Back to Journals » Nature and Science of Sleep » Volume 14
Comparison of Composite Lipid Indices in Patients with Obstructive Sleep Apnoea
Authors Bikov A , Frent S , Reisz D, Negru A, Gaita L , Breban Schwarzkopf D, Mihaicuta S
Received 6 February 2022
Accepted for publication 21 June 2022
Published 27 July 2022 Volume 2022:14 Pages 1333—1340
DOI https://doi.org/10.2147/NSS.S361318
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Andras Bikov,1,2 Stefan Frent,3 Daniela Reisz,4 Alina Negru,5,6 Laura Gaita,7 Daniel Breban Schwarzkopf,8 Stefan Mihaicuta3
1North West Lung Centre, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester, UK; 2Division of Immunology, Immunity to Infection and Respiratory Medicine, University of Manchester, Manchester, UK; 3Center for Research and Innovation in Precision Medicine of Respiratory Diseases, Department of Pulmonology, “Victor Babes” University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 4Department of Neurology, “Victor Babes” University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 5Department of Cardiology (II), “Victor Babes” University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 6Department of Clinical Research, Institute of Cardiovascular Diseases, Timisoara, Romania; 7Second Department of Internal Medicine, “Victor Babes” University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 8Department of Anatomy, “Victor Babes” University of Medicine and Pharmacy Timisoara, Timisoara, Romania
Correspondence: Stefan Frent; Daniela Reisz, Email [email protected]; [email protected]
Purpose: Obstructive sleep apnoea (OSA) is a recognised risk factor for cardiovascular disease. However, it is difficult to evaluate the risk of cardiovascular disease in patients with OSA due to multiple shared risk factors. Composite lipid indices, such as atherogenic index of plasma (AIP), visceral adiposity index (VAI) and lipid accumulation product (LAP) have been shown to predict cardiovascular disease better than their individual lipid components. This study aimed to evaluate these indices in patients with OSA.
Patients and Methods: Six hundred sixty-seven (667) patients with OSA and 139 non-OSA control volunteers participated in the study. Fasting serum triglycerides, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol (HDL-C) levels were measured, and AIP, LAP and VAI were calculated following cardiorespiratory polygraphy. The relationship between lipid parameters, OSA and its comorbidities was evaluated using receiver operating curve (ROC) analysis.
Results: We found a significant difference in all lipid parameters between OSA patients and controls. Comparing ROCs, LAP was significantly more strongly associated with OSA compared to all the other parameters. The optimal cut-off value for LAP to detect OSA was 76.4, with a sensitivity of 63% and a specificity of 76%. In addition, LAP was the best parameter to predict hypertension and diabetes in patients with OSA, and it was predictive for ischaemic heart disease together with HDL-C.
Conclusion: Our results support the use of LAP in clinical practice when evaluating cardiovascular risk in patients with OSA. However, the optimal cut-off value should be determined in large-scale follow-up studies.
Keywords: lipid indices, cholesterol, triglyceride, obstructive sleep apnea, cardiovascular risk
Introduction
Obstructive sleep apnoea (OSA) is a common condition characterised by repetitive partial or complete collapse of the pharynx during sleep resulting in intermittent hypoxaemia and sleep fragmentation. OSA is broadly recognised as a risk factor for cardiovascular morbidity and mortality. This association is driven by multiple processes induced by OSA, such as systemic inflammation and oxidative stress, sympathetic activity, hypertension and dyslipidaemia.1
Obstructive sleep apnoea can cause dyslipidaemia via various mechanisms. First, patients with OSA tend to consume more fat-rich meals.2 Second, OSA is associated with higher postprandial plasma levels of triglycerides, suggesting an accelerated lipid absorption and decreased lipoprotein lipase activity.3,4 Third, the hepatic synthesis of triglycerides is accelerated in OSA due to hypoxaemia-sensitive mechanisms.5 Forth, intermittent hypoxaemia triggers hormone sensitive lipases in the adipose tissues leading to the release of free fatty acids into circulation.6 Finally, the clearance of low-density lipoprotein cholesterol (LDL-C) from circulation is reduced in OSA.7 Not surprisingly, OSA is associated with high triglyceride (TGs) and low high-density lipoprotein cholesterol (HDL-C) levels.8 Of note, obesity is a well known risk factor for OSA9 and a significant confounder for the association between OSA and dyslipidaemia.10
The link between dyslipidaemia and cardiovascular risk cannot be entirely evaluated by investigating single lipid components due to the dynamic interaction between different lipoprotein fractions.11 To address this, the logarithmic ratio of high-density lipoprotein cholesterol (HDL-C) and triglycerides, the so called Atherogenic Index of Plasma (AIP) has been investigated in OSA before in several studies.12–15 In addition, visceral fat distribution was demonstrated to be a better predictor for cardiovascular disease than the body mass index (BMI).16 In line with this, composite indices which take into account both, lipid parameters and waist circumference, were better predictors for cardiovascular disease than single lipid components.17,18 These indices include the visceral adiposity index (VAI) and lipid accumulation product (LAP), both of which were investigated in OSA.19–24
Despite previous research, it remains unclear which index should be used in clinical practice in OSA. These indices were directly compared only in three studies;19,23,24 however, all were performed in Chinese populations. Notably, the clinical value of lipid indices depends on ethnicity,25 therefore these results are not fully translatable to a Caucasian population. Moreover, only one of these studies has investigated the prediction of metabolic consequences in OSA. Wei et al reported that LAP was more strongly related to insulin resistance than VAI, but only in normal-weight subjects.24
The aim of the current study was to analyse if AIP, VAI and LAP are more strongly related to OSA than the individual lipid components, how they change with disease severity and how they correlate with cardiovascular disease, hypertension and diabetes.
Materials and Methods
Study Design and Subjects
Eight hundred six (806) consecutive volunteers participated in the study. All patients were referred with symptoms and/or medical history suggestive for OSA (ie snoring, witnessed apnoeas, daytime somnolence, treatment-resistant comorbidities). Medical history was taken, blood pressure, height, weight, waist (WC), hip (HC) and neck circumference (NC) were measured, and the subjects filled out the Epworth Sleepiness Scale (ESS). Following an inpatient diagnostic sleep test, fasting blood sample was drawn for measuring total cholesterol, LDL-C, HDL-C and triglycerides. Comorbidities were defined by patient self-report, available medical records, and prescription charts. The study was approved by the local Ethics Committee (“Victor Babes” University of Medicine and Pharmacy Timisoara 22/2014/24.07.2019) and patients gave their informed consent before participation. All procedures performed in our study were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments.
Sleep Studies
Inpatient cardiorespiratory polygraphy was performed with Porti 7 SLEEP DOC DeVilbiss or Stardust Philips Respironics. Apnoea was defined as a drop in nasal flow of at least 90%, lasting for minimum 10 seconds. Hypopnoea was defined as a drop in nasal flow of at least 30%, associated with a drop in oxygen saturation of at least 3% lasting for minimum 10 seconds (according to the American Academy of Sleep Medicine Scoring Manual, version 2.5.). Apnoea-hypopnoea index (AHI) and oxygen desaturation index (ODI) were calculated and were used to define OSA severity. OSA was defined by AHI≥5/h, and patients with OSA were categorised into mild (AHI 5–14.9/h), moderate (AHI 15–29.9/h) and severe (AHI≥30/h) subgroups.
Measurement of Lipid Components and Composite Indices
The samples were analysed with a COBAS INTEGRA 400 plus analyzer (Roche Diagnostics, Switzerland). Lipid levels were estimated by the enzymatic colorimetric method with an inter-assay coefficient of variability of 1.6%, 0.5%, 1.3% and 1.13%, for triglyceride, total cholesterol, LDL-C and HDL-C, respectively. The laboratory is SR EN ISO 15189:2013 certified.
AIP was calculated as 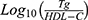 , VAI was calculated as
, VAI was calculated as 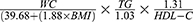 for men and
for men and 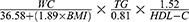 for women, while LAP was calculated as
for women, while LAP was calculated as 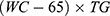 for men and
for men and 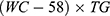 for women where WC was expressed in cm and TGs and HDL-C were both expressed in mmol/l.
for women where WC was expressed in cm and TGs and HDL-C were both expressed in mmol/l.
Statistical Analysis
JASP 0.14 (JASP Team, University of Amsterdam, The Netherlands) and MedCalc 19.5.3 (MedCalc Software Ltd, Belgium) were used for statistical analysis. The normality of the data was assessed with the Shapiro–Wilk test. The OSA and control groups were compared with Mann–Whitney and Chi-square tests. The relationship between lipids and OSA severity was investigated with the non-parametric analysis of covariance (ANCOVA) test adjusted for age, BMI and gender followed by the Dunn’s test where OSA severity groups were compared to the control group. In addition, the relationship between lipid parameters and OSA severity was also analysed with multiple linear regression analysis using AHI, age, gender and BMI as independent variables. The relationship between lipids and ESS was investigated with the Spearman’s test. The associations between AIP, LAP and VAI and OSA, and their predictive value for ischaemic heart disease, hypertension and diabetes were evaluated with the Receiver operating characteristic (ROC) analysis and compared to each other and to TGs, LDL-C and HDL-C using the DeLong test.26 The ROC analyses for comorbidities were performed only in patients with OSA. Data were expressed as median /interquartile range/. A p value <0.05 was considered statistically significant.
Results
Comparison of OSA and Control Groups
Six hundred sixty-seven (667) patients were diagnosed with OSA. Patients in OSA group were older, had higher WC, HC and NC values, higher total cholesterol, LDL-C and triglyceride levels and lower HDL-C levels. We found a higher prevalence of male gender, hypertension, ischaemic heart disease, and diabetes in the OSA group. There was no difference in smoking habits between the two groups. All three investigated composite lipid indices were significantly higher in OSA compared to controls. The patients’ characteristics are summarised in Table 1.
 |
Table 1 Subjects’ Characteristics |
The Relationship Between OSA Severity, Lipids and Lipid Indices
Eighty-one (81) patients were diagnosed with mild (AHI 5–14.9/h), 204 with moderate (AHI 15–29.9/h) and 382 with severe (AHI≥30/h) OSA. All lipid fractions were related to OSA severity (p<0.01); however, progressive changes were noticed only for HDL-C and LAP. A significant difference in total cholesterol level was noticed between severe OSA and control groups (p<0.05). LDL-C and LAP were different from the control group in mild, moderate and severe OSA subgroups (all p<0.05), while triglycerides, HDL-C, AIP and VAI were different from controls in moderate and severe OSA subgroups (all p<0.05, Table 1). The AHI independently related to triglycerides, total cholesterol, LDL-C and LAP (all p<0.05); however following adjustment for age, gender and BMI, there was no relationship between AHI and HDL-C (p=0.82), VAI (p=0.06) or AIP (p=0.09). All lipid parameters were related to ESS (ρ=0.18, ρ=0.12, ρ=0.14, ρ=−0.13, ρ=0.18, ρ=0.14, ρ=0.28, all p<0.01, for triglycerides, total cholesterol, LDL-C, HDL-C, AIP, VAI and LAP, respectively).
Receiver Operating Characteristic Analysis on the Association Between Lipid Indices and OSA
All lipid parameters were significantly associated with OSA; however, we found significant differences in the AUC of ROC curves for the lipid parameters and indices assessed (Table 2). The AUC for LAP was significantly larger than of triglycerides (p<0.01), total cholesterol (p<0.01), HDL-C (p<0.01), AIP (p<0.01) and VAI (p<0.01) and tended to be larger than of LDL-C (p=0.056). The AUC of AIP and LDL-C were larger than of VAI (both p=0.04) and of total cholesterol (both p<0.01). No further differences in the AUCs were observed. LAP was significantly more strongly associated with OSA compared to the other parameters, while AIP performed significantly better than VAI (Figure 1). The optimal cut-off for LAP to predict OSA was 76.4, that was associated with a 63% sensitivity and a 76% specificity.
 |
Table 2 Comparison of Lipid Parameters’ Receiver Operating Characteristic Curves to Detect OSA |
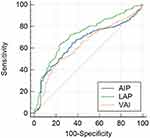 |
Figure 1 Comparison of lipid indices’ receiver operating characteristic curves to detect OSA. |
Receiver Operating Characteristic Analysis on the Association Between Lipid Indices and Comorbidities
Investigating the predictive value of lipid parameters for hypertension, ischaemic heart disease and diabetes, not all lipid parameters were significant (Table 3). Specifically, for hypertension the AUCs of triglycerides, AIP, VAI, and LAP were significant (all p<0.05), and the AUC of LAP was significantly larger than of all the other parameters (all p<0.01). For ischaemic heart disease, only the AUCs of HDL-C and LAP were significant, without any difference between the two AUCs (p=0.61). For diabetes, the AUCs of triglycerides, HDL-C, AIP, VAI and LAP were significant (p<0.05), and the AUC of LAP was significantly larger than of any other lipid parameter (p<0.01).
 |
Table 3 Comparison of Lipid Parameters’ Receiver Operating Characteristic Curves to Detect Comorbidities in Patients with OSA |
Discussion
In this study we have assessed several lipid parameters and composite lipid indices in subjects with or without OSA. We found that the lipid accumulation product (LAP) was most strongly associated with OSA, related to disease severity, and also had the highest predictive value for comorbidities.
The LAP was first introduced in 2005. The equation was derived from the National Health and Nutrition Examination Survey data, where it was found to be a better predictor for cardiovascular disease than the BMI.17 Subsequently, it was validated as a useful clinical marker to predict future cardiovascular events and cardiovascular mortality,27 and it was also proven to be superior to BMI in predicting diabetes.28 Regarding OSA, Bianchi et al reported higher LAP values in patients at high risk of OSA based on Berlin questionnaire, however in this study the diagnosis was not confirmed by a diagnostic sleep test.20 In patients with diabetes, LAP was associated with OSA, however, interestingly, it was not related to disease severity.29 When investigating only patients with OSA, LAP was related to insulin resistance in normal-weight male subjects.24 In our study, we reported that LAP was associated with hypertension, diabetes, and ischaemic heart disease. We did not investigate male and female, as well as normal and abnormal weight subjects separately, as the prevalence of comorbidities in some subgroups was low and the study was not powered to assess subgroups.
The visceral adiposity index was introduced in 2010 and it was demonstrated to be significantly associated with cardiovascular disease and metabolic syndrome.18 Several studies investigated VAI in OSA. Interestingly, Mazzuca et al reported no relationship between VAI and OSA, but concluded that it might be beneficial in predicting metabolic syndrome in patients with sleep apnoea.22 In line with this, VAI was not related to OSA in a large Chinese study.23 In contrast, Chen et al found that VAI was increased in patients with OSA, and it was related to disease severity and metabolic syndrome.21 Investigating patients with OSA, VAI was also shown to be related to insulin resistance in normal-weight female subjects.24 In our study, VAI was higher in patients with OSA compared to controls, but the difference was significant only in moderate-to-severe disease. In addition, when adjusting for age, gender and BMI, the association between VAI and OSA no longer became significant. Differences in OSA severity and patient characteristics between the previous studies could explain some of the contradictory results. We found VAI to be related to hypertension and diabetes, but not to cardiovascular disease. Taking into account the difficulty of calculating VAI compared to LAP, we suggest that this marker may have limited value in clinical practice when assessing patients with OSA.
A dynamic interaction has been observed between HDL and VLDL particles; therefore, HDL-C and triglyceride levels should not be interpreted in isolation.11 The AIP was introduced as a marker which reflects on the esterification rate of HDL particles.30 It was shown to better predict cardiovascular morbidity31 and mortality32 than the single lipid components. Cao et al reported that AIP was higher in OSA and was related to disease severity.12 These findings were in line with the study by Wysocki et al who reported a direct relationship between AIP and AHI.13 Shimizu et al found that AIP was related to OSA, but only in normal-weight subjects.14 In a previous study, we have reported that AIP was higher in OSA and was related to disease severity, however HDL-C alone more precisely discriminated patients with OSA from controls. Furthermore, compared to single lipid components, AIP did not show a better value in predicting cardiovascular disease or diabetes.15 In the current study, AIP was increased in OSA, but only in subjects with moderate-to-severe disease and related to hypertension and diabetes, while being inferior to LAP in detecting comorbidities and was less strongly associated with OSA than the LAP. In addition, AIP was not superior to single lipid components when evaluating cardiometabolic disease. Hence, our results suggest no additional value for this marker in clinical practice.
To date, composite lipid indices were compared to each other only in a few studies. In their report, Zhao et al have investigated LAP and VAI in a large cross-sectional cohort and in 86 patients who underwent bariatric surgery. The authors have concluded that LAP was more strongly associated with OSA than VAI.23 Zou et al compared VAI, LAP and the triglyceride glucose index (TyG) in another large Chinese cohort and found that LAP predicted OSA better than the two other composite indices.19 Our results are in line with these findings, however the optimal cut-off value was more than twice higher in our study than in theirs (76.4 vs 33.15). A possible reason for the difference could be ethnicity, as it was previously reported that OSA-related rise in triglycerides is genetically determined.33 Investigating only patients with OSA, Wei et al compared LAP, VAI and TyG. The authors have found that the TyG and LAP were most strongly associated with insulin resistance, but only in normal-weight subjects.24 Finally, Ramesh et al have evaluated numerous demographical and clinical data to compute a predictive model for OSA. Although LAP, VAI and TyG were all evaluated, only LAP was included in the final model.34
In our large group of subjects, lipid parameters were significantly different between the OSA and control groups. To compare the strength of associations between OSA and composite lipid indices we used ROC analysis followed by the DeLong test.26 This should not be interpreted as a prediction model analysis, as OSA should still be diagnosed with sleep studies. However, high LAP values should give indications to screen for OSA in routine clinical practice. The DeLong test allowed us to compare the predictive value of lipid indices to detect cardiovascular disease. In this respect, LAP has performed the best.
Our study has some limitations. First, although medications, diet and regular exercise could affect lipid values, these factors were not evaluated in the current study. Second, we used cardiorespiratory polygraphy over polysomnography as a diagnostic test for OSA. Polygraphy is an accepted diagnostic test, however it tends to underestimate disease severity.35 Third, this is a cross-sectional study, and the clinical value of lipid indices should be evaluated in interventional trials. We believe that our data could serve as a potential basis for the design of these studies.
Conclusion
We have demonstrated that compared to single lipid components and other composite indices, LAP was most strongly associated with OSA and its comorbidities. However, as its value could be affected by lifestyle factors, the causality between LAP and OSA need to be tested in interventional studies, such as randomized controlled trials. We suggest using this marker in the routine assessment of patients with OSA; however, the exact cut-off value for this marker needs to be further determined in follow-up studies.
Acknowledgments
Andras Bikov is supported by NIHR Manchester Biomedical Research Centre.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi:10.1016/S0140-6736(05)71141-7
2. Smith SS, Waight C, Doyle G, Rossa KR, Sullivan KA. Liking for high fat foods in patients with obstructive sleep apnoea. Appetite. 2014;78:185–192. doi:10.1016/j.appet.2014.03.019
3. Jun JC, Shin MK, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303:E377–88. doi:10.1152/ajpendo.00641.2011
4. Morin R, Mauger JF, Amaratunga R, Imbeault P. The effect of acute intermittent hypoxia on postprandial triglyceride levels in humans: a randomized crossover trial. J Transl Med. 2021;19:268. doi:10.1186/s12967-021-02933-z
5. Li J, Grigoryev DN, Ye SQ, et al. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol. 2005;99:1643–1648. doi:10.1152/japplphysiol.00522.2005
6. Barceló A, Piérola J, de la Peña M, et al. Free fatty acids and the metabolic syndrome in patients with obstructive sleep apnoea. Eur Respir J. 2011;37:1418–1423. doi:10.1183/09031936.00050410
7. Meszaros M, Kunos L, Tarnoki AD, Tarnoki DL, Lazar Z, Bikov A. The role of soluble low-density lipoprotein receptor-related protein-1 in obstructive sleep apnoea. J Clin Med. 2021;10:1494. doi:10.3390/jcm10071494
8. Barros D, García-Río F. Obstructive sleep apnea and dyslipidemia: from animal models to clinical evidence. Sleep. 2019;42. doi:10.1093/sleep/zsy236
9. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi:10.1093/aje/kws342
10. Guan J, Yi H, Zou J, et al. Distinct severity stages of obstructive sleep apnoea are correlated with unique dyslipidaemia: large-scale observational study. Thorax. 2016;71:347–355. doi:10.1136/thoraxjnl-2015-207403
11. Kjeldsen EW, Thomassen JQ, Frikke-Schmidt R. HDL cholesterol concentrations and risk of atherosclerotic cardiovascular disease - Insights from randomized clinical trials and human genetics. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867:159063. doi:10.1016/j.bbalip.2021.159063
12. Cao B, Fan Z, Zhang Y, Li T. Independent association of severity of obstructive sleep apnea with lipid metabolism of atherogenic index of plasma (AIP) and apoB/apoAI ratio. Sleep Breath. 2020;24:1507–1513. doi:10.1007/s11325-020-02016-1
13. Wysocki J, Balcerzak J, Prus M, Niemczyk K, Lachowska M. Sleep quality and atherogenic risk in sleep apnea patients. Eur J Gen Med. 2016;13:28–36.
14. Shimizu Y, Yoshimine H, Nagayoshi M, et al. Serum triglyceride levels in relation to high-density lipoprotein cholesterol (TG-HDL) ratios as an efficient tool to estimate the risk of sleep apnea syndrome in non-overweight Japanese men. Environ Health Prev Med. 2016;21:321–326. doi:10.1007/s12199-016-0532-4
15. Bikov A, Meszaros M, Kunos L, Negru AG, Frent SM, Mihaicuta S. Atherogenic index of plasma in obstructive sleep apnoea. J Clin Med. 2021;10:417. doi:10.3390/jcm10030417
16. Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44. doi:10.1046/j.1365-2796.2003.01177.x
17. Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26. doi:10.1186/1471-2261-5-26
18. Amato MC, Giordano C, Galia M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–922. doi:10.2337/dc09-1825
19. Zou J, Wang Y, Xu H, et al. The use of visceral adiposity variables in the prediction of obstructive sleep apnea: evidence from a large cross-sectional study. Sleep Breath. 2020;24:1373–1382. doi:10.1007/s11325-019-01980-7
20. Bianchi VE, Herbert WG, Myers J, Ribisl PM, Miller LE, Dalman RL. Relationship of obstructive sleep apnea and cardiometabolic risk factors in elderly patients with abdominal aortic aneurysm. Sleep Breath. 2015;19:593–598. doi:10.1007/s11325-014-1053-2
21. Chen GP, Qi JC, Wang BY, et al. Applicability of visceral adiposity index in predicting metabolic syndrome in adults with obstructive sleep apnea: a cross-sectional study. BMC Pulm Med. 2016;16:37. doi:10.1186/s12890-016-0198-0
22. Mazzuca E, Battaglia S, Marrone O, et al. Gender-specific anthropometric markers of adiposity, metabolic syndrome and visceral adiposity index (VAI) in patients with obstructive sleep apnea. J Sleep Res. 2014;23:13–21. doi:10.1111/jsr.12088
23. Zhao X, Xu H, Qian Y, et al. Abdominal obesity is more strongly correlated with obstructive sleep apnea than general obesity in China: results from two separated observational and longitudinal studies. Obes Surg. 2019;29:2535–2547. doi:10.1007/s11695-019-03870-z
24. Wei R, Gao Z, Xu H, et al. Body fat indices as effective predictors of insulin resistance in obstructive sleep apnea: evidence from a cross-sectional and longitudinal study: BFI as predictors of IR in OSA. Obes Surg. 2021;31:2219–2230. doi:10.1007/s11695-021-05261-9
25. Kim-Dorner SJ, Deuster PA, Zeno SA, Remaley AT, Poth M. Should triglycerides and the triglycerides to high-density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metabolism. 2010;59:299–304. doi:10.1016/j.metabol.2009.07.027
26. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi:10.2307/2531595
27. Ioachimescu AG, Brennan DM, Hoar BM, Hoogwerf BJ. The lipid accumulation product and all-cause mortality in patients at high cardiovascular risk: a PreCIS database study. Obesity. 2010;18:1836–1844. doi:10.1038/oby.2009.453
28. Yan G, Li F, Elia C, et al. Association of lipid accumulation product trajectories with 5-year incidence of type 2 diabetes in Chinese adults: a cohort study. Nutr Metab. 2019;16:72. doi:10.1186/s12986-019-0399-7
29. Dong L, Lin M, Wang W, et al. Lipid accumulation product (LAP) was independently associated with obstructive sleep apnea in patients with type 2 diabetes mellitus. BMC Endocr Disord. 2020;20:179. doi:10.1186/s12902-020-00661-x
30. Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34:583–588. doi:10.1016/S0009-9120(01)00263-6
31. Cai G, Shi G, Xue S, Lu W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine. 2017;96:e8058. doi:10.1097/MD.0000000000008058
32. Edwards MK, Blaha MJ, Loprinzi PD. Atherogenic index of plasma and triglyceride/high-density lipoprotein cholesterol ratio predict mortality risk better than individual cholesterol risk factors, among an older adult population. Mayo Clin Proc. 2017;92:680–681. doi:10.1016/j.mayocp.2016.12.018
33. Meszaros M, Tarnoki AD, Tarnoki DL, et al. Obstructive sleep apnea and hypertriglyceridaemia share common genetic background: results of a twin study. J Sleep Res. 2020;29:e12979. doi:10.1111/jsr.12979
34. Ramesh J, Keeran N, Sagahyroon A, Aloul F. Towards validating the effectiveness of obstructive sleep apnea classification from electronic health records using machine learning. Healthcare. 2021;9(11):1450. doi:10.3390/healthcare9111450
35. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479–504. doi:10.5664/jcsm.6506
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
