Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Non-Traditional Blood Lipid Indices for Metabolism Dysfunction-Associated Fatty Liver Disease Prediction in Non-Obese Type 2 Diabetes Mellitus
Authors Gao Q , Feng L, Zhou W , Li X , Yin L , Wang Y
Received 20 April 2023
Accepted for publication 30 July 2023
Published 7 August 2023 Volume 2023:16 Pages 2345—2354
DOI https://doi.org/10.2147/DMSO.S418020
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Muthuswamy Balasubramanyam
Qian Gao, Lei Feng, Weiling Zhou, Xiaoli Li, Lanzi Yin, Yuan Wang
Department of Endocrine and Metabolic Diseases, The Fourth Hospital of Hebei Medical University, Shijiazhuang, 050000, People’s Republic of China
Correspondence: Yuan Wang, Department of Endocrine and Metabolic Diseases, The Fourth Hospital of Hebei Medical University, 12 Jiankang Road, Shijiazhuang City, Hebei Province, People’s Republic of China, Tel +86 18531116018, Email [email protected]
Objective: This study aims to investigate the predictive value of non-traditional blood lipid indices for metabolism dysfunction-associated fatty liver disease (MAFLD) in non-overweight/obese patients with type 2 diabetes mellitus (T2DM).
Methods: A retrospective observational study was conducted, including non-overweight/obese patients with T2DM who visited the Fourth Hospital of Hebei Medical University between August 2018 and August 2022. The low-density lipoprotein cholesterol (LDL-C)/high-density lipoprotein cholesterol (HDL-C) ratio, the triacylglycerol-glucose index (TyG) multiplied by body mass index (BMI), and TyG/HDL-C ratio were calculated.
Results: The study involved 190 participants, of whom 34 were diagnosed with MAFLD (24 males and 10 females), while 156 did not have MAFLD (64 males and 92 females). Multivariable analysis revealed that aspartate transaminase (AST) (OR=1.216, 95% CI: 1.059– 1.374, P=0.006), blood uric acid (BUA) (OR=1.017, 95% CI: 1.002– 1.032, P=0.022), TyG*BMI (OR=1.231, 95% CI: 1.051– 1.442, P=0.010), and TyG/HDL-C (OR=3.162, 95% CI: 1.228– 8.141, P=0.017) were independently associated with MAFLD. The TyG*BMI index exhibited an area under the ROC curve (AUC) of 0.812, with 91.2% sensitivity and 69.2% specificity for MAFLD. The TyG/HDL-C index had an AUC of 0.929, with 85.3% sensitivity and 88.5% specificity for MAFLD.
Conclusion: The results indicate that TyG*BMI and TyG/HDL-C are independently associated with MAFLD in non-overweight/obese patients with T2DM.
Keywords: metabolism dysfunction-associated fatty liver disease, body mass index, type 2 diabetes mellitus, cholesterol, LDL-C, HDL-C, obesity, TyG
Introduction
Metabolism dysfunction-associated fatty liver disease (MAFLD), previously known as nonalcoholic fatty liver disease (NAFLD), is a progressive liver disorder that is closely linked to metabolic disorders, insulin resistance, lipotoxicity, inflammatory cell infiltration, activation of hepatic stellate cells (HSCs), fibrogenesis, and hepatic fat accumulation.1–3 MAFLD encompasses various pathological processes, including simple steatosis, nonalcoholic steatohepatitis (NASH), and fibrosis, with NASH posing a risk factor for the development of hepatocellular carcinoma (HCC). Recent studies have established a strong mechanistic connection between the NASH microenvironment and HCC development, indicating that immune response alterations are closely associated with the progression of NASH and subsequent HCC development.4,5
MAFLD is strongly associated with obesity, type 2 diabetes mellitus (T2DM), and other metabolic disorders, leading to a wide range of complications.2,3 The adoption of the name “MAFLD” instead of “NAFLD” highlights the importance of recognizing the metabolic nature of the disease and emphasizes the potential for therapeutic interventions targeting metabolic pathways to enhance disease awareness and clinical management. However, this change in nomenclature has not affected the disease’s prevalence.6,7 Globally, the prevalence of MAFLD is increasing, affecting approximately 24% to 45% of the global population.3 Furthermore, MAFLD is projected to become the leading cause of liver failure, liver cancer, and liver transplantation.8,9 By categorizing NAFLD as MAFLD, healthcare professionals can better identify the warning signs that increase the risks of morbidity and mortality, particularly when attempting to diagnose metabolic issues at an early stage.10,11
Currently, the clinical detection of fatty liver mainly relies on abdominal ultrasound, which has limitations due to factors such as instrument sensitivity and imaging technology. Liver biopsy is the most accurate diagnostic technique for MAFLD, but it is invasive and challenging to perform.12 Therefore, there is a significant need for non-invasive diagnostic markers that are simple, cost-effective, and highly accurate.
Several studies have revealed strong associations between NAFLD and non-traditional blood lipid parameters, including low-density lipoprotein cholesterol (LDL-C)/high-density lipoprotein cholesterol (HDL-C),13 the triacylglycerol-glucose index (TyG),14 blood uric acid (BUA),15 TyG multiplied by body mass index (BMI),16–18 and TyG/HDL-C.19 These markers have shown potential for predicting NAFLD. Notably, the TyG index has been used as an indicator to assess NAFLD in various research studies, such as the association of insulin resistance, type 2 diabetes mellitus, and NAFLD with bladder cancer.20 However, the extent to which these parameters can accurately predict MAFLD, considering the diverse metabolic considerations involved, remains an open question.13
Patients with NAFLD often have comorbid obesity and type 2 diabetes mellitus,2,3 and previous studies have predominantly focused on obese populations.13–16,19 Limited research has targeted non-obese populations, despite the occurrence of NAFLD in this group.21 Although non-obese NAFLD may be less severe, the metabolic profile of these patients poses a significantly higher risk of developing type 2 diabetes mellitus and metabolic syndrome compared to non-obese individuals without NAFLD.22 Therefore, further attention to this area is warranted to enhance our understanding of the disease and develop more effective treatments and preventive measures.23
The aim of this study was to investigate the predictive value of non-traditional lipid indices for MAFLD in non-overweight/obese patients with type 2 diabetes mellitus. The findings will contribute to the development of clinical diagnosis and treatment strategies for this patient population.
Methods
Study Design and Patients
This retrospective observational study included patients with type 2 diabetes mellitus (T2DM) who visited the Department of Endocrinology at the Fourth Hospital of Hebei Medical University between August 2018 and August 2022. The inclusion criteria were as follows: 1) Age ≥18 years, 2) BMI of 18.5–23.9 kg/m2,17,18 3) Diagnosed with T2DM according to the 1999 WHO criteria,24 and 4) Ultrasound examination indicating fatty liver.6 The exclusion criteria consisted of the following: 1) Patients with acute coronary syndrome, acute cerebral infarction, cerebral hemorrhage, cancer, serious infections, or severe liver or kidney dysfunction, 2) A history of liver operation, or 3) Mental health issues that limited their ability to comply with the study requirements.25
The study was conducted in accordance with the Declaration of Helsinki and received approval from the Ethical Committee of The Fourth Hospital of Hebei Medical University (2022KY384). Due to the retrospective nature of the study, the requirement for informed consent was waived.
Data Collection and Definitions
The researchers collected patient data, including sex, age, height, weight, BMI, duration of T2DM, history of alcohol consumption, history of hypertension, glycated hemoglobin (HbA1c), fasting blood glucose (FBG), alanine aminotransferase (ALT), albumin (ALB), aspartate aminotransferase (AST), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), triglycerides (TG), and blood uric acid (BUA). The LDL-C/HDL-C ratio,13 TyG*BMI (TyG multiplied by BMI),16 and TyG/HDL-C ratio19 were collected and calculated. The TyG index was calculated as ln (fasting triglyceride [mg/dl] × fasting glucose [mg/dl]/2).14
The diagnostic criteria for MAFLD were based on liver biopsy, imaging, and blood biomarkers, combined with one of the following conditions: overweight/obesity, T2DM, and metabolic dysfunction. The criteria included: 1) waist circumference: Asian men ≥90 cm and women >80 cm, 2) blood pressure: ≥130/85 mmHg or receiving blood pressure-lowering medications, 3) blood triglycerides: ≥1.70 mmol/L or receiving lipid-lowering drugs, 4) plasma HDL-C: men <1.0 mmol/L, women <1.3 mmol/L, or receiving lipid-regulating drugs, 5) prediabetes: FBG 5.6–6.9 mmol/L or 2-hour postprandial blood glucose 7.8–11.0 mmol/L or HbA1c 5.7%-6.4%, 6) evaluation of islet resistance index by the steady-state model: ≥2.5, and 7) blood hypersensitive C-reactive protein (hsCRP): >2 mg/L.6,26 In non-overweight/obese populations, patients with fatty liver disease (normal BMI) but with two or more risk factors for metabolic abnormalities should also be considered as diagnostic criteria for MAFLD.6,23
Statistical Analysis
Statistical analysis was conducted using IBM SPSS Statistics version 23.0 (IBM, Armonk, NY, USA). The continuous variables in this study were found to have a non-normal distribution and were presented as medians and quartiles. The Mann–Whitney U-test was used for analysis. Categorical variables were presented as counts and percentages and analyzed using the chi-square test. Multivariable logistic regression analysis was performed to examine the association between MAFLD and LDL-C/HDL-C, TyG*BMI, and TyG/HDL-C. The associations were adjusted for sex, drinking history, duration of diabetes, TG, ALP, AST, and BUA. Variables with a P-value <0.05 in the univariable analyses were entered into the multivariable logistic regression analysis. Receiver operating characteristics (ROC) curve analysis was used to assess and compare the parameters. Statistical significance was defined as a two-tailed P-value <0.05.
Results
This study included a total of 190 participants, with 34 diagnosed with MAFLD (24 males and 10 females, Figure 1). Compared to the non-MAFLD group, the MAFLD group had a higher proportion of males (70.6% vs 41.0%, P=0.002), a higher prevalence of patients with a history of drinking (23.5% vs 10.9%, P=0.048), a longer duration of diabetes (>10 years: 52.9% vs 20.5%, P=0.001), a higher BMI (23.66 vs 21.63 kg/m2, P<0.001), higher levels of LDL-C (3.81 vs 2.79 mmol/L, P=0.034), lower levels of HDL-C (0.72 vs 1.01 mmol/L, P=0.001), higher AST levels (28.1 vs 21.4 U/L), higher levels of alkaline phosphatase (ALP) (141.0 vs 73.7 U/L, P<0.001), higher levels of BUA (378.0 vs 288.8 µmol/L, P<0.001), higher TyG values (9.36 vs 8.99, P<0.001), higher TyG*BMI values (212.55 vs 203.00, P=0.002), higher TyG/HDL-C values (12.61 vs 9.31, P<0.001), and higher LDL-C/HDL-C values (3.96 vs 2.94, P<0.001) (Table 1).
 |
Table 1 Clinical Characteristics of the Patients |
 |
Figure 1 Flowchart of Patients. |
After adjusting for sex, drinking history, duration of diabetes, TG, ALP, AST, and BUA, the multivariable logistic analysis revealed that TyG*BMI (OR=1.231, 95% CI: 1.051–1.442, P=0.010) and TyG/HDL-C (OR=3.162, 95% CI: 1.228–8.141, P=0.017) were independently associated with MAFLD (Table 2).
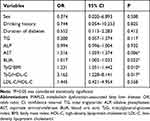 |
Table 2 The Multivariable Logistic Regression of MAFLD |
TyG*BMI demonstrated an area under the ROC curve (AUC) of 0.812, with a sensitivity of 91.2% and specificity of 69.2% for MAFLD. The Youden index for TyG*BMI was 0.604, indicating a cutoff value of 210.9743. For TyG/HDL-C, the AUC was 0.929, with a sensitivity of 85.3% and specificity of 88.5% for MAFLD. The Youden index for TyG/HDL-C was 0.738, corresponding to a cutoff of 10.6706. The Youden index is calculated as sensitivity plus specificity minus one (Table 3 and Figure 2).
 |
Table 3 Predictive Values of the TyG*BMI and TyG/HDL-C |
 |
Figure 2 Receiver-Operating Characteristics (ROC) Curve of Each Parameter for Predicting Metabolic Dysfunction-Associated Fatty Liver Disease. |
The subgroup analysis by sex using multivariate logistic regression demonstrated that TyG/HDL-C (OR=6.129, 95% CI: 1.011-7-1.135, P=0.049) was independently associated with MAFLD in female patients (Table 4). The AUC of TyG/HDL-C for MAFLD in females was 0.892, with a sensitivity of 87.5% and specificity of 78.5%. The Youden index for TyG/HDL-C in females was 0.66, indicating a cutoff value of 10.1509 (Table 5 and Figure 3).
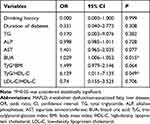 |
Table 4 The Multivariable Logistic Regression of MAFLD in Female Patients |
 |
Table 5 Predictive Values of the TyG/HDL-C |
 |
Figure 3 Gender Subgroup Analysis: ROC Curves for Parameters Predictive of Metabolic Dysfunction-Associated Fatty Liver Disease in Female Patients. |
Discussion
The findings of this study suggest that TyG*BMI and TyG/HDL-C are independently associated with MAFLD in non-overweight/obese patients with T2DM. These parameters exhibit high area under the curve (AUC) values for the diagnosis of MAFLD, indicating their potential as markers for early identification of the disease in this population.
MAFLD is characterized by elevated blood lipid levels, insulin resistance, and increased fasting and postprandial glucose levels.27 Although the prevalence of MAFLD is high, there are currently no specific drugs available for its treatment. Early diagnosis of this common disease is beneficial, considering the promising pipeline of drugs being investigated for the treatment of NAFLD, as demonstrated in recent studies. For instance, studies have shown the potential of SGLT-2 inhibitors in improving NAFLD through autophagy.28–31
The TyG index is widely recognized as a reliable measure of insulin resistance and has been established as an important tool for predicting insulin resistance in various patient groups.32,33 Moreover, it serves as an effective and cost-efficient diagnostic method for NAFLD.14,16 Body mass index (BMI) is commonly used to assess obesity levels and is associated with the likelihood of developing metabolic conditions, including insulin resistance, NAFLD, and other related diseases.34 Previous studies have demonstrated that TyG*BMI, which combines these two indices, is a more accurate indicator of insulin resistance in NAFLD and possesses a high diagnostic value.14,16,35
Furthermore, research has revealed that the ratio of triglycerides to high-density lipoprotein cholesterol (TG/HDL-C) is independently associated with insulin resistance, obesity, and metabolic syndrome. It can also be utilized as a predictive marker for NAFLD.16,19 LDL-C is commonly considered an unfavorable type of cholesterol, while HDL-C is regarded as a beneficial form of cholesterol. A study conducted on Chinese individuals who were neither overweight nor had abnormal cholesterol levels found that LDL-C/HDL-C was a robust indicator of NAFLD, surpassing other blood lipid measurements in terms of accuracy.13 Building upon these findings, the present study further evaluated the aforementioned parameters and compared their diagnostic value and accuracy for MAFLD. The results demonstrated that both TyG*BMI and TyG/HDL-C were capable of identifying MAFLD in this study. Among them, TyG/HDL-C exhibited the highest area under the receiver operating characteristic curve (AUC) with a value of 0.929 (95% CI 0.887–0.973) and a cutoff point of 10.607. This parameter demonstrated 85.3% sensitivity and 88.5% specificity. Subgroup analysis data revealed that TyG/HDL-C was able to identify MAFLD in female participants, yielding an AUC of 0.892 (95% CI 0.784–1.000). However, in male patients, the correlation analysis did not indicate an independent predictive value for these indicators.
The indices evaluated in this study rely on routine biochemistry parameters that do not require rare, difficult-to-obtain, or expensive tests. Their calculation is straightforward and simple, allowing for easy utilization in clinical settings to predict the presence of MAFLD. There is a certain risk of underdiagnosing MAFLD, as the previous nomenclature and diagnosis of NAFLD were based on exclusion criteria, leading to delays in diagnosis and underdiagnosis.36,37 Consequently, the concept of MAFLD was proposed, facilitating a more streamlined diagnosis by incorporating factors known to contribute to fatty liver.38,39 A better understanding of MAFLD and earlier diagnosis should contribute to a more favorable prognosis.40
This study has several limitations. It was conducted at a single center over a limited period, resulting in a relatively small sample size. Furthermore, due to its retrospective nature, establishing causality between MAFLD and the various indicators tested was not possible. Additionally, this study focused on Chinese adults with T2DM, and the diagnostic criteria for MAFLD based on BMI and waist circumference may vary among different nations, thus limiting the generalizability of the findings.
Conclusion
The results suggest that TyG*BMI and TyG/HDL-C were independently associated with MAFLD in non-overweight/obese patients with T2DM. TyG*BMI and TyG/HDL-C showed high AUCs for the diagnosis of MAFLD. These indices might be practical markers for the early identification of MAFLD.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Statement
The study was conducted in compliance with the principles outlined in the Declaration of Helsinki and received approval from the Ethical Committee of The Fourth Hospital of Hebei Medical University (2022KY384). The subjects’ personal information is strictly confidential. Testing blood and recording all other variables included in our analysis are routine, essential for diagnosis and treatment, and are not additional for research purposes, and the data is anonymous. Based on the standardization of program design and execution, we have reason to believe that there will be no adverse impact on the study object. For these reasons, informed written consent was not obtained from each subject.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research is supported by the Department of Science and Technology of Hebei Province of China (No.20221266).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Kuchay MS, Choudhary NS, Mishra SK. Pathophysiological mechanisms underlying MAFLD. Diabetes Metab Syndr. 2020;14(6):1875–1887. doi:10.1016/j.dsx.2020.09.026
2. Marchesini G, Day CP, Dufour JF, et al. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi:10.1016/j.jhep.2015.11.004
3. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. doi:10.1001/jama.2015.5370
4. Ali A, Amin MJ, Ahmed MU, Taj A, Aasim M, Tabrez E. Frequency of non-alcoholic fatty liver disease (NAFLD) and its associated risk factors among type-2 diabetics. Pak J Med Sci. 2022;38(1):28–33. PMID: 35035396; PMCID: PMC8713246. doi:10.12669/pjms.38.1.4968
5. Koo SY, Park EJ, Lee CW. Immunological distinctions between nonalcoholic steatohepatitis and hepatocellular carcinoma. Exp Mol Med. 2020;52(8):1209–1219. PMID: 32770081; PMCID: PMC8080649. doi:10.1038/s12276-020-0480-3
6. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
7. Zheng KI, Fan JG, Shi JP, et al. From NAFLD to MAFLD: a “redefining” moment for fatty liver disease. Chin Med J. 2020;133(19):2271–2273. doi:10.1097/cm9.0000000000000981
8. Yano K, Seko Y, Takahashi A, et al. Effect of sodium glucose cotransporter 2 inhibitors on renal function in patients with nonalcoholic fatty liver disease and type 2 diabetes in Japan. Diagnostics. 2020;10(2):86. doi:10.3390/diagnostics10020086
9. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi:10.1038/nrgastro.2017.109
10. Wong VW, Wong GL, Woo J, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clin Gastroenterol Hepatol. 2021;19(10):2161–2171.e5. doi:10.1016/j.cgh.2020.10.046
11. Zeng J, Qin L, Jin Q, et al. Prevalence and characteristics of MAFLD in Chinese adults aged 40 years or older: a community-based study. Hepatobiliary Pancreat Dis Int. 2022;21(2):154–161. doi:10.1016/j.hbpd.2022.01.006
12. Zhou X, Lin X, Chen J, et al. Clinical spectrum transition and prediction model of nonalcoholic fatty liver disease in children with obesity. Front Endocrinol. 2022;13:986841. PMID: 36120457; PMCID: PMC9471666. doi:10.3389/fendo.2022.986841
13. Zou Y, Zhong L, Hu C, Zhong M, Peng N, Sheng G. LDL/HDL cholesterol ratio is associated with new-onset NAFLD in Chinese non-obese people with normal lipids: a 5-year longitudinal cohort study. Lipids Health Dis. 2021;20(1):28. doi:10.1186/s12944-021-01457-1
14. Khamseh ME, Malek M, Abbasi R, Taheri H, Lahouti M, Alaei-Shahmiri F. Triglyceride glucose index and related parameters (triglyceride glucose-body mass index and triglyceride glucose-waist circumference) identify nonalcoholic fatty liver and liver fibrosis in individuals with overweight/obesity. Metab Syndr Relat Disord. 2021;19(3):167–173. doi:10.1089/met.2020.0109
15. Cui Y, Liu J, Shi H, Hu W, Song L, Zhao Q. Serum uric acid is positively associated with the prevalence of nonalcoholic fatty liver in non-obese type 2 diabetes patients in a Chinese population. J Diabetes Complications. 2021;35(5):107874. doi:10.1016/j.jdiacomp.2021.107874
16. Li N, Tan H, Xie A, et al. Value of the triglyceride glucose index combined with body mass index in identifying non-alcoholic fatty liver disease in patients with type 2 diabetes. BMC Endocr Disord. 2022;22(1):101. doi:10.1186/s12902-022-00993-w
17. Health Management Branch of the Chinese Medical Association CNBotCNS, Association. NaMBotNHIEM. Expert consensus on the weight management process for overweight or obese individuals. Chin J Health Manag. 2021;15(4):317–322.
18. Overweight/Obesity. ECWGoBQMPaPfCIPw. Expert consensus on body quality management pathways and processes for Chinese infertility patients with overweight/obesity. Chin J Reprod Contracept. 2020;40(12):965–971.
19. Babic N, Valjevac A, Zaciragic A, Avdagic N, Zukic S, Hasic S. The triglyceride/HDL ratio and triglyceride glucose index as predictors of glycemic control in patients with diabetes mellitus type 2. Med Arch. 2019;73(3):163–168. doi:10.5455/medarh.2019.73.163-168
20. Tarantino G, Crocetto F, Di Vito C, et al. Association of NAFLD and insulin resistance with non metastatic bladder cancer patients: a Cross-Sectional Retrospective Study. J Clin Med. 2021;10(2):346. PMID: 33477579; PMCID: PMC7831331. doi:10.3390/jcm10020346
21. Zeng J, Yang RX, Sun C, et al. Prevalence, clinical characteristics, risk factors, and indicators for lean Chinese adults with nonalcoholic fatty liver disease. World J Gastroenterol. 2020;26(15):1792–1804. doi:10.3748/wjg.v26.i15.1792
22. Zou ZY, Wong VW, Fan JG. Epidemiology of nonalcoholic fatty liver disease in non-obese populations: meta-analytic assessment of its prevalence, genetic, metabolic, and histological profiles. J Dig Dis. 2020;21(7):372–384. doi:10.1111/1751-2980.12871
23. Yoo JJ, Kim W, Kim MY, et al. Recent research trends and updates on nonalcoholic fatty liver disease. Clin Mol Hepatol. 2019;25(1):1–11. doi:10.3350/cmh.2018.0037
24. World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications-Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization; 1999.
25. Xing Y, Chen J, Liu J, Ma H. Associations between GGT/HDL and MAFLD: a Cross-Sectional Study. Diabetes Metab Syndr Obes. 2022;15:383–394. doi:10.2147/dmso.S342505
26. Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi:10.1053/j.gastro.2019.11.312
27. Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr Rev. 2019;40(5):1367–1393. doi:10.1210/er.2019-00034
28. Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism. 2022;126:154925. PMID: 34740573. doi:10.1016/j.metabol.2021.154925
29. Tarantino G, Balsano C, Santini SJ, et al. It is high time physicians thought of natural products for alleviating NAFLD. Is there sufficient evidence to use them? Int J Mol Sci. 2021;22(24):13424. PMID: 34948230; PMCID: PMC8706322. doi:10.3390/ijms222413424
30. Youssef ME, Yahya G, Popoviciu MS, Cavalu S, Abd-Eldayem MA, Saber S. Unlocking the full potential of SGLT2 inhibitors: expanding applications beyond glycemic control. Int J Mol Sci. 2023;24(7):6039. doi:10.3390/ijms24076039
31. Li L, Li Q, Huang W, et al. Dapagliflozin alleviates hepatic steatosis by restoring autophagy via the AMPK-mTOR pathway. Front Pharmacol. 2021;12:589273. PMID: 34093169; PMCID: PMC8176308. doi:10.3389/fphar.2021.589273
32. Kim JA, Hwang SY, Yu JH, et al. Association of the triglyceride and glucose index with low muscle mass: KNHANES 2008–2011. Sci Rep. 2021;11(1):450. doi:10.1038/s41598-020-80305-1
33. Luo E, Wang D, Yan G, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18(1):150. doi:10.1186/s12933-019-0957-3
34. Brody GH, Yu T, Chen E, Ehrlich KB, Miller GE. Racial discrimination, body mass index, and insulin resistance: a longitudinal analysis. Health Psychol. 2018;37(12):1107–1114. doi:10.1037/hea0000674
35. Malek M, Khamseh ME, Chehrehgosha H, Nobarani S, Alaei-Shahmiri F. Triglyceride glucose-waist to height ratio: a novel and effective marker for identifying hepatic steatosis in individuals with type 2 diabetes mellitus. Endocrine. 2021;74(3):538–545. doi:10.1007/s12020-021-02815-w
36. Pal SC, Méndez-Sánchez N. Screening for MAFLD: who, when and how? Ther Adv Endocrinol Metab. 2023;14:20420188221145650. doi:10.1177/20420188221145650
37. Ando Y, Jou JH. Nonalcoholic fatty liver disease and recent guideline updates. Clin Liver Dis. 2021;17(1):23–28. doi:10.1002/cld.1045
38. Mendez-Sanchez N, Arrese M, Gadano A, et al. The Latin American Association for the Study of the Liver (ALEH) position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol. 2021;6(1):65–72. doi:10.1016/s2468-1253(20)30340-x
39. Méndez-Sánchez N, Bugianesi E, Gish RG, et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol. 2022;7(5):388–390. doi:10.1016/s2468-1253(22)00062-0
40. Yilmaz Y, Byrne CD, Musso G. A single-letter change in an acronym: signals, reasons, promises, challenges, and steps ahead for moving from NAFLD to MAFLD. Expert Rev Gastroenterol Hepatol. 2021;15(4):345–352. doi:10.1080/17474124.2021.1860019
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
