Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Comparison of Inflammatory Markers in the Diagnosis of Metabolic Syndrome in Hemodialysis Patients: A Multicenter Observational Study
Authors Song P, Zhao Y, Zhang H, Chen X, Han P, Fang C, Yu C, Guo Q
Received 14 April 2022
Accepted for publication 28 June 2022
Published 4 July 2022 Volume 2022:15 Pages 1995—2002
DOI https://doi.org/10.2147/DMSO.S370835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Peiyu Song,1 Yinjiao Zhao,1,* Hui Zhang,1,* Xiaoyu Chen,2 Peipei Han,2 Chenghu Fang,1 Chen Yu,3 Qi Guo1,2
1Department of Rehabilitation Medicine, Jiangwan Hospital of Shanghai Hongkou District, Shanghai University of Medicine and Health Science Affiliated First Rehabilitation Hospital, Shanghai, People’s Republic of China; 2Department of Rehabilitation Medicine, Shanghai University of Medicine and Health Sciences, Shanghai, People’s Republic of China; 3Department of Nephrology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qi Guo, Department of Rehabilitation Medicine, Shanghai University of Medicine and Health Sciences Affiliated Zhoupu Hospital, 1500 Zhouyuan Road, Pudong New District, Shanghai, 201318, People’s Republic of China, Tel/Fax +86-22-8333-6977, Email [email protected] Chen Yu, Department of Nephrology, Tongji Hospital, School of Medicine, Tongji University, No. 389 Xincun Road, Shanghai, 200065, People’s Republic of China, Tel +86-13311996821, Email [email protected]
Objective: The purpose of this study is to observe the correlation between high sensitivity C-reactive protein (hs-CRP) and metabolic syndrome (MetS) in hemodialysis patients, determine its optimal cut-off point value, and compare the diagnostic ability of different inflammatory markers for MetS.
Methods: This cross-sectional study finally included 860 long-term hemodialysis patients (male 524, average age 61.5 years) from seven dialysis centers in Shanghai, China. The International Diabetes Federation metabolic syndrome guidelines were used to define MetS, including high waist circumference, elevated blood pressure, elevated fasting blood glucose, elevated triglycerides, and reduced HDL cholesterol. Serum hs-CRP was determined by the immunonephelometric assay. The association with MetS was observed according to the quartile of inflammatory markers, and then the optimal cut-off point value of the hs-CRP was determined by ROC analysis.
Results: The overall prevalence of MetS was 55.1% (46.6% in males and 68.5% in females). In the final logistic regression model, there was a significant, graded positive association between hs-CRP and MetS (p for trend = 0.010). The traditional inflammatory markers leukocytes, neutrophils, lymphocytes, monocytes and neutrophil-to-lymphocyte ratio (NLR) were not associated with MetS. The results of the ROC analysis showed that the optimal cut point value of hs-CRP for the diagnosis of MetS was 1.58 mg/L. In the components of MetS and hs-CRP was significantly positively associated with high waist circumference, elevated TG and low HDL (p < 0.05).
Conclusion: The increase in hs-CRP concentration is significantly associated with the risk of MetS, and the diagnostic ability of hs-CRP for MetS is better than traditional inflammatory markers.
Keywords: metabolic syndrome, inflammatory markers, high sensitivity C-reactive protein, hemodialysis
Introduction
Metabolic syndrome (MetS) is common in hemodialysis patients, with a prevalence of 40–74.5%.1–3 As an important risk factor for cardiovascular disease and death, the risk of cardiovascular events in dialysis patients is 6.42 times higher than in the normal population and the risk of cardiovascular death is 20 times higher than in the normal population.4,5 Given the high prevalence and serious consequences of MetS in the dialysis population, the identification of individuals at risk who are eligible for early intervention is of clinical and public health importance.
Although the mechanisms associated with the development of the MetS remain unclear, chronic low-grade inflammation is thought to be a central mechanism in the pathophysiology of the MetS and insulin resistance.6 As the best biomarker of inflammation, the increase in high-sensitivity C-reactive protein (hs-CRP) concentration is significantly associated with MetS.7 In addition, some studies have proposed to add hs-CRP as the clinical standard of MetS.8 However, some studies have shown that hs-CRP is not associated with metabolic syndrome after further adjusting obesity indicators, and the addition of hs-CRP to obesity indicators could not improve the ability to predict metabolic syndrome.9 Therefore, the relationship between hs-CRP and metabolic syndrome needs further study. In addition, chronic low-grade inflammation is a common comorbidity in dialysis patients and is independently associated with multiple adverse clinical outcomes.10 Studies have shown that the increase in inflammatory markers is also significantly associated with MetS in hemodialysis patients.11,12 However, the correlation between hs-CRP and MetS in hemodialysis patients has rarely been explored.
Therefore, the purpose of this study is to observe the correlation between hs-CRP and MetS in hemodialysis patients, determine its optimal cut-off point value, and compare the diagnostic ability of different inflammatory markers for MetS.
Methods
Study Participants
The cross-sectional study recruited hemodialysis patients who were older than 18 years of age from seven dialysis units in Shanghai, China, and had undergone hemodialysis at least 3 months between July 2020 and April 2021. Participants with the following conditions were excluded from the study: (1) unable to communicate with interviewers or to grant informed consent; (2) no blood samples were collected. Following these exclusions, the final analyzed population comprised 860 subjects (524 men). The study was approved by the Ethics Committee of Shanghai University of Medicine and Health Sciences and the methods were carried out in accordance with the principles of the Declaration of Helsinki. All participants were informed and signed consent prior to enrollment in the study.
Baseline Variable
Demographic characteristics (including age and gender) and health behaviors (including smoking and drinking) were obtained from a standardized questionnaire by face-to-face interview. Anthropometric parameters (including height and weight) were measured by trained personnel using standardized protocols. Physical activity was assessed using the short form of the International Physical Activity Questionnaire (IPAQ).13 Nutritional status assessment using the Malnutrition Inflammation Score (MIS).14 Charlson Comorbidity Index (CCI) was used to assess the comorbidity risk associated with several conditions.15 Pre-dialysis laboratory measures were examined, including serum uric acid, creatinine, hemoglobin, albumin, calcium, phosphate and Kt/V.
Inflammatory Markers
Inflammatory markers, including white blood cell (WBC), neutrophil, lymphocyte and monocyte counts, were assessed by a hematology analyzer XE-2100 (Sysmex), and NLR values were calculated. hs-CRP was determined by the immunonephelometric assay using the Roche/Hitachi 917 analyzer (Roche). The detection limit of the hs-CRP concentration was 0.01 mg/L.
Definition of MetS
According to the International Diabetes Federation (IDF), people with MetS are defined by having central obesity (WC ≥90 cm in men and ≥80 cm in women) along with two or more of the following abnormalities: (1) elevated triglycerides (≥150 mg/dL); (2) reduced HDL cholesterol (<40 mg/dL in men and <50 mg/dL in women); (3) elevated blood pressure (≥130/85 mm Hg or known treatment for hypertension); (4) elevated FPG (≥100 mg/dL, or known treatment for diabetes).16
Analysis of Blood Samples and Blood Pressure
Blood samples were obtained from the antecubital vein of patients who fasted overnight for at least 10 h. Blood sample analysis and blood pressure collection methods have been explained in our previous studies.17
Statistical Analysis
The continuous variables with a normal distribution are expressed as the mean ± standard deviation (SD), whereas data with an abnormal distribution are expressed as the median, with the 25–75% interquartile range given in parentheses. Differences in the characteristics according to MetS status were analyzed using t-tests, chi-square test, and Kruskal Wallis rank tests. Participants were divided into four groups by quadrisection based on the results of inflammatory markers.18,19 Logistic regression analysis was used to determine odds ratios (ORs) and 95% CIs and to assess whether participants with MetS were independently associated with inflammatory markers when compared with those without MetS. A linear trend across the quartiles of inflammatory markers were tested by using the median value of each category as an ordinal variable. Linear regression was used for calculating p for trend in the logistic binary models.20 ROC curves were constructed to assess the ability of the inflammatory markers in diagnosing MetS. The optimal cut-off points used are the peaks of the curve, where the sum of sensitivity and specificity is at maximum. Using logistic regression analysis to assess the ORs of MetS and its components associated with inflammatory markers, several confounding factors were adjusted: age, sex, BMI, Kt/V, dialysis vintage, smoking status, drinking, MIS, physical activity level and CCI. Differences were defined as significant when p < 0.05. All statistical analyses were performed with the SPSS V22.0 software.
Result
General characteristics of 860 participants (524 men; mean age: 61.5±12.6 years) are given in Table 1. The overall prevalence of MetS was 55.1% (46.6% in males and 68.5% in females). Subjects with MetS had a higher percentage of high waist circumference, elevated blood glucose, and dyslipidemia than subjects without MetS (all p<0.01). Moreover, compared with subjects free of MetS, those with MetS were older and tended to have higher BMI, WBC, neutrophils, hs-CRP, phosphorus and incidence rate of diabetes and hyperlipidemia (all p<0.05). However, subjects with MetS had significantly lower Kt/V, hemoglobin, and albumin than subjects without MetS (all p<0.05).
 |
Table 1 General Characteristics of Participants with and without MetS |
The association between WBC, neutrophils, and hs-CRP and MetS is displayed in Table 2. The results showed that in the unadjusted model, although elevated hs-CRP was associated with increased risk of MetS, the trend was not statistically significantly different (p for trend >0.05). In the final logistic regression model, subjects with higher quartiles for hs-CRP had a significantly higher risk of MetS than those with the lowest quartile, as shown below: 1.76(0.86–3.61); 2.49(1.22–5.08); and 4.31(2.03–9.14) (p for trend <0.05). The association of WBC and neutrophil counts with MetS was not statistically significant (p for trend >0.05).
 |
Table 2 Logistic Regression Analyses of the Association of WBC, Neutrophils, and hs-CRP Quartiles with MetS |
Receiver operating characteristic (ROC) curves were used to quantify the sensitivity, specificity, area under the ROC curve (AUC) and optimal threshold of hs-CRP, and the results are shown in Figure 1 and Table 3. The results showed a certain correlation between hs-CRP and MetS, with an area under the curve of 0.638. To evaluate the diagnostic performance of hs-CRP for MetS, the ROC yielded an optimal threshold value of 1.58 mg/L, with sensitivity and specificity of 74.4% and 46.8%, respectively.
 |
Table 3 AUC and Cutoff Point of hs-CRP in Identifying Subjects with Metabolic Syndrome |
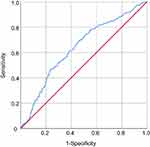 |
Figure 1 Receiver operating characteristic (ROC) curves of hs-CRP in identifying subjects with metabolic syndrome. |
Table 4 presents the odds ratios for MetS and its components associated with hs-CRP. After multifactorial adjustment, elevated hs-CRP was significantly associated with MetS, high waist circumference and elevated triglyceride (TG), with OR values of 2.46(1.44–4.18), 2.20(1.29–3.77), and 1.55(1.06–2.26), respectively.
 |
Table 4 Logistic Regression Analyses of the Association of hs-CRP with MetS and Its Components |
Discussion
This cross-sectional study examined the association between inflammatory markers such as WBC, neutrophils and hs-CRP and MetS in a population of hemodialysis patients. A significant, graded inverse association between hs-CRP and the risk of MetS was observed. However, there was no significant association between leukocytes, neutrophils, lymphocytes and NLR and the risk of metabolic syndrome. When the cut point values were divided according to the area under the ROC curve, elevated hs-CRP was significantly associated with high waist circumference and elevated TG.
There is a high prevalence of MetS in hemodialysis patients. According to the current epidemiological survey, the prevalence is between 40% and 74.5%.1,2 The major difference in prevalence is due to the difference in diagnostic criteria of MetS. In our study, the overall prevalence of MetS was 55.1%, slightly higher than that of a hemodialysis population in Southwest China.21 This may be that the average age of our population is slightly higher than that of patients in the region (61.5 vs 54.6 years). Among all MetS components, the prevalence of central obesity, elevated blood pressure, elevated glucose, elevated triglycerides, and reduced HDL were 56.8%, 98.4%, 75.7%, 50.1%, and 74.0%, respectively. Elevated blood pressure contributed the most to the cause of MetS in hemodialysis patients, which was consistent with the results of previous studies. Young et al found high prevalence of hypertension (99%) and low HDL (66%) in dialysis patients.22 These differences in results across studies can be attributed to the different populations and healthcare settings between these studies and the current study.
The association between hs-CRP, the best marker of chronic low-grade inflammation, and MetS and cardiovascular disease has been demonstrated in several studies.23 However, the association with MetS in hemodialysis patients has not been agreed upon in several studies. An Iranian hemodialysis population study showed a significant linear increase in hs-CRP levels based on the number of MeS components.11 In contrast, no significant association was shown between hs-CRP and MetS in the Japanese study.24 The association between hs-CRP and MetS in hemodialysis patients has not been further investigated. In our study, the risk of MetS gradually increased with increasing hs-CRP concentrations, confirming the positive association between hs-CRP and MetS in the hemodialysis patient population. The above differences may be the reason for the small sample size in the Japanese study. There are only 20 hemodialysis patients. In our study, the association between hs-CRP and MetS has been verified in a multicenter and large patient population, but its mechanism still needs further research. Furthermore, serum uric acid levels were shown to be associated with an elevated risk of death in the general population and in hemodialysis patients25,26 and were significantly associated with ejection fraction preserved heart failure and exercise-induced pulmonary hypertension,27,28 which are common in hemodialysis patients. In our study, serum uric acid levels were also significantly higher in hemodialysis patients with MetS than in patients without MetS; therefore, serum uric acid was further added to the adjusted model, and the final results remained stable. The possible mechanisms by which hs-CRP affects the MetS are as follows: first, a large body of data suggests that hs-CRP induces endothelial activation and dysfunction in vitro and in vivo,29,30 and that various components of the MetS are associated with endothelial dysfunction.31 Second, oxidative stress, mainly superoxide, plays a key role in the pathogenesis of MetS parameters.32 Recent studies suggest that CRP stimulates superoxide production in macrophages through the upregulation of NADPH oxidase and may have implications for the risk of MetS.33
Traditional inflammatory markers such as leukocytes, neutrophils, and lymphocytes were also included in our study. Although leukocyte and lymphocyte counts were significantly higher in the MetS group compared to non-MetS group, no significant trend was observed in association with MetS based on quartile distribution. This finding differs from the results in non-hemodialysis patients.34 The possible reasons for this are as follows: a previous Japanese study showed that hs-CRP was superior to WBC as an inflammatory component of MetS, suggesting a more stable association with the MetS. At the same time, considering the complexity of disease levels in hemodialysis patients, ultimately, hs-CRP preserves a stable cascade association with the MetS.35
This study has several significant advantages. First, to our knowledge, this is the first large-scale study based on hemodialysis population in China to observe the diagnostic efficiency of hs-CRP for MetS and compare it with other traditional inflammatory markers. In addition, the best cut-off value for identifying MetS in Chinese hemodialysis population also has guiding value in clinical practice. Finally, our study is of great significance for the treatment and prevention of MetS. As an important natural immune system regulator, hs-CRP may play an important role in the pathogenesis of MetS. Therefore, the development of drugs targeting hs-CRP may be an effective method for the treatment of MetS.
Although this study has its advantages, it still has some limitations. First, this is a cross-sectional study, so we cannot determine the causal relationship between inflammatory markers and MetS. Secondly, some potentially important markers, such as tumor necrosis factor-α, Interleukin-6, leptin and other proinflammatory cytokines have not been studied. Finally, all participants in the present study came from one city, which means this study has a certain degree of regional limitations. Future work will need to increase the sample size, perform follow-up in this population to further verify the causal relationship, and validate these findings in other populations.
Conclusion
The increase of hs-CRP concentration is significantly associated with the risk of MetS, and the diagnostic ability of hs-CRP for MetS is better than traditional inflammatory markers.
Data Sharing Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from corresponding author (Q.G.) upon reasonable request.
Statement of Ethics
The study was approved by the Ethics Committee of Shanghai University of Medicine and Health Sciences and the methods were carried out in accordance with the principles of the Declaration of Helsinki. All participants were informed and signed consent prior to enrollment in the study.
Acknowledgments
We thank all the medical staff at the multi-center dialysis for their generous technical assistance and guidance. We also thank all the study participants for their kind participation and cooperation.
Author Contributions
P.S., Y.Z. and H.Z. contributed equally to this work and should be considered the co-first authors. Q.G., C.Y., P.S., Y.Z. and H.Z. conceived the concept and design of the study. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article has been submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was funded by the National Natural Science Foundation of China (No. 82172552).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Vogt BP, Ponce D, Caramori JC. Anthropometric indicators predict metabolic syndrome diagnosis in maintenance hemodialysis patients. Nutr Clin Pract. 2016;31(3):368–374. doi:10.1177/0884533615601849
2. Tsangalis G, Papaconstantinou S, Kosmadakis G, Valis D, Zerefos N. Prevalence of the metabolic syndrome in hemodialysis. Int J Artif Organs. 2007;30(2):118–123. doi:10.1177/039139880703000206
3. Duong TV, Wong TC, Chen HH, et al. Inadequate dietary energy intake associates with higher prevalence of metabolic syndrome in different groups of hemodialysis patients: a clinical observational study in multiple dialysis centers. BMC Nephrol. 2018;19(1):236. doi:10.1186/s12882-018-1041-z
4. Sanguankeo A, Upala S. Metabolic syndrome increases mortality risk in dialysis patients: a systematic review and meta-analysis. Int J Endocrinol Metab. 2018;16(2):e61201. doi:10.5812/ijem.61201
5. Cozzolino M, Mangano M, Stucchi A, Ciceri P, Conte F, Galassi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33(suppl_3):iii28–iii34. doi:10.1093/ndt/gfy174
6. Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res. 2016;167(1):257–280. doi:10.1016/j.trsl.2015.06.017
7. Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol. 2009;20(3):182–189. doi:10.1097/MOL.0b013e32832ac03e
8. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi:10.1056/NEJM200003233421202
9. Saisho Y, Hirose H, Roberts R, Abe T, Kawabe H, Itoh H. C-reactive protein, high-molecular-weight adiponectin and development of metabolic syndrome in the Japanese general population: a longitudinal cohort study. PLoS One. 2013;8(9):e73430. doi:10.1371/journal.pone.0073430
10. Nowak KL, Chonchol M. Does inflammation affect outcomes in dialysis patients? Semin Dial. 2018;31(4):388–397. doi:10.1111/sdi.12686
11. Shahrokh S, Heydarian P, Ahmadi F, Saddadi F, Razeghi E. Association of inflammatory biomarkers with metabolic syndrome in hemodialysis patients. Ren Fail. 2012;34(9):1109–1113. doi:10.3109/0886022X.2012.713280
12. Park JT, Chang TI, Kim DK, et al. Association of white blood cell count with metabolic syndrome in patients undergoing peritoneal dialysis. Metabolism. 2009;58(10):1379–1385. doi:10.1016/j.metabol.2009.05.002
13. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi:10.1249/01.MSS.0000078924.61453.FB
14. Jagadeswaran D, Indhumathi E, Hemamalini AJ, Sivakumar V, Soundararajan P, Jayakumar M. Inflammation and nutritional status assessment by malnutrition inflammation score and its outcome in pre-dialysis chronic kidney disease patients. Clin Nutr. 2019;38(1):341–347. doi:10.1016/j.clnu.2018.01.001
15. Rattanasompattikul M, Feroze U, Molnar MZ, et al. Charlson comorbidity score is a strong predictor of mortality in hemodialysis patients. Int Urol Nephrol. 2012;44(6):1813–1823. doi:10.1007/s11255-011-0085-9
16. Song P, Zhang Y, Wang Y, et al. Clinical relevance of different handgrip strength indexes and metabolic syndrome in Chinese community-dwelling elderly individuals. Arch Gerontol Geriatr. 2020;87:104010. doi:10.1016/j.archger.2020.104010
17. Han P, Yu H, Ma Y, et al. The increased risk of sarcopenia in patients with cardiovascular risk factors in Suburb-Dwelling older Chinese using the AWGS definition. Sci Rep. 2017;7(1):9592. doi:10.1038/s41598-017-08488-8
18. Ma Y, Fu L, Jia L, et al. Muscle strength rather than muscle mass is associated with osteoporosis in older Chinese adults. J Formos Med Assoc. 2018;117(2):101–108. doi:10.1016/j.jfma.2017.03.004
19. Yao Z, Gu Y, Zhang Q, et al. Estimated daily quercetin intake and association with the prevalence of type 2 diabetes mellitus in Chinese adults. Eur J Nutr. 2019;58(2):819–830. doi:10.1007/s00394-018-1713-2
20. Gu Y, Li X, Zhang Q, et al. Grip strength and depressive symptoms in a large-scale adult population: the TCLSIH cohort study. J Affect Disord. 2021;279:222–228. doi:10.1016/j.jad.2020.08.023
21. Zhou C, Zhan L, Yuan J, Tong X, Peng Y, Zha Y. Comparison of visceral, general and central obesity indices in the prediction of metabolic syndrome in maintenance hemodialysis patients. Eat Weight Disord. 2020;25(3):727–734. doi:10.1007/s40519-019-00678-9
22. Young DO, Lund RJ, Haynatzki G, Dunlay RW. Prevalence of the metabolic syndrome in an incident dialysis population. Hemodial Int. 2007;11(1):86–95. doi:10.1111/j.1542-4758.2007.00158.x
23. den Engelsen C, Koekkoek PS, Gorter KJ, van den Donk M, Salome PL, Rutten GE. High-sensitivity C-reactive protein to detect metabolic syndrome in a centrally obese population: a cross-sectional analysis. Cardiovasc Diabetol. 2012;11:25. doi:10.1186/1475-2840-11-25
24. Rasic-Milutinovic Z, Perunicic G, Pljesa S, Gluvic Z, Ilic M, Stokic E. Metabolic syndrome in HD patients: association with body composition, nutritional status, inflammation and serum iron. Intern Med. 2007;46(13):945–951. doi:10.2169/internalmedicine.46.0092
25. Zawada AM, Carrero JJ, Wolf M, et al. Serum uric acid and mortality risk among hemodialysis patients. Kidney Int Rep. 2020;5(8):1196–1206. doi:10.1016/j.ekir.2020.05.021
26. Pugliese NR, Mengozzi A, Virdis A, et al. The importance of including uric acid in the definition of metabolic syndrome when assessing the mortality risk. Clin Res Cardiol. 2021;110(7):1073–1082. doi:10.1007/s00392-021-01815-0
27. Antlanger M, Aschauer S, Kopecky C, et al. Heart failure with preserved and reduced ejection fraction in hemodialysis patients: prevalence, disease prediction and prognosis. Kidney Blood Press Res. 2017;42(1):165–176. doi:10.1159/000473868
28. Pugliese NR, Mazzola M, Madonna R, et al. Exercise-induced pulmonary hypertension in HFpEF and HFrEF: different pathophysiologic mechanism behind similar functional impairment. Vascul Pharmacol. 2022;144:106978. doi:10.1016/j.vph.2022.106978
29. Grad E, Golomb M, Mor-Yosef I, et al. Transgenic expression of human C-reactive protein suppresses endothelial nitric oxide synthase expression and bioactivity after vascular injury. Am J Physiol Heart Circ Physiol. 2007;293(1):H489–95. doi:10.1152/ajpheart.01418.2006
30. Teoh H, Quan A, Lovren F, et al. Impaired endothelial function in C-reactive protein overexpressing mice. Atherosclerosis. 2008;201(2):318–325. doi:10.1016/j.atherosclerosis.2008.02.034
31. Rodriguez CJ, Miyake Y, Grahame-Clarke C, et al. Relation of plasma glucose and endothelial function in a population-based multiethnic sample of subjects without diabetes mellitus. Am J Cardiol. 2005;96(9):1273–1277. doi:10.1016/j.amjcard.2005.06.070
32. Keaney JF, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–439. doi:10.1161/01.ATV.0000058402.34138.11
33. Devaraj S, Dasu MR, Singh U, Rao LV, Jialal I. C-reactive protein stimulates superoxide anion release and tissue factor activity in vivo. Atherosclerosis. 2009;203(1):67–74. doi:10.1016/j.atherosclerosis.2008.05.060
34. Yang XJ, Tian S, Ma QH, Sun HP, Xu Y, Pan CW. Leukocyte-related parameters in older adults with metabolic syndrome. Endocrine. 2020;68(2):312–319. doi:10.1007/s12020-020-02243-2
35. Oda E, Kawai R. Comparison between high-sensitivity C-reactive protein (hs-CRP) and white blood cell count (WBC) as an inflammatory component of metabolic syndrome in Japanese. Intern Med. 2010;49(2):117–124. doi:10.2169/internalmedicine.49.2670
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
