Back to Journals » Cancer Management and Research » Volume 10
Comparative efficacy of different targeted therapies plus fulvestrant for advanced breast cancer following progression on prior endocrine therapy: a network meta-analysis
Authors Zhang T , Feng F , Zhao W, Yao Y, Tian J, Zhou C, Zang C, Liu C , Wang X, Sun C
Received 13 June 2018
Accepted for publication 19 September 2018
Published 16 November 2018 Volume 2018:10 Pages 5869—5880
DOI https://doi.org/10.2147/CMAR.S176172
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Tingting Zhang,1,* Fubin Feng,2,* Wenge Zhao,3 Yan Yao,3 Jinhui Tian,4 Chao Zhou,2 Chuanxin Zang,1 Cun Liu,1 Xue Wang,5 Changgang Sun2,6
1College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, People’s Republic of China; 2Department of Oncology, Weifang Traditional Chinese Hospital, Weifang, Shandong, People’s Republic of China; 3Clinical Medical College, Weifang Medical University, Weifang, Shandong, People’s Republic of China; 4Evidence-Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu, People’s Republic of China; 5Medical Colleges, Qingdao University, Qingdao, Shandong, People’s Republic of China; 6Department of Oncology, Affiliated Hospital of Weifang Medical University, Weifang, Shandong, People’s Republic of China
*These authors contributed equally to this work
Background: We performed a network meta-analysis of randomized controlled trials (RCTs) to indirectly compare the efficacy of different targeted agents with fulvestrant for patients with hormone-receptor-positive (HR+) and human epidermal growth factor receptor type 2-negative (HER2–) advanced breast cancer (ABC) following progression on prior endocrine therapy.
Methods: The titles/abstracts were searched from the PubMed, EMBASE, and the Cochrane Library databases for RCTs to evaluate the efficacy of palbociclib plus fulvestrant vs alternative targeted therapies plus fulvestrant for postmenopausal HR+/HER2– ABC following progression on prior endocrine therapy. In addition, the primary measured outcome was progression-free survival (PFS) and objective response rate. The surface under the cumulative ranking (SUCRA) value of each treatment was calculated to achieve the best ranking for each treatment.
Results: A total of 11 studies, including 4,178 patients in the network meta-analysis, were included and analyzed. In terms of the pooled hazard ratios (HRs) for PFS, palbociclib plus fulvestrant was superior to other target agents plus fulvestrant (HR=0.62, 95% credible interval [CrI]: 0.40–0.96; HR=0.62, 95% CrI: 0.47–0.96; for pictilisib plus fulvestrant and buparlisib plus fulvestrant, respectively). Ribociclib plus fulvestrant has no difference in abemaciclib plus fulvestrant and palbociclib plus fulvestrant (HR =1.02, 95% CrI =0.72–1.45; HR =1.22, 95% CrI =0.84–1.78). In terms of objective response rate, compared with placebo plus fulvestrant, abemaciclib plus fulvestrant, dovitinib plus fulvestrant, buparlisib plus fulvestrant, and palbociclib plus fulvestrant had a significant difference (odds ratio [OR] =2.84, 95% CrI =1.91– 4.31; OR =3.62, 95% CrI =1.21–12.48; OR =1.80, 95% CrI =1.25–2.60; and OR =2.52, 95% CrI =1.43– 4.72, respectively).
Conclusion: According to the present study, palbociclib plus fulvestrant may be the optimal treatment for HR+/HER2– postmenopausal women with ABC after disease progression following endocrine therapy.
Keywords: advanced breast cancer, endocrine therapy, targeted therapy, progression-free survival, objective response rate, network meta-analysis
A Letter to the Editor has been received and published for this article.
Introduction
Breast cancer is the most common malignant disease in women and its incidence increases in postmenopausal individuals.1 Among the postmenopausal patients with advanced breast cancer (ABC; locally advanced or metastatic), the majority are hormone receptor positive (HR+) and human epidermal growth factor receptor type 2 negative (HER2–).2–4 At present, endocrine therapy plays a crucial part in HR+/HER2– ABC. There are three types of commonly used endocrine therapy drugs: selective estrogen-receptor modulators, aromatase inhibitors (AIs), and selective estrogen-receptor downregulators.5 Previous meta-analysis have demonstrated that AIs are more effective than tamoxifen in postmenopausal women with ABC in terms of objective response rate (ORR) and complete response (CR).6 However, therapeutic options for patients who failed after tamoxifen or AI treatment are unclear. Fulvestrant is a selective estrogen-receptor downregulator different from other endocrine agents. It binds with 100-fold greater affinity than tamoxifen, and in terms of inhibiting estrogen signaling, it is more effective than tamoxifen and AIs.7–9 These evidence suggested that it could be a better platform for combination with other targeted pathways. The Phase III EFECT trial showed that fulvestrant loading dose (loading dose is 500 mg on day 0, 250 mg on days 14 and 28, and 250 mg every 28 days thereafter) was as effective as exemestane for postmenopausal women with ABC who have experienced progression or recurrence during treatment with a nonsteroidal AIs.10 The CONFIRM and FINDER2 trials demonstrated that fulvestrant 500 mg has efficacy superior to fulvestrant 250 mg in treatment for estrogen-receptor positive (ER+) ABC with progression after previous endocrine therapy.11–13 Therefore, for patients who experience disease progression after tamoxifen or AI therapy, fulvestrant could be a second-line therapeutic option.14 Fulvestrant monotherapy was used to treat HR+ ABC patients with good tolerance but limited efficacy.11,15 With the application of fulvestrant in HR+/HER2– ABC patients, numerous new targeted agents in combination with fulvestrant are in clinical development, providing therapeutic options for patients with endocrine resistance. For instance, the combination of palbociclib and fulvestrant was associated with greater median progression-free survival (PFS) compared with fulvestrant plus placebo (9.5 vs 4.6 months; hazard ratio [HR] =0.46, 95% CI =0.36–0.59, P<0.0001) for patients with metastatic breast cancer who had progressed on previous endocrine therapy.15 Furthermore, the Food and Drug Administration approved palbociclib for use in combination with fulvestrant for the treatment of women with HR+/HER2– advanced or metastatic breast cancer with disease progression following endocrine therapy.16 Undeniably, previous network meta-analysis17 has compared the efficacy of endocrine-based therapies following progression on nonsteroidal AI in patients with postmenopausal HR+/HER2– metastatic breast cancer. The result strongly demonstrated that in this circumstance, patients who received palbociclib plus fulvestrant, everolimus plus AI, or everolimus plus fulvestrant prolonged PFS compared with those who received fulvestrant or AI alone.
So far, there has been no direct comparison between palbociclib plus fulvestrant vs other targeted therapies plus fulvestrant for patients with HR+ ABC following progression or recurrence on prior endocrine therapy. Thus, a summary of these trials is needed. The purpose of the present study was to perform a network meta-analysis of the existing literature to indirectly compare the efficacy of different targeted agents with fulvestrant as a second-line therapy for HR+/HER2– ABC.
Materials and methods
Literature and search strategy
The network meta-analysis was designed, and the trial was reported, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension to network meta-analysis.18
We searched the titles/abstracts from the following databases inception to June 5, 2018: PubMed, EMBASE, and the Cochrane Library. We used the Medical Subject Headings/Emtree terms combined with keywords properly adjusted for the different databases in all the search strategies. Detailed information on the search strategies of different databases is provided in Supplementary materials. The network meta-analysis was only restricted to articles of randomized controlled trials (RCTs) published in the English language. For a more comprehensive search strategy and to identify more relevant literature, we manually searched the reference lists of multiple articles, including published meta-analysis and reviews.
Selection criteria
Included participants were HR+/HER2– women with ABC who have progressed or recurrence after previous endocrine therapy or targeted therapy. The type of intervention was fulvestrant plus any of the following treatments: palbociclib, abemaciclib, buparlisib, dovitinib, ribociclib, vandetanib, and everolimus, pictilisib, bortezomib, selumetinib, and placebo. Eligible studies were RCTs that assessed the effectiveness of fulvestrant plus any targeted therapy. The main outcomes were PFS and ORR. Studies involving loading dose of fulvestrant (loading dose is 500 mg on day 0, 250 mg on days 14 and 28, and 250 mg every 28 days) were excluded. We also excluded these studies of fulvestrant plus targeted therapy used as adjuvant treatment. If several articles were based on the same trial, then only the most informative study and/or the primary publication of the results was included in the present network meta-analysis.
Two investigators (TT Zhang and FB Feng), working independently, scanned all titles and abstracts, excluding obviously unmatched articles, and the remaining full texts were read for further identification. For any discrepancies between authors, a third author provided arbitration (Y Yao).
Data extraction and quality assessments
Two authors (CX Zang and WG Zhao) independently extracted the data from eligible studies by using a predefined and standard data form based on Excel spreadsheet. Data were extracted on an intention-to-treat basis. For crossover trials, only first period data were extracted. The information of studies that met the inclusion criteria was extracted as follows: first author’s name, year of publication, pathway inhibited, disease stage, characteristics of trial participants (median age, postmenopausal status, HR status, prior endocrine therapy, Eastern Cooperative Oncology Group performance status, median follow-up), type of treatment (type, dose, duration, and frequency), and primary outcomes. The primary outcomes in the present study were PFS and ORR. After extracting all the information, the two authors crosschecked the extracted information to guarantee accuracy of the information.
The quality of studies was evaluated by two authors (Y Yao and WG Zhao) by using the Cochrane Collaboration’s tool for assessing risk of bias evaluating six domains19: 1) selection bias, 2) performance bias, 3) detection bias, 4) attrition bias, 5) reporting bias, and 6) other bias. The studies were judged as unclear, high risk, or low risk of bias. Two authors independently extracted the data and assessed risk of bias, and any discrepancies between the two authors were resolved by consensus through discussion.
Statistical methods
The HR was ultimately utilized for pooling effect sizes because the outcome was time-to-event outcomes. We mainly derived the digitized HR and the corresponding of 95% CI from publications. If the HR is not given in the publications directly, the Engauge Digitizer 4.1 (http://digitizer.sourceforge.net/) is used to extract the survival information from the Kaplan–Meier curve.20 Additionally, the odds ratio (OR) was utilized for pooling effect sizes for ORR. If only the percentage of ORR is reported in the article, we need to convert it to decimal to carry out four rounds of five entries.
To ensure that all data were normally distributed, we used the log HR for analysis and a Bayesian approach to evaluate efficacy, according to Welton et al.21 It applies to ORR as well. Furthermore, the model parameter was conducted using the Markov chain Monte Carlo technique with WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK). The WinBUGS sampler was run with three chains using randomly different chosen initial values for 10,000 iterations after a burn-in of 5,000. According to the Deviance Information Criteria (DIC) value, we selected a fixed model or random model. The smaller the DIC value, the more suitable the model.
The summary treatment effect size (HR or OR), as a point estimate, was considered as the median of the posterior distribution, and a 95% credible interval (CrI), which derived from the 2.5 and 97.5 percentiles, was presented. We judged whether it was meaningful or not according to whether the 95% CrI was included. In addition, we adopted the surface under the cumulative ranking (SUCRA) value to rank all treatments. Finally, we used these SUCRAs to determine which is the best treatment. The SUCRA is closer to 100, indicating that it is in the first place. If a loop connecting three arms existed, a node-splitting approach was adopted to assess the inconsistency among direct and indirect evidence.
The risk of bias in individual studies was assessed by Review Manager, version 5.3 (The Nordic Cochrane Centre: The Cochrane Collaboration, Copenhagen, Norway). A network of different interventions was plotted, and the SUCRA was calculated by STATA, version 13.0 (Stata Corporation, College Station, TX, USA).
Results
Overview of the literature search
A total of 2,627 articles were identified, among which 207 records were duplicates. After screening the titles and abstracts, 2,369 studies were discarded because they obviously did not meet the predefined inclusion criteria. By reviewing the full text, we further excluded 41 publications for the following reasons: 17 papers were derived from one study, 1 paper was repetitive, 19 papers did not report relevant outcomes on data, 3 papers used the loading dose of fulvestrant, and 1 paper had no appropriate participants. Furthermore, we obtained an eligible paper by tracking relevant references manually. Ultimately, 11 eligible papers were included in the present study.15,22–31 The details of the study selection and the results are shown in Figure 1.
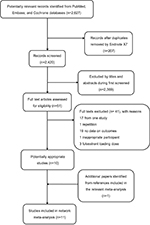  | Figure 1 Flowchart for search results and selection details. |
Characteristics of included studies
All trials were multicenter and only had two groups. The 11 papers included 11 trials and comprised 4,178 patients with advanced or locally advanced or metastatic breast cancer. The majority of trials are compared with placebo plus fulvestrant; however, one30 of the trials is compared with fulvestrant alone. The publication time of the included studies ranged from 2014 to 2018. The median ages were between 56 and 69 years across all studies. The percentage of ER+/progesterone-receptor positive patients ranged from 10% to 100%. Moreover, regarding the percentage of postmenopausal status, only two papers reported the specific percentage, which ranged from 79% to 83.2%, and the remaining papers did not report the specific percentage. In previous endocrine therapy, the included participants had received AIs and/or tamoxifen therapy. The median PFS is from 2.69 to 20.3 months. The characteristics of the eligible studies are summarized in Table 1. Detailed information is shown in Supplementary materials.
The assessment of the risk of bias
The risk of bias of individual studies included in the present network meta-analysis is presented in Figure 2. In general, the included studies were high quality in methodological. The majority of studies had reported random sequence generation. In addition to one study, the remaining studies did not explicitly propose specific ways to allocate concealment. Eight trials were funded by pharmaceutical companies. All included studies did not report selectively.
  | Figure 2 Cochrane risk of bias tool assessment (green: low risk of bias; red: high risk of bias; and yellow: unclear risk of bias). |
Network meta-analysis
The evidence network of eligible comparisons is shown in Figure 3, of which the network of PFS is shown in Figure 3A, and the network of ORR is shown in Figure 3B. The combination of fulvestrant plus placebo was the most frequently investigated regimen (11 comparisons). This regimen was respectively compared with abemaciclib plus fulvestrant, dovitinib plus fulvestrant, everolimus plus fulvestrant, buparlisib plus fulvestrant, pictilisib plus fulvestrant, palbociclib plus fulvestrant, vandetanib plus fulvestrant, ribocilib plus fulvestrant, bortezomib plus fulvestrant, and selumetinib plus fulvestrant. As is demonstrated in Figure 4, there is no closed loop. Therefore, consistency check is not performed.
  | Figure 3 Network of eligible comparisons for network meta-analysis for PFS (A) and ORR (B). Abbreviations: FP, placebo+fulvestrant; ORR, objective response rate; PFS, progression-free survival. |
PFS was reported as a primary outcome in all 11 studies. The network meta-analysis results were based on a fixed-effect model because there was no significant difference of the DIC between the fixed-effects model (DIC =1.954) and the random-effects model (DIC =2.779). Among which, compared with placebo plus fulvestrant, palbociclib plus fulvestrant, ribociclib plus fulvestrant, abemaciclib plus fulvestrant, everomilus plus fulvestrant, and buparlisib plus fulvestrant had a significant advantage (HR =0.46, 95% CrI =0.36–0.59; HR =0.56, 95% CrI =0.43–0.74; HR =0.55, 95% CrI =0.45–0.68; HR =0.61, 95% CrI =0.40–0.93; and HR =0.74, 95% CrI =0.66–0.84, respectively). Palbociclib plus fulvestrant was superior to other target agents plus fulvestrant (HR =0.62, 95% CrI =0.40–0.96; HR =0.62, 95% CrI =0.47–0.96 for pictilisib plus fulvestrant and buparlisib plus fulvestrant, respectively). Burparlisib plus fulvestrant and vandetanib plus fulvestrant were inferior to abemaciclib plus fulvestrant (HR =1.34, 95% CrI =1.06–1.71 and HR =1.69, 95% CrI =1.10–2.62, respectively). Ribociclib plus fulvestrant has no difference in abemaciclib plus fulvestrant and palbociclib plus fulvestrant (HR =1.02, 95% CrI =0.72–1.45; HR =1.22, 95% CrI =0.84–1.78). ORR was reported as a primary outcome in all eight studies. The network meta-analysis results were based on fixed-effect model (DIC =101.099) rather than random-fixed model (DIC =101.299). Abemaciclib plus fulvestrant, dovitinib plus fulvestrant, buparlisib plus fulvestrant, and palbociclib plus fulvestrant had a significant difference (OR =2.84, 95% CrI =1.91–4.31; OR =3.62, 95% CrI =1.21–12.48; OR =1.80, 95% CrI =1.25–2.60; and OR =2.52, 95% CrI =1.43–4.72, respectively) when compared with placebo plus fulvestrant. Selumetinib plus fulvestrant was inferior to abemaciclib plus fulvestrant, dovitinib plus fulvestrant, and palbociclib plus fulvestrant (OR =0.08, 95% CrI =0.00–0.80; OR =0.06, 95% CrI =0.00–0.77; and OR =0.08, 95% CrI =0.00–0.92, respectively). More network meta-analysis results of PFS and ORR are summarized in Table 2.
The SUCRAs for the outcomes of PFS in the network meta-analysis are shown in Figure 4A. Moreover, the size of SUCRA value determined which treatment is the best. Palbociclib plus fulvestrant had the biggest SUCRA (SUCRA =94.2%), suggesting that this regimen may be the optimal treatment in terms of PFS. The second highest percentage of treatment was the combination of abemaciclib and fulvestrant (SUCRA =78.1%), followed by ribociclib plus fulvestrant (SUCRA =75.3%), everolimus plus fulvestrant (SUCRA =66.3%), and dovitinib plus fulvestrant (SUCRA =53.4%). The SUCRAs for the outcomes of ORR in the network meta-analysis are shown in Figure 4B. In terms of ORR, dovitinib plus fulvestrant may be the optimal treatment because it has the biggest SUCRA (SUCRA =84.7%). The ranks of second and third are abemaciclib and fulvestrant (SUCRA =80.6%) and palbociclib plus fulvestrant (SUCRA =73.0%).
Discussion
The use of endocrine therapy for the treatment of HR+/HER2– postmenopausal women with ABC has been well established. Clinically, resistance to endocrine therapies is also common in HR+/HER2− disease, and most patients inevitably face disease progression.32 However, for postmenopausal women with HR+/HER2– ABC who previously failed after prior endocrine therapy, the optimal sequence of endocrine treatment remains unclear. Here, we performed a network meta-analysis on the efficacy of the available studies involving different targeted therapy with fulvestrant as a second-line therapy for HR+/HER2– ABC.
According to our network meta-analysis, we derived the following findings in the present analysis: the combination of palbociclib plus fulvestrant significantly improved PFS when compared with fulvestrant plus placebo and abemaciclib plus fulvestrant also improved PFS when compared with fulvestrant plus placebo, but to a lesser degree. However, palbociclib plus fulvestrant had not significantly prolonged PFS comparing with abemaciclib plus fulvestrant. As far as we are concerned, dovitinib plus fulvestrant was more effective than other targeted therapies plus fulvestrant in terms of ORR.
In this case, the combination of endocrine therapy and targeted agents (eg, palbociclib, abemaciclib) to block cell signaling pathways that interact with the ER increased clinical benefits compared with single endocrine therapy.15,31,33 Furthermore, a previous report indicated that estrogen activity and cyclin-dependent kinase (CDK) 4/6/retinoblastoma protein pathway activity are closely related.34 Palbociclib, a selective CDK4/6 inhibitor, preferentially inhibited the proliferation of ER+ cancer cells in preclinical studies.35 A Phase III randomized trial indicated that the combination of palbociclib and fulvestrant could be considered as an option for patients with HR+/HER2– ABC who have progressed on prior endocrine therapy.15 Moreover, in a previous network meta-analysis, we found that the combination of palbociclib plus fulvestrant was associated with longer PFS than single endocrine therapies, such as anastrozole, letrozole, exemestane, and megestrol acetate.36 Following failure on previous endocrine therapy, many patients are offered chemotherapy as a second-line therapeutic option.3 However, the combination of palbociclib plus fulvestrant also improved PFS relative to all other chemotherapy treatments.37 Furthermore, endocrine therapy has a lower toxicity than chemotherapy. But due to the same signal pathway of palbociclib and abemeciclib, they may be no significant difference in prolonging PFS..
In addition, dovitinib plus fulvestrant is more effective than other targeted therapis plus fulvestrant. Dovitinib belongs to a small molecule inhibitor of fibroblast growth factor receptor 1 (FGFR1), FGFR2, and FGFR3.38 The abnormal regulation of FGF and FGFR signals is associated with oncogenesis activity,39 increases the risk of developing breast cancer,40–42 and is related to the resistance to endocrine therapy.43 In addition to inhibiting cell proliferation, dovitinib also showed antineoplastic activity in FGFR-amplified xenograft models.44 This difference in signal transduction pathway may be responsible for the difference between dovitinib and palbociclib on the basis of ORR.
Previous meta-analysis showed that fulvestrant plus targeted therapies showed ORR and PFS benefit in patients with ABC.45 However, different doses of fulvestrant on the effects of combined therapy were not considered in that study, and as a result, there may be greater heterogeneity. In the present study, we only included trials on high dose of fulvestrant, which ruled out the effect of different doses of fulvestrant on results. Furthermore, in terms of PFS and ORR, we ranked different targeted agents plus fulvestrant to determine the optimal treatment. To our knowledge, there are currently no head-to-head trials evaluating the efficacy of different targeted therapies in combination with fulvestrant in patients who experienced disease progression on prior endocrine therapy. The present network meta-analysis indirectly compared different targeted therapies in combination with fulvestrant. Thus, the present study could provide the higher level of evidence for physicians and patients. The network meta-analysis provided an insight into the use of fulvestrant combined with different targeted agents for patients with HR+/HER2– ABC following failure on prior endocrine therapy. However, there are also several limitations in the present study. First, for all treatment comparisons in the present network meta-analysis, no direct evidence was available and thus, it was impossible to evaluate incoherence (ie, the extent of disagreement between direct and indirect evidence). Second, adverse events of targeted agents varied across trials. The assessment of adverse events is relative to the acceptability of patients, and unfortunately, the collected data did not facilitate an evaluation of the adverse events; thus, the toxicity of the targeted agents was ultimately not evaluated. Third, only a limited number of regimens (n=11) and trials (n=11) were included, and the combination of palbociclib plus fulvestrant has only been reported in one study, thereby weakening the validity of the present analysis.
Conclusion
The present study showed that palbociclib plus fulvestrant may be the optimal treatment for HR+/HER2– postmenopausal women with ABC after disease progression following endocrine therapy. However, direct comparisons are still needed to examine differences among different targeted agents plus fulvestrant.
Acknowledgments
The authors would like to acknowledge Dr Shengjie Dong and all the members of Department of Oncology of Weifang Traditional Chinese Hospital.
This study was supported by grants from the National Natural Science Foundation of China (numbers 81473513 and 81673799).
Author contributions
Changgang Sun, Tingting Zhang, and Fubin Feng were involved in the concept and design of the study. Tingting Zhang drafted the manuscript. All authors were involved in acquisition, analysis, and interpretation of the data, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Stat Fact Sheets SE. Female breast cancer; 2017. Available from: seer.cancer.gov/statfacts/html/Breast/. Assessed January 16, 2018. | ||
Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Ann Oncol. 2014;25(10):1871–1888. | ||
Migliaccio I, Malorni L, Hart CD, Guarducci C, Di Leo A. Endocrine therapy considerations in postmenopausal patients with hormone receptor positive, human epidermal growth factor receptor type 2 negative advanced breast cancers. BMC Med. 2015;13:46. | ||
Joy AA, Ghosh M, Fernandes R, Clemons MJ. Systemic treatment approaches in her2-negative advanced breast cancer-guidance on the guidelines. Curr Oncol. 2015;22(Suppl 1):S29–S42. | ||
Fan W, Chang J, Fu P. Endocrine therapy resistance in breast cancer: current status, possible mechanisms and overcoming strategies. Future Med Chem. 2015;7(12):1511–1519. | ||
Xu HB, Liu YJ, Li L. Aromatase inhibitor versus tamoxifen in postmenopausal woman with advanced breast cancer: a literature-based meta-analysis. Clin Breast Cancer. 2011;11(4):246–251. | ||
Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396–3403. | ||
Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20(16):3386–3395. | ||
Howell A, Robertson JF, Abram P, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004;22(9):1605–1613. | ||
Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26(10):1664–1670. | ||
di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594–4600. | ||
Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337. | ||
Pritchard KI, Rolski J, Papai Z, et al. Results of a phase II study comparing three dosing regimens of fulvestrant in postmenopausal women with advanced breast cancer (FINDER2). Breast Cancer Res Treat. 2010;123(2):453–461. | ||
Cope S, Ouwens MJ, Jansen JP, Schmid P. Progression-free survival with fulvestrant 500 mg and alternative endocrine therapies as second-line treatment for advanced breast cancer: a network meta-analysis with parametric survival models. Value Health. 2013;16(2):403–417. | ||
Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. | ||
Walker AJ, Wedam S, Amiri-Kordestani L, et al. FDA approval of palbociclib in combination with fulvestrant for the treatment of hormone receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res. 2016;22(20):4968–4972. | ||
Ayyagari R, Tang D, Patterson-Lomba O, et al. Progression-free survival with endocrine-based therapies following progression on non-steroidal aromatase inhibitor among postmenopausal women with hormone receptor positive, human epidermal growth factor receptor-2 negative metastatic breast cancer: a network meta-analysis. Curr Med Res Opin. 2018;34(9):1645–1652. | ||
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. | ||
Higgins JP, Altman DG, Gotzsche PC, et al; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. | ||
Zhou ZR, Zhang TS, Li B, et al. Extracting and transforming of appropriate data of meta-analysis in survival curve. Chin J Evid Based Cardiovasc Med. 2014;6:243–247. | ||
Welton NJ, Sutton AJ, Cooper NJ, Abrams KR, Ades AE. Evidence synthesis for decision making in healthcare. Chichester (UK): John Wiley & Sons, Inc.; 2012. | ||
Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. | ||
Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2– advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. | ||
Di Leo A, Johnston S, Lee KS, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(1):87–100. | ||
Baselga J, Im SA, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(7):904–916. | ||
Zaman K, Winterhalder R, Mamot C, et al. Fulvestrant with or without selumetinib, a MEK 1/2 inhibitor, in breast cancer progressing after aromatase inhibitor therapy: a multicentre randomised placebo-controlled double-blind phase II trial, SAKK 21/08. Eur J Cancer. 2015;51(10):1212–1220. | ||
Musolino A, Campone M, Neven P, et al. Phase II, randomized, placebo-controlled study of dovitinib in combination with fulvestrant in postmenopausal patients with HR+, HER2– breast cancer that had progressed during or after prior endocrine therapy. Breast Cancer Res. 2017;19(1):18. | ||
Krop IE, Mayer IA, Ganju V, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17(6):811–821. | ||
Clemons MJ, Cochrane B, Pond GR, et al. Randomised, phase II, placebo-controlled, trial of fulvestrant plus vandetanib in postmenopausal women with bone only or bone predominant, hormone-receptor-positive metastatic breast cancer (MBC): the OCOG ZAMBONEY study. Breast Cancer Res Treat. 2014;146(1):153–162. | ||
Adelson K, Ramaswamy B, Sparano JA, et al. Randomized phase II trial of fulvestrant alone or in combination with bortezomib in hormone receptor-positive metastatic breast cancer resistant to aromatase inhibitors: a New York Cancer Consortium trial. NPJ Breast Cancer. 2016;2:16037. | ||
Kornblum N, Zhao F, Manola J, et al. Randomized Phase II trial of fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer resistant to aromatase inhibitor therapy: results of PrE0102. J Clin Oncol. 2018;36(16):1556–1563. | ||
Wilson S, Chia SK. Treatment algorithms for hormone receptor-positive advanced breast cancer: applying the results from recent clinical trials into daily practice-insights, limitations, and moving forward. Am Soc Clin Oncol Educ Book. 2013;33:e20–e27. | ||
Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res Treat. 2017;166(1):41–54. | ||
Butt AJ, McNeil CM, Musgrove EA, Sutherland RL. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: the potential roles of c-Myc, cyclin D1 and cyclin E. Endocr Relat Cancer. 2005;12 Suppl 1(Suppl 1):S47–S59. | ||
Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. | ||
Chirila C, Mitra D, Colosia A, et al. Comparison of palbociclib in combination with letrozole or fulvestrant with endocrine therapies for advanced/metastatic breast cancer: network meta-analysis. J Cell Biochem. 2017;33(8):1457–1466. | ||
Wilson FR, Varu A, Mitra D, Cameron C, Iyer S. Systematic review and network meta-analysis comparing palbociclib with chemotherapy agents for the treatment of postmenopausal women with HR-positive and HER2-negative advanced/metastatic breast cancer. Breast Cancer Res Treat. 2017;166(1):167–177. | ||
Lopes de Menezes DE, Peng J, Garrett EN, et al. CHIR-258: a potent inhibitor of FLT3 kinase in experimental tumor xenograft models of human acute myelogenous leukemia. Clin Cancer Res. 2005;11(14):5281–5291. | ||
Heinzle C, Sutterlüty H, Grusch M, Grasl-Kraupp B, Berger W, Marian B. Targeting fibroblast-growth-factor-receptor-dependent signaling for cancer therapy. Expert Opin Ther Targets. 2011;15(7):829–846. | ||
Easton DF, Pooley KA, Dunning AM, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447(7148):1087–1093. | ||
Ruiz-Narváez EA, Haddad SA, Lunetta KL, et al. Gene-based analysis of the fibroblast growth factor receptor signaling pathway in relation to breast cancer in African American women: the AMBER consortium. Breast Cancer Res Treat. 2016;155(2):355–363. | ||
André F, Cortés J. Rationale for targeting fibroblast growth factor receptor signaling in breast cancer. Breast Cancer Res Treat. 2015;150(1):1–8. | ||
Rugo H, Vidula N, Ma C. Improving response to hormone therapy in breast cancer: new targets, new therapeutic options. Am Soc Clin Oncol Educ Book. 2016;35:e40–e54. | ||
André F, Bachelot T, Campone M, et al. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res. 2013;19(13):3693–3702. | ||
Lin WZ, Xu QN, Wang HB, Li XY. Fulvestrant plus targeted agents versus fulvestrant alone for treatment of hormone-receptor positive advanced breast cancer progressed on previous endocrine therapy: a meta-analysis of randomized controlled trials. Breast Cancer. 2017;24(3):345–352. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



