matching (PSM).
Materials and Methods
Patient Selection
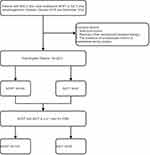
Data from 281 ESCC patients who underwent NICT or NCRT combined with esophagectomy between January 2016 and May 2022 at our study institution were retrospectively analyzed. All eligible patients demonstrated ESCC pathology, received only NICT or NCRT before esophagectomy, and did not receive adjuvant therapy postoperatively. The following outcomes were reported: tumor regression grade (TRG) status, whether resected as R0, whether pCR was achieved, survival, and surgical complications. Patients were excluded if they had unresectable tumors or metastases during exploratory surgery or if they received other neoadjuvant-targeted therapies (Figure 1). The International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) tumor-node-metastasis (TNM) staging method, 8th edition, was employed.
Neoadjuvant Treatment Regimens
For patients undergoing NCRT, the radiation dose is 40–50.4 Gy (in units of 1.8–2.0 Gy). Radiotherapy (RT) techniques include intensity-modulated radiotherapy (IMRT) and three-dimensional conformal radiotherapy (3D-CRT). All patients were positioned under large-aperture computed tomography (CT) with the patient in the supine position with their hands on either side of their body, then a large mask was in a fixed place, laser light posed, enhanced CT scan performed, and, finally, CT positioning images were transmitted to the Varian Eclipse planning system. Each patient was positioned by a radiotherapist and radiotherapy physiotherapist. The target area was dominated by the area involved in the lesson. The preoperative chemotherapy regimen mainly consisted of treatment with platinum-based drugs and fluorouracil (PF regimen), or platinum-based drugs and paclitaxel/albumin paclitaxel (TP regimen) via intravenous injection (IV). The PF regimen consisted of platinum-based drugs (nedaplatin at 75 mg/m2, carboplatin at an area under the curve of 5, or cisplatin at 25 mg/m2 on days 1–3) along fluorouracil at 50 mg/m2 on days 1–5. The TP regimen consisted of paclitaxel at 135–175 mg/m2 or albumin-paclitaxel at 260 mg/m2. Patients received 1–3 preoperative chemotherapy (PF or TP regimens) every three weeks, and the average usage cycle was two in the NICT and NCRT groups. For patients who chose NICT, the preoperative immunotherapy regimen was 1–3 cycles of intravenous programmed cell death-1 (PD-1) inhibitor (pembrolizumab at a dose of 200 mg, camrelizumab at a dose of 200 mg, toripalimab at a dose of 240 mg, or sintilimab at a dose of 200 mg) every three weeks and the preoperative chemotherapy regimen is the same as that for NCRT. The chemotherapy dose is appropriately adjusted according to the patient’s tolerance.
Surgical Treatment
Patients enrolled in this study were clinically evaluated as suitable for radical esophagectomy. All patients underwent esophagectomy and mediastinal lymph node dissection under general anesthesia 4–6 weeks after the end of the last neoadjuvant treatment. Surgery was performed through thoracotomy or minimally invasive esophagectomy, including two and three incisions, with a two-field lymph node dissection as the standard of care. Patients with suspected cervical lymph node enlargement underwent standard three-field lymph node dissection.
Follow-Up
All selected patients underwent a regular outpatient review and telephone follow-up after admission, with routine physical examination, enhanced CT of the chest and abdomen, esophagography, and, if necessary, ultrasound, endoscopy, positron emission tomography (PET)/CT, magnetic resonance imaging, or whole-body bone imaging during the follow-up period. For patients whose last follow-up was recorded in the case system less than one month before the cut-off time of this study, a telephone follow-up was performed to ask the patients for details of their progress and survival. The follow-up ended on April 1, 2023, with a median follow-up time of 18 months for all patients, ranging from to 4–82 months.
Study Endpoints
The primary study endpoints included TRG, R0 resection rate, pCR rate, failure mode, and surgical complications. The secondary endpoints were disease-free survival (DFS) and OS. TRG was graded according to the CAP/AJCC criteria for pathological assessment after neoadjuvant therapy:16 grade 0 (complete response), no surviving cancer cells; grade 1 (moderate response), single or small clusters of cancer cells remaining; grade 2 (mild response), residual cancer foci with extensive interstitial fibrosis; and grade 3 (no response), little or no cancer cell necrosis with a large number of cancer cells remaining. R0 resection meant the tumor was completely removed with all microscopic margins negative. pCR refers to the absence of residual tumor cells at the primary site of the surgical sample and in the resected lymph nodes. This study also analyzed the failure patterns in both the NCRT and NICT groups. In the failure model, locoregional recurrences (LRR) was defined as primary tumor or local lymph node recurrence, and metastasis was defined as non-regional lymph node metastasis, systemic metastasis, malignant pleural effusion, or peritoneal metastasis. DFS was defined as the interval between esophagectomy and the first recording of recurrence, metastasis, death due to any cause, or last follow-up. OS was defined as the time from the start of the first cycle of neoadjuvant therapy to death from any cause or the last follow-up visit.
Statistical Analysis
PSM was used to form a well-balanced cohort using the full range of available explanatory factors.17 Therefore, this study used R software (version 4.2.1) to perform a 1:2 matching analysis between the NICT and NCRT groups to adjust for existing explanatory factors that might affect the results. A logistic regression model was used to calculate the propensity scores, including age, sex, Karnofsky Performance Status (KPS) score, tumor location, history of smoking, history of alcohol consumption, concomitant diseases, family history, number of chemotherapy cycles, clinical stage, and the use of endoscopic ultrasound (EUS) or PET/CT. The Kruskal–Wallis or independent samples t-test was employed to compare the R0 resection rates, pCR rates, surgical complications, and failure patterns. The Kaplan-Meier method was employed to assess DFS and OS, and a Log rank test was used to compare them. R software (version 4.2.1) and SPSS 25.0, were used for statistical analysis. Beyond the utilization of PSM, we have employed IPTW to adjust the baseline factors for both groups.
Results
Patients’ Baseline Characteristics
Between March 2016 and May 2022, a total of 281 ESCC patients underwent NICT or NCRT combined with radical esophagectomy at our study institution, with 81 in the NICT group and 200 in the NCRT group. Patients in the NCRT group ranged in age from 43 to 73 years, while those in the NICT group were between the ages of 45 and 73 years. To balance the potential bias, a 1:2 PSM was performed on the NICT and NCRT groups, and 110 patients receiving NCRT and 55 patients receiving NICT were selected for the final analysis. The area under the curve of the propensity score estimated by the logistic regression model is 0.704 (Figure 2). Furthermore, considering the distribution of propensity score in both groups, as depicted by the histogram, we believe the ability of propensity scores to discriminate between the two groups is satisfactory (Figure 2). The baseline characteristics are listed in Table 1. The clinical characteristics of post-PSM patients were more balanced and included age, sex, KPS, tumor site, family history, concomitant disease, history of smoking, history of alcohol consumption, clinical T stage, clinical N stage, clinical TNM stage, number of cycles of neoadjuvant therapy, presence of EUS, and presence of PET/CT, surgical interval. The baseline characteristics prior to and following the implementation of IPTW are presented in Supplemental Table 1.
 |
Table 1 Baseline Characteristics Before and After PSM |
Neoadjuvant Treatment and Surgical Treatment Outcome
All patients underwent esophagectomy and mediastinal lymph node dissection under general anesthesia 4–8 weeks after the last neoadjuvant treatment, with no delays in surgery due to treatment-related adverse events. None of the patients died within 30 or 90 days after surgery. As shown in Table 2, better results were obtained for the pathological response in the NCRT group compared to the NICT group, with a total of 63 (57.3%) patients in the NCRT group and 18 (32.7%) in the NICT group having a TRG grade of 0. This difference was statistically significant (P=0.003). No statistically significant difference was observed in the R0 resection rate between the NCRT and NICT groups (97.3% vs 98.2%, P=1.000). The pCR rates were 48.2% in the NCRT group and 29.1% in the NICT group (P=0.030). The NCRT group also exhibited a significantly higher complete pathological remission rate for primary lesions than the NICT group, yet no statistically significant differences were observed in the complete pathological remission rate of the lymph node (54.5% for primary lesions compared to 32.7%, P=0.013; 76.4% compared to 67.3% for lymph node lesions, P=0.290). The number of lymph nodes removed was 16.9±6.5 in the NCRT group and 22.8±8.0 in the NICT group (P<0.001). Patients in the NCRT group had a significantly longer postoperative length of stay (POLS) than those in the NICT group (17.7±8.2 vs 14.8±9.0, P=0.045), and the incidence of lymphovascular invasion (LVSI) was significantly lower in the NCRT group than in the NICT group, but no significant difference was seen in the rate of nerve invasion (1.8% vs 10.9%, P=0.010 and 12.7% vs 5.5%, P=0.239, respectively). The incidence of postoperative complications, such as anastomotic leak and pneumonia, was slightly higher in the NCRT group than in the NICT group, but the difference was not statistically significant (24.5% vs 12.7%, P=0.082) (Table 3).
 |
Table 2 Comparative Analysis of Pathological Outcomes Between NCRT and NICT Before and After PSM |
 |
Table 3 Postoperative Information |
The comparative analysis of pathological outcomes between NCRT and NICT before and after the implementation of IPTW is illustrated in Supplemental Table 2. Further, the postoperative information subsequent to the IPTW implementation is detailed in Supplemental Table 3. These conclusions were corroborated consistently via two statistical methods.
Progression and Survival Outcomes
The median follow-up period in this study was 18 months (range 4–82 months). In the NCRT group, the median follow-up period was 24 months (range 7–82 months), and 12 months (range 4–26 months) in the NICT group. As shown in Figure 3, the results of the 12-month follow-up of this study showed that patients in the NCRT group had significantly better DFS and OS than those in the NICT group (12-month DFS rate: 94.3% vs 81.8%, P=0.006; 12-month OS rate: 100.0% vs 95.4%, P=0.032). However, the results of the 24-month follow-up showed that there was also a statistically significant difference in DFS between the two groups, but there was no statistically significant difference in OS (P=0.026; P=0.359). In all patients, DFS was significantly better in those with postoperative pCR than in those with Non-pCR (P<0.001) (Figure 4). This study analyzed the pattern of failure after surgery in both groups and showed that after PSM, LRR occurred in four patients (3.6%) in the NCRT group, all outside the irradiated field, which was significantly lower than the seven patients (12.7%) in the NICT group who had LRR (P=0.027). Before PSM, LRR occurred in eight patients (4.0%) in the NCRT group, with one patient (0.5%) occurring within the irradiated field and the remaining seven patients (3.5%) occurring outside the irradiated field, all with LRR predominantly in the regional lymph nodes. Of the patients with LRR, two patients (1.0%) had postoperative pCR and six patients (3.0%) had postoperative non-pCR. However, there was no significant difference in the probability of metastasis between patients in the NCRT group and those in the NICT group (10.0% vs 1.8%, P=0.057) (Table 4).
 |
Table 4 Failure Modes After Radical Esophagectomy |
The Kaplan-Meier survival analyses for DFS and OS comparing NCRT and NICT, both pre and post IPTW, are delineated in Supplemental Figure 1. Additionally, the Kaplan-Meier survival analyses for DFS and OS comparing pCR and non-pCR groups, before and after the application of IPTW, are presented in Supplemental Figure 2. The modes of failure following radical esophagectomy prior to and subsequent to IPTW implementation are illustrated in Supplemental Table 4. The findings from this extensive analysis are bolstered by the coherence of results derived from two distinct statistical methodologies.
Discussion
Surgical treatment alone is inadequate in patients with locally advanced ESCC, and radical resection is not feasible in some patients. In recent years, the neoadjuvant mode of multidisciplinary combination therapy has become popular and applied to esophageal cancer, and neoadjuvant therapy combined with radical esophagectomy has become the standard treatment for patients with locally advanced ESCC.18 However, the most appropriate preoperative treatment regimen for patients with EC has been controversial, and no studies have compared the outcomes of patients with ESCC treated with NCRT or NICT. In this study, 110 patients who underwent NCRT and 55 who underwent NICT combined with esophagectomy were selected for the final analysis after PSM.
Both neoadjuvant regimens of NCRT and NICT are recommended in the Chinese Society of Clinical Oncology guideline of EC,19 and our present study was a retrospective analysis using PSM to maintain consistency in baseline characteristics. We found that there were more patients with aged ≥60 years and more patients with tumors located in the mid-upper esophagus in the NIRT group than in the NCRT group before matching, considering that this may be due to surgeons’ concern that more surgical complications such as anastomotic fistula may be associated with radiation-induced fibrosis to the mid-upper esophagus. The individualized neoadjuvant treatment strategies are more appropriate for patients, taking into account their individual choices and multidisciplinary opinions. Whether NICT can replace NCRT in clinical application, large prospective clinical trials to further demonstrate the effectiveness and safety are necessary.
In our study, we apply PSM to reduce the impact of potential confounding factors. The chemotherapy regimen was not a PSM factor in our study. Firstly, a study comparing chemotherapy regimens for locally advanced ESCC patients receiving NCRT found that the pCR rates were 24.6% and 35.5% for the Carboplatin and Paclitaxel regimen and Cisplatin and 5-Fluorouracil regimen, respectively (P=0.154), with a median survival of 16.7 and 32.7 months, respectively (P=0.083). There is no statistical difference in survival or clinicopathological outcome between both groups.20 Secondly, no significant difference in OS and progression-free survival between paclitaxel combined with fluorouracil, paclitaxel combined with carboplatin, and paclitaxel combined with cisplatin in the comparison of paclitaxel-based chemotherapy regimens in Professor Kuaile Zhao’s study of radical CCRT for locally advanced ESCC.21 We also did not perform further subgroup analyses of the immunological drugs in this study, which were all selected from Chinese domestic PD-1 inhibitors, and by default, the basic pharmacological mechanism of action of PD-1 inhibitors is the same. On the other hand, the small sample size limits our further subgroup analysis, which is also a limitation of our research.
Both before and after matching, the NCRT group had an advantage in pCR compared to the NICT group, with a statistically significant difference. Patients in the NCRT group had a significantly better DFS than those in the NICT group, and the risk of LRR was significantly lower in NCRT than in NICT. However, in terms of OS between the two groups, there was a statistically significant difference in the results of the 12-month follow-up, but not the 24-month follow-up, which we considered to be due to the shorter follow-up period. NCRT was significantly better than NICT in terms of both the pathological response and prognosis. This study considered RT as a local treatment modality second only to surgery, which can damage local tumor lesions better than IT and provide better local control of tumors; therefore, the post-treatment response of local tumor lesions was more pronounced in patients who received NCRT and also confirmed the predictive significance of postoperative pathological pCR on patient prognosis.22 Among all patients in this study, those with postoperative pCR had significantly better DFS than those with non-pCR, which reminds us that higher pCR rates remain the focus of neoadjuvant treatment strategies. However, improving systemic tumor control with neoadjuvant therapy and thus reducing the risk of metastasis should also be considered. In the pre-PSM NCRT group, LRR occurred within the irradiated field in only one of the eight patients, providing evidence that RT has good control over localized lesions and can effectively reduce the risk of LRR. Although NCRT is superior to NICT in terms of treatment response, it has a higher incidence of postoperative complications, especially postoperative anastomotic fistulas. Although the difference is not statistically significant, this is one of the critical limitations of the application of NCRT, which should also be noted. Therefore, we recommend that when clinical practitioners apply NCRT in combination with surgery to treat ESCC, they should limit the radiation dose at future anastomoses to minimize the incidence of postoperative anastomotic leakage. A review of the data demonstrates that there was a significantly increased length in hospital stay associated with an esophageal leak.23 The incidence of postoperative complications, such as anastomotic leak and pneumonia, was slightly higher in the NCRT group than in the NICT group, this may be one reason for the longer hospital stay after surgery. On the other hand, the NCRT group had a lower proportion of MIE than NICT (NCRT:63.6%; NICT:76.4%), which may also lead to a prolonged hospital stay. Therefore, the reduction of perioperative complications is an area of concern.
We observed the advantages of NCRT in the preoperative treatment of patients with resectable locally advanced ESCC in terms of postoperative pCR rate, risk of postoperative recurrence, and DFS. The CROSS study, a milestone in EC treatment, showed after a median follow-up time of 147 months that patients receiving NCRT had a longer OS compared to surgery alone (hazard ratio [HR]=0.70, 95% confidence interval [95% CI]=[0.55–0.89]), with an absolute benefit of 13% in 10-year OS rate (38% vs 25%). The risk of recurrence assessment showed that patients in the NCRT group had a lower risk of local recurrence alone (HR=0.40, [95% CI]= [0.21–0.72]) and concurrent local and distant recurrence (HR=0.43, [95% CI]=[0.26–0.72]), thus establishing NCRT as the standard of care for locally advanced EC. The results of the NEOCRTE5010 clinical trial showed that preoperative NCRT helps downstage tumors, increases R0 resection rates, and improves patient prognosis. The long-term follow-up results of these two studies further strengthen the position of NCRT in locally advanced EC.
Although we did not see an advantage of NICT in terms of pathological response and prognosis, we saw the potential of NICT as a preoperative neoadjuvant therapy for patients with locally advanced ESCC. Preclinical studies have shown that chemotherapeutic agents can promote the immune response by disrupting immunosuppressive cell activity, immunogenic death, and upregulation of MHC class 1 molecule expression.24–27 In neoadjuvant therapy, ICIs are thought to eliminate micrometastases and improve patient survival by activating the immune system. In this study, patients in the NICT group had a lower risk of metastasis than those in the NCRT group, although no statistical difference was observed. The advantages shown by NICT in controlling systemic tumors and the fact that patients in the NICT group had a lower incidence of postoperative complications and a better safety profile than those in the NCRT group have led to an increasing interest in NICT. In recent years, several studies have reported that NICT combined with radical esophagectomy can be considered an effective treatment for patients with locally advanced ESCC, with an increased pCR of 25%–39.2%.28–31 A multicenter, single-arm, phase II trial to evaluate the safety and efficacy of camrelizumab in combination with chemotherapy as neoadjuvant therapy for locally advanced ESCC achieved R0 resection in 50 patients (98.0%) and identified pCR in 20 patients (39.2%). The TD-NICE study reported the efficacy of tirelizumab in combination with albumin-paclitaxel + carboplatin. A total of 36 patients were treated with surgery, with major pathologic remission (MPR) and pCR rates of 72% and 50%, respectively, and 75% of these patients achieved a step-down in neoadjuvant therapy. The keynote-001 study, which included 42 patients, 29 of whom underwent robotic McKeown radical surgery, all with R0 resection, showed a high MPR rate of 72.4% (21/29) in patients receiving pablizumab combined with the TP regimen, with 12 of them achieving pCR (ypT0N0, 41.4%).
However, in the present study, no statistically significant differences in 24 months-OS were observed between the two groups, either before or after pairing, which may be due to several reasons. First, while the risk of recurrence was significantly lower in the NCRT group than in the NICT group, the risk of metastasis was higher in the NCRT group than in the NICT group, and NICT provided better control of systemic tumors than NCRT. Therefore, no statistically significant difference in OS was observed. Second, the study period was long, and the follow-up period for patients in the NICT group was relatively short compared to that for patients in the NCRT group; therefore, the survival of patients in the NICT group may not have been observed within the limited follow-up period. It is also possible that NCRT is more mature than NICT and that NCRT may be more likely to be chosen in patients with larger lesions and later stages, and to minimize potential confounding bias due to non-random selection data, we used PSM analysis to compare the two groups before-and-after to minimize the impact of this condition.
The ability of RT to damage local tumor tissue is more suitable for preoperative neoadjuvant treatment of patients with resectable locally advanced ESCC, allowing better control of local lesions, lowering tumor stage, reducing the difficulty of surgery, and improving patient prognosis; therefore, the use of NCRT in the neoadjuvant treatment of ESCC remains unassailable. However, the control of systemic tumors is inferior to that of NICT, and radiotherapy can lead to increased tissue adhesion and fragility. In recent years, IT has made unprecedented advances in cancer treatment with good efficacy and acceptable side effects in the management of ESCC, and combining IT with NCRT is an effective strategy for improving the prognosis of locally advanced ESCC.32 The PALACE-1 study33 used pembrolizumab in combination with radiotherapy for locally advanced resectable ESCC, with a 90% surgery rate and a high pCR rate of 55.6% after neoadjuvant treatment in 20 patients, higher than the 43.2% in the NEOCRTEC5010 trial4 and the 49% in the CROSS trial.3 In addition, studies such as PERFECT and PALACE-1 have reported that IT combined with NCRT has similar adverse events to NCRT.33,34 This suggests that the combination of IT and NCRT will be an inevitable trend in the future for the neoadjuvant treatment of ESCC. In our study, patients within the NCRT group reported a higher pCR rate and a reduced likelihood of LRR compared to those in the NICT group. Meanwhile, the NICT group demonstrated a lower probability of distant metastasis than the NCRT group. These findings suggest that chemoradiotherapy effectively suppresses local tumor activity, while IT effectively thwarts systemic tumor progression. Consequently, the integration of NCRT with IT emerges as a compelling direction for future research. To our knowledge, this is the first study to compare the efficacy and safety of NICT and NCRT in combination with radical esophagectomy in patients with locally advanced ESCC, providing a reference for future clinical trials and treatments. Some unanswered questions were also raised. First, whether it is necessary to continue surgical treatment for this group of patients who have already achieved pCR after neoadjuvant therapy. Second, if patients who have already achieved pCR can be treated without surgery and how to accurately predict whether patients after neoadjuvant treatment will achieve pCR before surgery. Finally, how to choose the optimal dose of RT for the esophagus to kill the tumor to the maximum extent possible while reducing the incidence of postoperative complications.
This study has certain limitations: (1) this is a retrospective analysis although we tried to improve the comparability of the two groups using PSM methods. The propensity score allows one to design and analyze an observational (nonrandomized) study so that it mimics some of the particular characteristics of a randomized controlled trial.17 (2) the sample size is not yet sufficiently large, and (3) the follow-up period is relatively short.
Conclusion
In comparison to NICT, NCRT demonstrates considerable advantages in improving the pCR rate in patients identified with locally advanced ESCC. Short-term follow-up results show that NCRT has a significantly better prognosis than NICT. Notably, the safety profiles of both NICT and NCRT are largely comparable. Future confirmation of this conclusion will require large-scale Phase III clinical trials.
Ethical Approval and Consent to Participate
This study has been approved by the Ethics Committee of Cancer Hospital Affiliated to Shandong First Medical University. Given its retrospective nature, the committee has waived the informed consent requirement for this study. We declare that patients information will be kept confidential and that we adhere to the principles of the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
2. Shah MA, Kennedy EB, Catenacci DV, et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol. 2020;38:2677–2694. doi:10.1200/JCO.20.00866
3. Eyck BM, van Lanschot JJB, Hulshof M, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. 2021;39:1995–2004. doi:10.1200/JCO.20.03614
4. Yang H, Liu H, Chen Y, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. 2021;156:721–729. doi:10.1001/jamasurg.2021.2373
5. de Miguel M, Calvo E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell. 2020;38:326–333. doi:10.1016/j.ccell.2020.07.004
6. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi:10.1126/science.aar4060
7. Zhao Q, Yu J, Meng X. A good start of immunotherapy in esophageal cancer. Cancer Med. 2019;8:4519–4526. doi:10.1002/cam4.2336
8. Kojima T, Doi T. Immunotherapy for esophageal squamous cell carcinoma. Curr Oncol Rep. 2017;19:33. doi:10.1007/s11912-017-0590-9
9. Salem ME, Puccini A, Xiu J, et al. Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist. 2018;23:1319–1327. doi:10.1634/theoncologist.2018-0143
10. Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2007;17:38–44. doi:10.1016/j.semradonc.2006.09.007
11. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant Pd-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–1986. doi:10.1056/NEJMoa1716078
12. Yan X, Duan H, Ni Y, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, Phase II Study (TD-NICE). Int J Surg. 2022;103:106680. doi:10.1016/j.ijsu.2022.106680
13. Xu J, Bai Y, Xu N, et al. Tislelizumab plus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma and gastric/gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2020;26:4542–4550. doi:10.1158/1078-0432.CCR-19-3561
14. Shang X, Zhao G, Liang F, et al. Safety and effectiveness of pembrolizumab combined with paclitaxel and cisplatin as neoadjuvant therapy followed by surgery for locally advanced resectable (Stage III) esophageal squamous cell carcinoma: a study protocol for a prospective, single-arm, single-center, open-label, Phase-II Trial (Keystone-001). Ann Transl Med. 2022;10:229. doi:10.21037/atm-22-513
15. Yang P, Zhou X, Yang X, et al. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study. World J Surg Oncol. 2021;19:333. doi:10.1186/s12957-021-02446-5
16. Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:855–883. doi:10.6004/jnccn.2019.0033
17. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi:10.1080/00273171.2011.568786
18. Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. 2020;50:12–20. doi:10.1007/s00595-019-01878-7
19. Wang LH, Huang J, Han YT. The Chinese Society of Clinical Oncology (CSCO): Clinical Guidelines for the Diagnosis and Treatment of Esophageal Cancer. China: People’s Health Press. 2022
20. Wong IYH, Lam KO, Zhang RQ, et al. Neoadjuvant chemoradiotherapy using cisplatin and 5-Fluorouracil (PF) versus Carboplatin and Paclitaxel (CROSS Regimen) for Esophageal Squamous Cell Carcinoma (ESCC): a Propensity Score-Matched Study. Ann Surg. 2020;272:779–785. doi:10.1097/SLA.0000000000004329
21. Ai D, Ye J, Wei S, et al. Comparison of 3 paclitaxel-based chemoradiotherapy regimens for patients with locally advanced esophageal squamous cell cancer: a Randomized Clinical Trial. JAMA Netw Open. 2022;5:e220120. doi:10.1001/jamanetworkopen.2022.0120
22. Wan T, Zhang XF, Liang C, et al. The prognostic value of a pathologic complete response after neoadjuvant therapy for digestive cancer: systematic review and meta-analysis of 21 studies. Ann Surg Oncol. 2019;26:1412–1420. doi:10.1245/s10434-018-07147-0
23. Gopaldas RR, Bhamidipati CM, Dao TK, et al. Impact of surgeon demographics and technique on outcomes after esophageal resections: a Nationwide study. Ann Thorac Surg. 2013;95:1064–1069. doi:10.1016/j.athoracsur.2012.10.038
24. Pol J, Vacchelli E, Aranda F, et al. Trial watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4:e1008866. doi:10.1080/2162402X.2015.1008866
25. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3:436–443. doi:10.1158/2326-6066.CIR-15-0064
26. Kepp O, Galluzzi L, Martins I, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–69. doi:10.1007/s10555-011-9273-4
27. Wu J, Waxman DJ. Immunogenic chemotherapy: dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018;419:210–221. doi:10.1016/j.canlet.2018.01.050
28. Shen D, Chen Q, Wu J, et al. The safety and efficacy of neoadjuvant Pd-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol. 2021;12:1–10. doi:10.21037/jgo-20-599
29. Wu Z, Zheng Q, Chen H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Dis. 2021;13:3518–3528. doi:10.21037/jtd-21-340
30. Liu J, Yang Y, Liu Z, et al. Multicenter, Single-Arm, Phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10:e004291. doi:10.1136/jitc-2021-004291
31. Yang W, Xing X, Yeung SJ, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10:e003497. doi:10.1136/jitc-2021-003497
32. Sihag S, Ku GY, Tan KS, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg. 2021;161:836–843.e1. doi:10.1016/j.jtcvs.2020.11.106
33. Li C, Zhao S, Zheng Y, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. 2021;144:232–241. doi:10.1016/j.ejca.2020.11.039
34. van den Ende T, de Clercq NC, van Berge Henegouwen MI, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: a Single-Arm Phase II Feasibility Trial (PERFECT). Clin Cancer Res. 2021;27:3351–3359. doi:10.1158/1078-0432.CCR-20-4443
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.