Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 15
Comparative Efficiency for in vitro Transfection of Goat Undifferentiated Spermatogonia Using Lipofectamine Reagents and Electroporation
Authors Nakami WN, Nguhiu-Mwangi J, Kipyegon AN, Ogugo M, Muteti C, Kemp S
Received 12 January 2022
Accepted for publication 8 April 2022
Published 10 May 2022 Volume 2022:15 Pages 11—20
DOI https://doi.org/10.2147/SCCAA.S356588
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Wilkister Nabulindo Nakami,1– 3 James Nguhiu-Mwangi,2 Ambrose Ng’eno Kipyegon,2 Moses Ogugo,1,3 Charity Muteti,1,3 Stephen Kemp1,3
1Livestock Genetics, International Livestock Research Institute, ILRI, Nairobi, Kenya; 2Department of Clinical Studies, Faculty of Veterinary Medicine, University of Nairobi, Nairobi, Kenya; 3Centre for Tropical Livestock Genetics and Health (CTLGH), ILRI, Nairobi, Kenya
Correspondence: Wilkister Nabulindo Nakami, Livestock Genetics, International Livestock Research Institute, ILRI, 30709-00100, Nairobi, Kenya, Tel +254 711 761 459, Email [email protected]; [email protected]
Introduction: Spermatogonial stem cells (SSC), also referred to as undifferentiated spermatogonia, are the germline stem cells responsible for continuous spermatogenesis throughout a male’s life. They are, therefore, an ideal target for gene editing. Previously, SSC from animal testis have been isolated and transplanted to homologous recipients resulting in the successful reestablishment of donor-derived spermatogenesis.
Methods: Enhanced green fluorescent protein (eGFP) gene transfection into goat SSC was evaluated using liposomal carriers and electroporation. The cells were isolated from the prepubertal Galla goats testis cultured in serum-free defined media and transfected with the eGFP gene. Green fluorescing of SSC colonies indicated transfection.
Results: The use of lipofectamineTM stem reagent and lipofectamineTM 2000 carriers resulted in more SSC colonies expressing the eGFP gene (25.25% and 22.25%, respectively). Electroporation resulted in 15% ± 0.54 eGFP expressing SSC colonies. Furthermore, cell viability was higher in lipofectamine transfection (55% ± 0.21) as compared to electroporation (38% ± 0.14).
Conclusion: These results indicated that lipofectamine was more effective in eGFP gene transfer into SSC. The successful transient transfection points to a possibility of transfecting transgenes into male germ cells in genetic engineering programs.
Keywords: eGFP, culture, pre-pubertal
Introduction
Spermatogonial stem cells are the precursor cells that produce spermatozoa. Their ability to proliferate in vitro and reestablish spermatogenesis in recipient testes make them ideal for genetic manipulation.1 Utilization of SSC in the production of genome-edited animals is increasingly becoming an area of research interest.2,3 Intra-testicular transplantation of SSC that carries desired transgenes, can result in the production of donor genotype-derived transgenic spermatozoa.4,5 Transfection of genes into the germline stem cells shortens the generational interval for the production of transgenic animals.6
Previously, viral vectors and carrier molecules transfection techniques have been deployed for gene transfer.4 Despite successful reports of efficient viral transduction, the method risks viral gene transcription and insertion into the host genome.6,7 Non-viral methods, use of liposome carriers -lipofection-and electroporation have been used to transfer the foreign DNA into testicular cells and SSC cells in sheep and bovine.6,8,9
Lipofection has been used to transfer foreign DNA into spermatogonial stem cells.8 The DNA-lipid complex is introduced into the cell culture wells, and the liposome transports the foreign DNA into the cells.10 Bovine SSC have successfully been transfected with eGFP plasmid lipofectamine reagent.8
The electroporation process involves the exposure of cells to high-intensity electric field pulses. This creates temporary pores in the cell membrane through which exogenous DNA diffuse into the cytoplasm, or nucleus.6,11 Electroporation of sheep testicular cells6 and porcine spermatogonial stem cells has been reported.9
There is paucity of data on the comparative efficiency of various transfection methods in goat SSC. Here, we compare the efficiency of eGFP gene transfer through lipofection and electroporation methods.
Materials and Methods
Study Animals
Ten prepubertal Galla bucks aged 3–6 months were obtained from the International Livestock Research Institute (ILRI) farm. Prepubertal bucks are ideal for spermatogonial stem cells isolation.12 The study was approved by the Institutional Animal Care and Use Committees (IACUC Ref no: 2018–15) of the International Livestock Research Institute (ILRI) in Kenya and the University of Nairobi (Ref: FVM BAUEC/2019/243). The handling of the animals adhered strictly to animal welfare considerations by using local anaesthesia recommended for castration and pain management post-surgery.
Testicular Cell Isolation
The prepubertal goats were castrated, and the testes were transported on ice to ILRI laboratory within three hours in Hanks Balanced Salt Solution (HBSS, GibcoTM Grand Island, New York, USA). The transport media was supplemented with 100 IUmL−1 penicillin (Sigma-Aldrich St Louis, Missouri USA) and 100 ug/mL streptomycin (Sigma-Aldrich St Louis, Missouri USA). A testicular cell isolation procedure was performed as described by Oatley et al with minor adaptation.13 Briefly, testes were washed in HBSS and gently disentangled to expose seminiferous tubules. In a water bath at 37°C, 150–200 mg of testicular tissue was digested in 0.25mg/mL of collagenase type IV enzyme (GibcoTM Grand Island, New York, USA) and 7 mg/mL DNase 1(ROCHE Sandhoferstrasse,Mannheim Germany) in HBSS for 5–7 minutes. To eliminate interstitial cells, gravity sedimentation of seminiferous tubules was performed on ice and the supernatant discarded. The sedimentation on ice and washing process was repeated five times.
Digestion of Seminiferous Tubules to Obtain Single-Cell Testicular Population
The seminiferous tubules were incubated in 0.25% Trypsin/0.04 EDTA (GibcoTM Grand Island, New York, USA) and DNase 7 mg/mL in a 37°C water bath for 30 minutes. The trypsin reaction was terminated by adding Foetal Bovine Serum (FBS, GibcoTM Brazil). The cell suspension was passed through a 40μm cell-strainer and washed twice in HBSS through centrifugation. Somatic cells were removed through differential plating on gelatin-coated plates. The somatic cells are attached to the plate bottom. The floating SSC-rich population was collected and washed through centrifugation. The cell pellet was resuspended in Stempro serum-free medium (Gibco™ Grand Island, New York, USA) containing bovine serum albumin with Stempro nutrient supplement (Gibco™ Grand Island, New York, USA) and seeded on 96-well laminin-coated cell culture plates for four days. The medium was refreshed on alternate days. Trypan blue exclusion staining was performed to evaluate the viability of primary isolated cells. The total number of cells present in the suspension was determined using a hemocytometer observed under a light microscope.
Lipofectamine Transfection of SSC
Lipofectamine 2000 (Invitrogen by Life Technologies, Renfrew, United Kingdom) and Lipofectamine Stem (Invitrogen by Life Technologies, Renfrew, UK) reagents were used for transfection. Commercially available Enhanced Green Fluorescent Protein (eGFP) plasmid DNA (NepaGene, Clontech, Japan) was transferred to the cells. The protocol described by Tajik et al was adopted with minor modifications. Briefly, the cells were recovered from the bottom of the culture wells, and clumps were broken up by pipetting gently. Cells were washed and resuspended in Dulbecco's Modified Eagle Medium (DMEM, Sigma-Aldrich St Louis, Missouri USA) serum and antibiotic-free medium, then plated into 24-well cell culture plates (Corning®, Glendale, Arizona USA).
Experimental Groups
There were four experimental groups, treated as follows (Table 1).
 |
Table 1 Description of Culture Experimental Groups |
eGFP Gene Transfer: Lipofectamine Transfection
To transfer eGFP into goat SSC, lipofectamine 2000 DNA transfection reagent and lipofectamine stem reagent liposomal carriers were used. After the four-day culture, the SSC clumps were recovered and plated at a concentration of 0.5–2×105 cells in 500μL of serum-free and antibiotic-free growth medium 24 hours prior to transfection. On the day of transfection, the cells were detached from the bottom of the plate by gently pipetting and washing through centrifugation at 600xg for 7 minutes at 4°C. The cells were then re-suspended in an antibiotic-free, serum-free DMEM medium and seeded on 24 and 96-well culture plates. Various lipofectamine concentrations and DNA were prepared as shown in Tables 2 and 3.
 |
Table 2 Experimental Design for Lipofection with Lipofectamine Stem Reagent |
 |
Table 3 Experimental Design for Lipofection with Lipofectamine 2000 Reagent |
DMEM medium was used to dilute both the lipofectamine reagent and DNA plasmid. The diluted lipofectamine was added to the diluted DNA in a sterile micro-tube and incubated at room temperature for 5–10 minutes. The lipofectamine-DNA complex was added to each well and mixed gently by rocking the plate. The cells were incubated at 37°C in a 5% CO2 incubator for 48 hours before testing for transgene expression. The medium was substituted with a serum-free culture medium after 24 hours. The cells were examined under an inverted fluorescent microscope (EVOS M5000, Thermo Fisher Scientific) using UV radiation of 460–500 nm wavelengths (blue filter) to determine successful gene transfer. Successful gene transfer was indicated by green fluorescence.
Electroporation of Spermatogonial Stem Cells with eGFP
The four-day cultured SSCs were harvested and washed twice by centrifugation at 600g for 7 minutes at 4°C. The cells were counted and re-suspended in ice-cold OPTI-MEM (Gibco™ Grand Island, New York, USA) buffer without serum or antibiotic at a concentration of 1×10^6 cells/in 98μL. A 5µg/ul concentration of enhanced green fluorescent plasmid (eGFP-N1, Clontech, Japan) was added into the tube with cells and mixed gently. The OPTI-MEM buffer with cells was pipetted into 0.4mm gap electroporation cuvettes (Nepa Gene, Japan). The cuvettes were placed into the shocking chamber of the electroporator and, and the parameters were set. After the burst, the cells were placed in a pre-warmed culture medium and incubated at 37°C, 5% CO2 for 48 hours.
Evaluation of eGFP Expression
The transfection was evaluated under a fluorescent microscope (EVOS M5000 Thermo Fisher Scientific, Waltham Massachusetts, USA) with an excitation wavelength of 450–490 nm and emission wavelength of 515 nm.
The cells were harvested gently pipetting and re-suspending in ice-cold antibiotic and Ca/Mg-free PBS. Cells were counted and attached on poly-L-lysine coated slides using a cytospin (Shandon™ Cytospin 4™, Thermo Fisher Scientific Waltham Massachusetts, USA) at a concentration of 30×104 cells per slide. For nuclei staining, the slides were stained with DAPI anti-fade mountant (Thermo Fisher Scientific, Waltham Massachusetts, USA). The mean fluorescence of SSC colonies was evaluated by examining at least 10 microscope fields for quantitative expression of the eGFP gene. Cell images were analysed by Celleste 5.0 image analysis software.
Results
eGFP Gene Transfer to the Spermatogonial Stem Cell Colonies
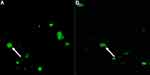
Green fluorescence cell colonies indicated successful eGFP gene transfer (Figures 1A and B). Transfected cells were stained with DAPI (diamidino-2-phenylindole) to exclude non-nuclei staining (Figure 2). Fluorescent colonies were absent in the control group (Figure 3). The SSC colonies incubated with only DNA plasmid without liposome carriers had 0.6% colonies expressing eGFP. In comparison, eGFP expression in SSC colonies incubated with liposome carriers and eGFP plasmid was significantly higher (18–25% eGFP expression, p <0.05) (Table 4 and Figure 4). When the ratio of 1–1.6:2, DNA (2–2.5 μg) to lipofectamine stem or lipofectamine 2000 reagent (4 μL) was used, the percentage of SSC colonies expressing eGFP was 25.25% and 22.25% respectively (Figure 4). Lipofectamine transfection reduced cell viability from an initial live cell percentage of 80% to 55% ± 0.21 viable cells (Tables 4 and 5).
 |
Table 4 Percentage Spermatogonia Stem Cell Colonies Expressing eGFP and Percentage Cell Viability After Transfection with Lipofectamine Stem Reagent as the Gene Carrier |
 |
Table 5 Percentage of Spermatogonia Stem Cell Colonies Expressing eGFP as Well as Percentage Cell Viability After Transfection with Lipofectamine 2000 Reagent as a Gene Carrier |
 |
Figure 2 Blue DAPI (4′,6-diamidino-2-phenylindole) staining of the transfected cells as shown by the white arrow. |
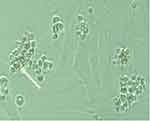 |
Figure 3 Spermatogonial stem cell colonies cultured without DNA and liposomal carriers no expression of green fluorescence as shown by the white arrow. |
Green fluorescing SSC colonies were observed in electroporated cells. (Figure 5). The highest eGFP expression in SSC cells was detected at 100 volts for 2.5 milliseconds (15% ± 0.54), while the lowest expression was at 150 volts for 5 milliseconds groups (2% ± 0.20%) (Table 6). Reduction in the poring pulse voltage to 100 reduced percentage of cell death. Conversely, this improved the gene transfer, indicated by an increase in the percentage of eGFP-expressing colonies from 2% ± 0.20 to 15% ± 0.54.
 |
Table 6 Percentage of Spermatogonia Stem Cell Colonies Expressing eGFP Gene and the Resulting Percentage Cell Viability After Transfection Through Electroporation |
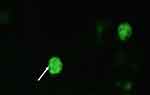 |
Figure 5 Green fluorescence as shown by white arrows signifying eGFP gene transfer to the spermatogonial stem cells through electroporation. |
Lipofectamine transfection yielded a higher percentage of eGFP-expressing SSC colonies than electroporation (Figure 6). In the transfected SSC population, the cell viability was more increased in lipofectamine transfection than electroporation with a mean of 55% ± 0.21 and 38% ± 0.14, respectively.
Discussion
In this study, foreign DNA (pCMV-eGFP) was introduced into the cytosol of enriched and cultured goat SSC through electroporation and lipofectamine reagents, followed by evaluation of transfection efficiency. The spermatogonial stem cells were transfected at the logarithmic growth phase peak, which was shown in a previous report to be the most appropriate stage for foreign gene uptake.8
There was a 0.6% uptake of eGFP plasmid in the SSC cultures incubated with eGFP only, without carrier molecules. This finding agrees with previous findings in which there was 1.6% uptake of eGFP plasmid by SSC when no liposomal carrier was used for transfection.8 Similar results have also shown a lack of uptake of foreign DNA by rodent spermatozoa in the absence of lipid carriers.14 The current study’s findings further confirm that liposomal carriers facilitate the movement of foreign genes across the cell membranes of host cells and incorporation into the host cell genome.
The lipofectamine transfection efficiency was lower than the 37% ± 6.5 reported in bovine SSC using Lipofectamine 2000 carrier.8 Comparatively, the low lipofection transfection efficiency of < 1.5% and survival rates of < 80.0% were reported with porcine SSC.9 The weakening of the SSC membrane resulting from chemical stimulation may contribute to the low survival rates.8
The current study achieved the highest transfection efficiency of 15% with cell viability of 50% after electroporation of goat SSC. This was consistent with the previously reported findings that obtained a transfection efficiency of 25.3% ± 2.4% with a cell viability rate of 78.5%.6 Similarly, the transfection efficiency of > 7.5% and survival rates > 80% for porcine spermatogonial stem cells was reported.9 Another consistent finding was reported in cock spermatogonial stem cells in which 106 cells were efficiently electroporated with 1–1.6 µg of plasmid.15
Electroporation efficiency can be modified by adjusting electrical stimulation parameters, mainly voltage, length of the pulse application, and electric pulse frequency.16,17 Electric stimulation causes the formation of tiny pores on the cell membrane due to the microseconds of cell polarisation. The tiny pores allow large gene constructs and molecules to enter the cell cytosol through simple diffusion.9 The efficiency of transfection using electroporation is lower than that of lipofectamine and the use of viral vectors because of irreversible damage to the cell membranes that occurs in some cells.9 However, the simplicity, buffer-free usage, and the easy use of electroporation necessitate optimisation of its parameters for electroporators that are easy to use and do not require the use of buffer solutions.
In the current study, electroporation resulted in a drastic decrease in cell viability from 80% to 25%. Some of the surviving eGFP expressing cells showed unusual morphological changes in the cell membrane and nuclei fragmentation, suggesting apoptosis. This was similarly reported with electroporation of sheep testicular cells6 and in mouse SSC.18 Apoptosis is caused by irreversible changes in membrane permeability, causing the inflow of ions and molecules leading to cell bursting.19
In conclusion, the use of lipofectamine for transfection of eGFP gene into goat spermatogonial stem cells was more effective than the use of electroporation and resulted in better preservation of cell viability. To our knowledge, this is the first study to compare transient gene transfer into goat SSC using the two methods. The parameters optimised in the study provide a baseline for further research and utilisation of these techniques in gene delivery in genome editing and transgenesis in livestock.
The strength of this study is the comparative indication of the better method for transfection and viability preservation of goat spermatogonial stem cells. At the same time, the limitation was that germline transmission of the eGFP gene from parents to offspring was not carried out. This will be done in a follow-up study.
With optimized culture systems for SSC, these cells can be used for genetic modification and transplanted into multiple surrogate males to produce transgenic semen. This will enable a significant multiplier effect and accelerate the rate of genetic gain.20
Acknowledgments
This study was funded by CGIAR Research Program on Livestock (https://livestock.cgiar.org/) and the Centre for Tropical Livestock Genetics and Health (Grant number: OPP01127286).
Disclosure
The authors report no conflicts of interest in relation to this work and declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Miao XY. Production of transgenic animals using spermatogonial stem cells. Agric Sci China. 2011;10(5):762–768. doi:10.1016/S1671-2927(11)60060-6
2. Niu Y, Liang S. Progress in gene transfer by germ cells in mammals. J Genet Genomics. 2008;35(12):701–714. doi:10.1016/S1673-8527(08)60225-8
3. Shirazi MS, Heidari B, Naderi MM, et al. Transplantation of goat spermatogonial stem cells into the mouse rete testis. Int J Anim Biol. 2015;1(3):61–68.
4. Zeng W, Tang L, Bondareva A, et al. Viral transduction of male germline stem cells results in transgene transmission after germ cell transplantation in pigs. Biol Reprod. 2013;88(1):27. doi:10.1095/biolreprod.112.104422
5. Brinster RL. Germline stem cell transplantation and transgenesis. Science. 2002;296(5576):2174–2176. doi:10.1126/science.1071607
6. Niakan S, Heidari B, Akbari G, Nikousefat Z. Comparison of different electroporation parameters on transfection efficiency of sheep testicular cells. Cell J. 2016;18(3):425–437. doi:10.22074/cellj.2016.4571
7. Whitelaw CBA, Lillico SG, King T. Production of transgenic farm animals by viral vector-mediated gene transfer. Reprod Domest Anim. 2008;43(SUPPL.2):355–358. doi:10.1111/j.1439-0531.2008.01184.x
8. Tajik P, Hoseini Pajooh K, Fazle Elahi Z, et al. Transfection of bovine spermatogonial stem cells in vitro. Iran J Vet Res. 2017;18(2):113–118. doi:10.22099/ijvr.2017.4095
9. Kim MS, Park MH, Park JE, et al. Establishment of an electroporation-mediated gene delivery system in porcine spermatogonial stem cells. Vitro Cell Dev Biol Anim. 2019;55(3):177–188. doi:10.1007/s11626-019-00326-7
10. Garrett FE, Goel S, Yasul J, Koch RA. Liposomes fuse with sperm cells and induce activation by delivery of impermeant agents. Biochim Biophys Acta Biomembr. 1999;1417(1):77–88. doi:10.1016/S0005-2736(98)00258-2
11. Rae JL, Levis RA. Single-cell electroporation. Pflugers Arch Eur J Physiol. 2002;443(4):664–670. doi:10.1007/s00424-001-0753-1
12. Sharma A, Shah SM, Tiwari M, et al. Propagation of goat putative spermatogonial stem cells under growth factors defined serum-free culture conditions. Cytotechnology. 2020;72(3):489–497. doi:10.1007/s10616-020-00386-8
13. Oatley KAV, Yang Q-E, Waqas MS, Oatley JM. Conditions for long-term culture of cattle undifferentiated spermatogonia. Biol Reprod. 2016;95(1):14. doi:10.1095/biolreprod.116.139832
14. Yonezawa T, Furuhata Y, Hirabayashi K, Suzuki M, Yamanouchi K, Nishihara M. Protamine-derived synthetic peptide enhances the efficiency of sperm-mediated gene transfer using liposome-peptide-DNA complex. J Reprod Dev. 2002;48(3):281–286. doi:10.1262/jrd.48.281
15. Kalina J, Kolmanová A, Mikuš T, Mičáková A, Trefil P. Transfection of cock spermatogonial cells via electroporation and lipofection. Czech J Anim Sci. 2003;48(7):279–284.
16. Cukjati D, Batiuskaite D, André F, Miklavčič D, Mir LM. Real time electroporation control for accurate and safe in vivo non-viral gene therapy. Bioelectrochemistry. 2007;70(2):501–507. doi:10.1016/j.bioelechem.2006.11.001
17. Guo H, Hao R, Wei Y. Optimization of electrotransfection conditions of mammalian cells with different biological features. J Membr Biol. 2012;245(12):789–795. doi:10.1007/s00232-012-9480-0
18. Kanatsu-shinohara M, Toyokuni S, Shinohara T. Genetic selection of mouse male germline stem cells in vitro: offspring from single stem cells 1 methods for generating stable animal transgenics or for germline. Biol Reprod. 2005;240(1):236–240. doi:10.1095/biolreprod.104.035659
19. Li LH, McCarthy P, Hui SW. High-efficiency electrotransfection of human primary hematopoietic stem cells. FASEB J. 2001;15(3):586–588. doi:10.1096/fj.00-0447fje
20. Gottardo P, Gorjanc G, Battagin M, et al. A strategy to exploit surrogate sire technology in livestock breeding programs. G3 Genes Genomes Genet. 2019;9(1):203–215. doi:10.1534/g3.118.200890
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.