Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Comparative Effectiveness of Umeclidinium/Vilanterol versus Indacaterol/Glycopyrronium on Moderate-to-Severe Exacerbations in Patients with Chronic Obstructive Pulmonary Disease in Clinical Practice in England
Authors Requena G , Czira A , Banks V , Wood R , Tritton T, Castillo C , Yeap J, Wild R , Compton C , Rothnie KJ , Herth FJ , Quint JK , Ismaila AS
Received 9 March 2023
Accepted for publication 31 August 2023
Published 15 September 2023 Volume 2023:18 Pages 2039—2054
DOI https://doi.org/10.2147/COPD.S408688
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jill Ohar
Gema Requena,1 Alexandrosz Czira,1 Victoria Banks,2 Robert Wood,2 Theo Tritton,2 Catherine Castillo,2 Jie Yeap,2 Rosie Wild,2 Chris Compton,1 Kieran J Rothnie,1 Felix JF Herth,3 Jennifer K Quint,4 Afisi S Ismaila5,6
1GSK, R&D Global Medical, Brentford, Middlesex, UK; 2Real-World Evidence, Adelphi Real World, Bollington, Cheshire, UK; 3Department of Pneumology and Critical Care Medicine, Thoraxklinik, University of Heidelberg and Translational Lung Research Center Heidelberg, Heidelberg, Germany; 4National Heart and Lung Institute, Imperial College London, London, UK; 5Value Evidence and Outcomes, GSK, Collegeville, PA, USA; 6Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada
Correspondence: Gema Requena, Epidemiology, Value Evidence and Outcomes, R&D Global Medical, GSK, Brentford, Middlesex, UK, Tel +44 20 80476893, Email [email protected]
Purpose: Chronic obstructive pulmonary disease (COPD) exacerbations are associated with significant morbidity and mortality and increased economic healthcare burden for patients with COPD. Long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) dual therapy is recommended for patients receiving mono-bronchodilator therapy who experience exacerbations or ongoing breathlessness. This study compared two single-inhaler LAMA/LABA dual therapies, umeclidinium/vilanterol (UMEC/VI) and indacaterol/glycopyrronium (IND/GLY), on moderate-to-severe exacerbation rates in patients with COPD in England.
Patients and Methods: This retrospective cohort study used linked primary care electronic health record data (Clinical Practice Research Datalink-Aurum) and secondary care data (Hospital Episode Statistics) to assess outcomes for patients with COPD who had a first prescription for single-inhaler UMEC/VI or IND/GLY (index date) between 1 January 2015 and 30 September 2019 (indexing period). Analyses compared UMEC/VI and IND/GLY on moderate-to-severe, moderate, and severe exacerbations, healthcare resource utilization (HCRU), and direct costs at 6, 12, 18, and 24 months, and time-to-first on-treatment exacerbation up to 24 months post-index date. Following inverse probability of treatment weighting (IPTW), non-inferiority and superiority of UMEC/VI versus IND/GLY were assessed.
Results: In total, 12,031 patients were included, of whom 8753 (72.8%) were prescribed UMEC/VI and 3278 (27.2%) IND/GLY. After IPTW, for moderate-to-severe exacerbations, weighted rate ratios were < 1 at 6, 12, and 18 months and equal to 1 at 24 months for UMEC/VI; around the null value for moderate exacerbations and < 1 at all timepoints for severe exacerbations. UMEC/VI showed lower HCRU incidence rates than IND/GLY for all-cause Accident and Emergency visits and COPD-related inpatient stays and associated all-cause costs at 6 months post-indexing. Time-to-triple therapy was similar for both treatments.
Conclusion: UMEC/VI demonstrated non-inferiority to IND/GLY in moderate-to-severe exacerbation reduction at 6, 12 and 18 months. These results support previous findings demonstrating similarity between UMEC/VI and IND/GLY on reduction of moderate-to-severe exacerbations.
Plain Language Summary: Sudden exacerbations, or flare-ups, of chronic obstructive pulmonary disease (COPD) are linked with worsening health and increased risk of death, as well as increased healthcare costs for people with COPD. Long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) dual therapy is recommended for patients with COPD who take LAMA or LABA monotherapy but continue to experience flare-ups or ongoing breathlessness. This study compared two single-inhaler LAMA/LABA dual therapies, umeclidinium/vilanterol (UMEC/VI) and indacaterol/glycopyrronium (IND/GLY), in terms of flare-ups in patients with COPD in England.
We used two linked databases of de-identified medical records from general practitioners and hospitals for patients with COPD who had a first prescription for UMEC/VI or IND/GLY between 1 January 2015 and 30 September 2019. We compared the two treatments on COPD flare-ups, healthcare resource utilization and related costs, and changes in medication over the 2 years following starting treatment.
We found that the treatments were comparable for moderate-to-severe flare-ups. Patients taking UMEC/VI had less Accident and Emergency (A&E) visits in total and less inpatient stays related to their COPD, and had a lower overall cost of healthcare for A&E visits and inpatient stays than patients taking IND/GLY. Changes to treatment and time before their first flare-up were similar for all patients, regardless of their prescribed treatment.
This study showed that UMEC/VI is as effective as IND/GLY at preventing moderate-to-severe flare-ups. These results support previous findings demonstrating similarity between UMEC/VI and IND/GLY in reducing the rate of moderate-to-severe exacerbations after starting treatment.
Keywords: COPD dual therapy, LABA/LAMA new users, healthcare resource utilization, exacerbations, comparative effectiveness, single-inhaler dual therapy
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death globally, affecting 5–22% of adults aged 40 and above, and is a leading cause of hospitalizations worldwide.1 The burden of COPD is expected to grow in the coming years due to factors such as increased global exposure to tobacco and noxious particles, aging populations, and lack of awareness and access to diagnosis.1–3 In the United Kingdom (UK) alone, COPD is the second most common lung disease after asthma, with an estimated 1.2 million people (2% of the population) having been diagnosed.2 Each year in the UK, COPD costs the National Health Service (NHS) approximately £1.9 billion, which constitutes 29% of the total cost of respiratory illness, second only to asthma (£3 billion).3 Exacerbations are associated with significant morbidity and mortality, as well as increased economic burden of healthcare for patients with COPD.4,5
For patients receiving maintenance therapy with a long-acting muscarinic antagonist (LAMA) or a long-acting β2-agonist (LABA), but who still experience exacerbations, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategic report recommends escalating to LAMA/LABA or inhaled corticosteroid (ICS)/LABA dual therapy.5 In the UK, the National Institute for Health and Care Excellence (NICE) guidelines recommend offering LAMA/LABA dual therapy for patients with dyspnea or exacerbations despite the use of a short-acting bronchodilator.6 For patients who develop further exacerbations on dual therapy with eosinophil counts ≥100 cells/µL, the GOLD strategy report recommends escalation to ICS/LAMA/LABA triple therapy.5 NICE guidelines recommend escalation to triple therapy in patients who experience one severe (requiring hospitalization) or two moderate exacerbations over the course of a year.6
A similar benefit in terms of frequency and proportion of patients experiencing exacerbations between umeclidinium/vilanterol (UMEC/VI), indacaterol/glycopyrronium (IND/GLY) and aclidinium/formoterol (ACL/FOR) was observed in the non-interventional DETECT study.7 The study showed clinical benefits in terms of lung function, quality of life, and early morning symptoms of COPD in patients with COPD across multiple sites in Germany, which improved following indexing on UMEC/VI, IND/GLY, and ACL/FOR.7 However, there is little evidence comparing LAMA/LABA dual therapies in a UK patient population.
This study compares exacerbation outcomes, healthcare resource utilization (HCRU) and costs in patients with COPD newly initiating single-inhaler LAMA/LABA dual therapy with either UMEC/VI or IND/GLY in England in a routine primary care setting.
Methods
Study Design
This was a new-user, active comparator, retrospective cohort study (GSK study 214887) using anonymized primary care electronic health record data from the Clinical Practice Research Datalink (CPRD-Aurum) linked on a patient level using an 8-stage algorithm to secondary care administrative Hospital Episode Statistics (HES) data in England (Figure 1).
 |
Figure 1 Study design. Abbreviations: GLY, glycopyrronium; IND, indacaterol; UMEC, umeclidinium; VI, vilanterol. |
The index date was defined as the date of the first single-inhaler UMEC/VI or IND/GLY prescription within the indexing period. The indexing period spanned from 1 January 2015 to 30 September 2019. This ensured that the overall study period did not include the severe acute respiratory syndrome coronavirus 2 (COVID-19) pandemic, as management of patients with COPD would not be representative during this period and the changes in HCRU were not under study. The minimum baseline for assessment of patient characteristics was 12 months before the index date. The follow-up period for assessment of clinical endpoints was variable, spanning from the index date to patient death, study period end date, or end of patient data availability, up to a maximum of 24 months.
Study Population
Inclusion criteria were applied prior to patient inclusion in the study. Patients needed ≥1 diagnostic code of COPD in primary care as an adult (≥35 years of age aligning with guidance from NICE6), ≥1 prescription of single-inhaler UMEC/VI or IND/GLY within the indexing period, and forced expiratory volume in one second/forced vital capacity (FEV1/FVC) <0.7 at any time prior to and including index date for study inclusion. They also had to have ≥12 months of continuous registration with a general practitioner (GP) prior to their index date, and healthcare data which were eligible for linkage to HES.
Patients were excluded from the study if they had a diagnosis of a medical condition incompatible with a COPD diagnosis at any time prior to and including index date. These included conditions related to lung developmental anomalies, degenerative processes such as cystic fibrosis, and other conditions potentially interfering with clinical COPD diagnosis or changing the natural history of the disease, such as pulmonary resections (Supplementary Table 1). Other exclusion criteria were prescription for both UMEC/VI and IND/GLY at index date, concomitant use of ICS at index date (two ICS prescriptions with ≤30-day gap between end of last supply date to subsequent new supply, overlapping the index date), and ≥1 prescription of any single-inhaler or open combinations of LAMA/LABA prior to index date.
Patients were classified by indexed therapy (UMEC/VI or IND/GLY).
Outcomes
Outcomes were assessed up to a maximum of 24 months after the index date. The primary outcome was rate of moderate-to-severe exacerbations in the 6, 12, 18, and 24 months following treatment initiation in patients with COPD newly initiating UMEC/VI versus those initiating treatment with IND/GLY.
Secondary outcomes were rate of moderate exacerbations, rate of severe exacerbations, COPD-related and all-cause HCRU and direct healthcare costs at 6, 12, 18, and 24 months, and time-to-first on-treatment exacerbation (including moderate-to-severe, moderate, and severe exacerbations) up to 24 months. An exploratory endpoint of time-to-triple therapy up to 24 months was also investigated.
Statistical Analysis
Differences in baseline characteristics between UMEC/VI and IND/GLY were calculated using t-test (continuous), chi-squared or Fisher’s Exact (for categorical), or Mann–Whitney (for ordinal) statistical comparisons. Rates of exacerbation were calculated by dividing the number of exacerbations observed by the total days at-risk across all patients, reaching the number of exacerbations per person per day. This could then be multiplied to calculate the rate of exacerbation per 1000 persons per day. Non-inferiority (NI) of UMEC/VI versus IND/GLY was assessed for the primary endpoint of moderate-to-severe exacerbations via rate ratio (RR) of exacerbations at 6, 12, 18, and 24 months; RRs were obtained using a negative binomial regression model. An NI margin of 10% was prespecified, as differences of >10% are widely regarded as clinically important in related respiratory studies.8 If NI was met, superiority was also assessed with a margin of <0. A sample size of 998–1342 and 448–499 patients receiving UMEC/VI and IND/GLY, respectively, was determined to be needed to demonstrate NI in the rate of exacerbations between the UMEC/VI and IND/GLY cohorts. A sample size of 1392–1875 and 625–696 patients receiving UMEC/VI and IND/GLY, respectively, was determined to be needed to demonstrate superiority of UMEC/VI in the rate of exacerbations compared with IND/GLY.
Moderate-to-severe exacerbations were identified according to an existing algorithm previously validated against physician notes.9,10 Baseline demographics and clinical characteristics were evaluated and compared between treatment cohorts whereby subgroup counts and percentages were calculated for categorical variables, and means and standard deviations (SD) were calculated for continuous variables.
Inverse probability of treatment weighting (IPTW) was used whereby weights were derived from the propensity score (PS) to create a pseudo-population in which the distribution of covariates in the population is independent of treatment assignment. Covariates considered for inclusion in the IPTW model included baseline sociodemographic and clinical characteristics, exacerbations, treatment use (including ICS use), and HCRU/costs. Selection of covariates was primarily based on background knowledge of the association of pre-treatment variables on the outcomes of interest.
The IPTW model allowed estimation of the average treatment effect in the entire population by accounting for the effects of the covariates on the results. Prior to outcomes assessment, covariate balance was assessed using standardized mean differences in the unweighted and weighted cohorts, with a standardized difference of <10% in the weighted cohort being judged as indicative of adequate balance.11
When evaluating the treatment effect, so as not to increase the probability of a type 1 error, an intention-to-treat (ITT) analysis was conducted whereby patients were classified into treatment groups at index and remained in the cohort for their indexed treatment for the entire follow-up period (maximum 24 months) and were not censored for any reason other than a treatment switch to the comparator treatment.
An on-treatment sensitivity analysis was also conducted whereby patients were classified into treatment groups at index and censored at the time of the first prescription for any non-indexed, long-acting maintenance medication, or discontinuation of their indexed therapy.
When reporting outcomes, results based on small numbers of patients (n < 5) were suppressed to protect patient confidentiality. Secondary suppression was also implemented, where required, to protect primary suppression.
Results
Study Population and Baseline Characteristics
In total, 12,031 eligible patients were included in the study, of whom 8753 (72.8%) were indexed on UMEC/VI and 3278 (27.2%) were indexed on IND/GLY. Sample attrition following application of inclusion and exclusion criteria is shown in Figure 2.
Demographic and clinical characteristics for both groups are shown in Table 1. Mean (SD) age at index was 69.6 years (10.7) for patients indexed on UMEC/VI and 69.4 years (10.3) for those indexed on IND/GLY. Distribution of patients per region differed between treatments. The UMEC/VI treatment group contained a greater proportion of patients from the South Central, South West, West Midlands, and Yorkshire and the Humber regions compared with the IND/GLY group, while the IND/GLY treatment group contained a greater proportion of patients from the East of England, London, North East, and South East Coast than the UMEC/VI group. The treatments included similar proportions of patients from the East Midlands and North West.
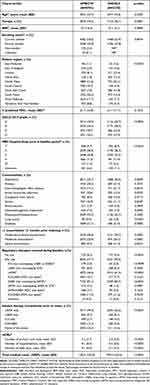 |
Table 1 Baseline Patient Characteristics |
The UMEC/VI treatment group also contained more patients in the GOLD grade A category compared with the IND/GLY treatment group (43.0% vs 36.7%), and fewer patients across the other grades. A smaller proportion of patients indexed on UMEC/VI had moderate-to-severe exacerbations in the 12 months prior to indexing: 30.6% compared with 34.2% for IND/GLY. Patients indexed on UMEC/VI also had lower mean (SD) total medical costs per patient, at £158.40 (163.80) compared with £190.90 (163.60) for those indexed on IND/GLY. LAMA monotherapy usage during baseline was also less common for patients indexed on UMEC/VI than those on IND/GLY (46.6% vs 61.4%, respectively). Mean (SD) % predicted FEV1 was similar for both treatments, at 61.7 (16.8) for UMEC/VI and 61.1 (17.1) for IND/GLY.
After IPTW, covariates were well balanced for all outcomes with all showing sufficient balance at all timepoints. No weighted analysis was done for the exploratory objective time-to-triple therapy.
Moderate-to-Severe Exacerbations
Across all timepoints, the moderate-to-severe exacerbation rates were lower in the UMEC/VI cohort than in the IND/GLY cohort in both unweighted and weighted analyses, except for the weighted analysis at 24 months where the rates were equivalent (Figure 3A). Unweighted RRs of moderate-to-severe exacerbations (95% confidence interval [CI]) were below 1 at all timepoints, ranging from 0.78 (0.70, 0.88) at 6 months, to 0.90 (0.82, 1.00) at 24 months. The weighted RR (95% CI) at 6 months was 0.90 (0.79, 1.03), 0.97 (0.87, 1.08) at 12 months, 0.96 (0.87, 1.05) at 18 months, and 1.00 (0.89, 1.12) at 24 months (Figure 3B). NI was met for UMEC/VI at every timepoint apart from 24 months, where the rates were equivalent. Across all timepoints, superiority was not observed for any comparison. On-treatment sensitivity analyses showed similar results (Supplementary Table 2). In the 12 and 24 months post-index, the number of exacerbations for both treatment groups was similar with a mean number (SD) of 0.4 (0.7) and 0.4 (0.8) at 12 months and 0.3 (0.7) for both groups at 24 months. The proportion of patients with exacerbations in the 12 months post index was 26.8% for those indexed on UMEC/VI and 30.2% for those indexed on IND/GLY. At 24 months post index, the proportion of patients with exacerbations was 20.2% for those on UMEC/VI and 23.5% for those on IND/GLY (data not shown).
Moderate Exacerbations
At all timepoints, unweighted rates of moderate exacerbations for UMEC/VI were consistently lower than for IND/GLY (Supplementary Table 3). Unweighted RRs were below 1 at all timepoints, while weighted RRs were above 1 at 6 and 12 months post-indexing, and equivalent at 18 and 24 months (Figure 4). On-treatment sensitivity analyses showed similar results (Supplementary Table 2).
 |
Figure 4 Rate ratio of moderate exacerbations at 6, 12, 18, and 24 months. Abbreviations: CI, confidence interval; GLY, glycopyrronium; IND, indacaterol; UMEC, umeclidinium; VI, vilanterol. |
Severe Exacerbations
Across all timepoints, the severe exacerbation rates were lower in the UMEC/VI cohort than in the IND/GLY cohort in both unweighted and weighted analyses (Supplementary Table 4). Unweighted and weighted RRs of severe exacerbations were both consistently below 1; it was statistically significant at 6 months (Figure 5). On-treatment sensitivity analyses showed RRs remaining <1 at all timepoints (Supplementary Table 2).
 |
Figure 5 Rate ratio of severe exacerbations at 6, 12, 18, and 24 months. Abbreviations: CI, confidence interval; GLY, glycopyrronium; IND, indacaterol; UMEC, umeclidinium; VI, vilanterol. |
Time-to-First On-Treatment Exacerbation
Time-to-first on-treatment exacerbation (moderate-to-severe, moderate, and severe exacerbations) occurred at a steady and relatively unchanging rate from indexing until 24 months post-indexing for both unweighted and weighted analyses; median survival (the point at which half of patients had experienced an on-treatment exacerbation) was not reached for either treatment (Supplementary Figure 1). In general, unweighted and weighted analyses showed no significant difference between UMEC/VI and IND/GLY in time-to-first on-treatment exacerbation, with the only exception being unweighted time-to-severe exacerbation, which was significantly greater for UMEC/VI versus IND/GLY (Figure 6). On-treatment sensitivity analyses showed similar results (Supplementary Table 2).
HCRU
In general, at 6 months post-indexing, the incidence rates of COPD-related and all-cause HCRU were lower among patients indexed on UMEC/VI than on those indexed on IND/GLY, with the exception of all-cause prescriptions (Supplementary Table 5). Statistical significance was met for number of all-cause A&E visits and COPD-related inpatient stays. After IPTW, at 6 months post-indexing, the weighted RRs were largely below 1, but only all-cause A&E visits and COPD-related inpatient stays were statistically significant in favor of UMEC/VI (Figure 7). A similar trend was observed at 12, 18, and 24 months post-indexing (Supplementary Table 5). On-treatment sensitivity analyses showed similar results for all elements except all-cause inpatient stays and A&E visits (Supplementary Table 2).
Direct Treatment Costs
At 6 months post-indexing, COPD-related and all-cause costs per-patient per-year were lower for all HCRU elements among patients indexed on UMEC/VI than for patients indexed on IND/GLY in both unweighted and weighted analyses (Supplementary Table 6). Patients indexed on UMEC/VI had significantly lower costs for inpatient stays and A&E visits versus IND/GLY in unweighted (COPD-related and all-cause costs) and weighted analyses (all-cause costs) (Figure 8). Lower costs for inpatient stays and A&E visits were also observed at 12, 18, and 24 months post-indexing (Supplementary Table 6). Total COPD-related costs were numerically lower and all-cause costs were significantly lower for patients indexed on UMEC/VI than IND/GLY at all timepoints (Figure 9, Supplementary Table 6). On-treatment sensitivity analyses showed similar results (Supplementary Table 2).
Time-to-Triple Therapy
Time-to-triple therapy was similar for patients indexed on UMEC/VI and IND/GLY. Approximately 9% of patients had escalated to triple therapy by 6 months post-indexing, 15% by 12 months and 20% by 18 months, irrespective of indexed treatment. Median survival was not reached for either indexed treatment (Supplementary Figure 2).
Discussion
In this study, we demonstrated NI on the rate of moderate-to-severe exacerbations in patients with COPD newly prescribed UMEC/VI versus IND/GLY in England. Across all timepoints, the rate of moderate-to-severe exacerbations was lower with UMEC/VI than with IND/GLY in both unweighted and weighted analyses, except for the weighted 24-month analysis where rates were equivalent. Treatment with UMEC/VI may result in a greater reduction of severe exacerbations in comparison with IND/GLY, as evidenced by the significantly lower incidence rates of UMEC/VI at 6 months post-indexing. This was possibly due to a reduced delay in accrued long-term benefit of treatment compared with IND/GLY.
The demonstration of NI of UMEC/VI in moderate-to-severe exacerbations in this study is in line with the non-interventional DETECT study, which reported no significant difference in the frequency of overall exacerbations between patients indexed on UMEC/VI and those indexed on IND/GLY.7
Two network meta-analyses including 16 randomized controlled trials and 49 studies, respectively, also found no significant differences in the number of moderate-to-severe exacerbations over at least 48 weeks12 or annualized moderate-to-severe exacerbation rates over 24 weeks13 between UMEC/VI and IND/GLY. Our study therefore supports previous findings concerning similarity between UMEC/VI and IND/GLY in moderate-to-severe exacerbations specifically.
There were no significant differences in weighted comparisons between cohorts for time-to-first on-treatment exacerbation regardless of exacerbation severity. These results suggest that despite the numerical reduction in rate of moderate-severe exacerbations for UMEC/VI versus IND/GLY in the 2 years following treatment initiation, there is no delay between treatments as to when the exacerbations begin. This is in line with a recent network meta-analysis which found similar results for UMEC/VI, IND/GLY and GLY/formoterol in hazard ratio of time-to-first exacerbation.13 The results presented here also support findings from a recent cohort study that collected data from patients with COPD in Taiwan, which found comparable time-to-first exacerbation rates between UMEC/VI and IND/GLY, along with lower annualized exacerbation rates with UMEC/VI versus IND/GLY.14
Time-to-triple therapy was similar between indexed treatments. Given the similarity in baseline demographic and clinical characteristics between the treatment groups, as well as comparable rates of exacerbations and time-to-first exacerbation, it is unsurprising that there was not a significant difference in time-to-triple therapy between the treatments, as a step up to triple therapy is recommended by both the GOLD strategic report and NICE guidelines for patients on LAMA/LABA dual therapy who continue to experience exacerbations.5,6 In this study, UMEC/VI was associated with a reduced rate of COPD-related inpatient stays and all-cause A&E visits, significantly lower all-cause medical costs, and numerically lower COPD-related medical costs than IND/GLY. The lower costs reported here may suggest that UMEC/VI is more effective than IND/GLY at lowering the burden of healthcare for patients with COPD, particularly regarding inpatient stays, which have been shown to drive COPD-related costs for patients with COPD newly initiating treatment with a LAMA/LABA in primary care in England.15
This study had several strengths. Firstly, an extensive and robust process was used to identify suitable covariates for inclusion into the PS models, ensuring that covariate balance in all models presented was sufficient for effective comparison of the groups. The suitability of the covariates used is demonstrated by the finding that across all objectives, PS models achieved high or satisfactory balance across all covariates once weighting had been applied. Secondly, use of IPTW to adjust for relevant covariates allowed for effective direct comparison between the two cohorts included in this study, despite the use of observational data. Finally, electronic medical records such as CPRD-Aurum represent an observation of effects in the real-world rather than under optimal conditions, providing the findings of this study with relevance in clinical practice.16
Limitations of this study included generalizability of results to the wider UK population being potentially impacted as the linkage to HES data reduces the patient sample to only those registered at a GP practice in England, and CPRD-Aurum data covers <10% of UK practices, although the sample population is considered highly representative of the UK population.17 There was also missing secondary care data due to poor linkage from outpatient HES data to CPRD-Aurum, although as most treatments initially prescribed in secondary care are continued in primary care. As such, this may not have been greatly consequential but may have led to the index date being slightly later than actual initiation of dual therapy in some cases. Also, patients with recorded asthma diagnoses were not excluded from this study so as not to exclude those with asthma-COPD overlap syndrome, possibly increasing the risk of misclassification due to reduced diagnostic accuracy of COPD for patients with asthma.18 It also cannot be known for certain that medication was specifically prescribed for treatment of COPD, as many COPD medications can also be indicated to treat asthma, although a diagnosis of COPD was required for patient inclusion in this study, in line with a definition of COPD validated against patient notes.18 Further, head-to-head clinical studies would provide additional evidence to confirm the findings from retrospective database studies.
Conclusions
Across all timepoints, the incidence rates of exacerbations were lower in the UMEC/VI cohort than in the IND/GLY cohort in both unweighted and weighted analyses, except for the weighted 24-month analysis where the rates were equivalent. In this study, UMEC/VI demonstrated NI to IND/GLY with regard to moderate-to-severe exacerbation reduction at 6, 12, and 18 months post-indexing. These results support previous findings demonstrating similarity between UMEC/VI and IND/GLY in effectiveness concerning reduction of moderate-to-severe exacerbations after treatment initiation.
Abbreviations
A&E, Accident and Emergency; ACL, aclidinium; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID, coronavirus; CPRD, Clinical Practice Research Datalink; FDC, fixed-dose combination; FEV1, forced expiratory volume in one second; FOR, formoterol; FVC, forced vital capacity; GBP, British Pound Sterling; GLY, glycopyrronium; GOLD; Global Initiative for Chronic Obstructive Lung Disease; GP, general practitioner; HCRU, healthcare resource utilization; HES; Hospital Episode Statistics; ICS; inhaled corticosteroid; IND, indacaterol; IPTW, inverse probability of treatment weighting; ITT, intention-to-treat; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MRC, Medical Research Council; NHS, National Health Service; NI, noninferiority; NICE, National Institute for Health and Care Excellence; PS, propensity score; RR, rate ratio; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist; SD, standard deviation; UK, United Kingdom; UMEC; umeclidinium; VI, vilanterol.
Data Sharing Statement
This study is based in part on data from the CPRD obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone.
Data from HES Copyright © (2022), re-used with the permission of The Health & Social Care Information Centre. All rights reserved. Authors had access to the study data for the purposes of this work only. Data were accessed through an existing GSK license to address pre-specified research questions only. Therefore, the data cannot be broadly disclosed or made publicly available at this time. Access to each database can be requested via the respective websites.
Ethics Approval and Informed Consent
Approval of this study was provided by the GSK Protocol Review Committee and by the Clinical Practice Research Datalink (CPRD) Research Data Governance [RDG] process, which reviewed the protocol and approved access to CPRD data (protocol no. 21_000616). No personal subject contact or primary collection of individual human data occurred, and anonymized patient-level data were used in this analysis; patient consent was therefore not required.
This study complied with all applicable laws regarding subject privacy. No direct subject contact or primary collection of individual human subject data occurred. Personal identifiers and personal identifiable information were removed by the database provider prior to receipt by the study team. Study results are in tabular form and aggregate analyses that omit subject identification; therefore, informed consent, ethics committee or Institutional Review Board (IRB) approval were not required. This study was designed, implemented, and reported in accordance with the Guidelines for Good Pharmacoepidemiology Practices (GPP) of the International Society for Pharmacoepidemiology (ISPE 2008), the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines, and with the ethical principles laid down in the Declaration of Helsinki.
Acknowledgments
Editorial support (in the form of writing assistance including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Christopher Heath, PhD, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK. An abstract based on this study was previously presented as a poster presentation at the 2022 ERS Congress, 4–6 September 2022, Barcelona, Spain, and then as an encore at the 2023 Kongress der Deutschen Gesellschaft für Innere Medizin (DGIM), 22–25 April 2023, Wiesbaden, Germany.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This study was funded by GSK (GSK study 214887) and approved by the GSK Protocol Review Committee and by the Clinical Practice Research Datalink (CPRD) Research Data Governance (RDG) process, which reviewed the protocol and approved access to CPRD data. GSK-affiliated authors had a role in study design, data analysis, data interpretation, and writing of the report and GSK funded the article processing charges and open access fee.
Disclosure
GR, CCo, KJR, and ASI are employees of GSK and hold stocks and shares at GSK. ASI also holds an unpaid faculty position at McMaster University. AC was an employee of GSK and held stocks and shares at GSK at the time of the study. FJFH is an employee of the Translational Lung Research Center Heidelberg, part of the Germany lung research Foundation (DZL). JKQ holds a position at Imperial College London and has received grants or contracts paid to this institution outside the scope of the submitted work from the Medical Research Council, Health Data Research UK, GSK, Boehringer Ingelheim, Asthma + Lung UK, and AstraZeneca. JKQ has also received payment for advisory board participation, teaching or lectures from GSK, AstraZeneca, and Insmed. CCa, TT, RWo, and RWi are employees of Adelphi Real World. VB and JY were employees of Adelphi Real World at the time of the study. Adelphi Real World is a business that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations which received funding from GSK to conduct the study. Adelphi Real World employees work with a variety of companies and organizations and are expressly prohibited from receiving any payment or honoraria directly from these organizations for services rendered. The authors report no other conflicts of interest in this work.
References
1. Akwe J, Fair N, Fongeh T. Overview of epidemiology, pathophysiology, diagnosis and staging with 2020 Updates. Med Res Arch. 2020;8(2). doi:10.18103/mra.v8i2.2046
2. Chronic obstructive pulmonary disease (COPD) statistics: British Lung Foundation. Availablr from: https://statistics.blf.org.uk/copd.
3. The 2nd Atlas of variation in risk factors and healthcare for respiratory disease in England. Availablr from: https://fingertips.phe.org.uk/documents/Introduction.pdf.
4. Shah CH, Onukwugha E, Zafari Z, Villalonga-Olives E, Park JE, Slejko JF. Economic burden of comorbidities among COPD Patients hospitalized for acute exacerbations: an analysis of a commercially insured population. Expert Rev Pharmacoecon Outcomes Res. 2022;22(4):683–690. doi:10.1080/14737167.2021.1981291
5. Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for prevention, diagnosis and management of COPD; 2021. Availablr from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
6. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease (acute exacerbation): antimicrobial prescribing; 2018. Availablr from: https://www.nice.org.uk/guidance/ng114.
7. Plate T, Friedrich FW, Beier J. Effectiveness and tolerability of LABA/LAMA fixed-dose combinations Aclidinium/formoterol, glycopyrronium/indacaterol and umeclidinium/vilanterol in the treatment of COPD in daily practice–results of the non-interventional DETECT study. Int J Chron Obstruct Pulmon Dis. 2020;15:1335. doi:10.2147/COPD.S252354
8. Chapman KR, Bergeron C, Bhutani M, et al. Do we know the minimal clinically important difference (MCID) for COPD exacerbations? J Chronic Obstructive Pulmonary Dis. 2013;10(2):243–249. doi:10.3109/15412555.2012.733463
9. Rothnie KJ, Müllerová H, Thomas SL, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin Epidemiol. 2016;8:771. doi:10.2147/CLEP.S117867
10. Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11(3):e0151357. doi:10.1371/journal.pone.0151357
11. Austin PC. Goodness‐of‐fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf. 2008;17(12):1202–1217. doi:10.1002/pds.1673
12. Lee HW, Park J, Jang EJ, Lee C-H. Comparisons of exacerbations and mortality among LAMA/LABA combinations in stable chronic obstructive pulmonary disease: systematic review and Bayesian network meta-analysis. Respir Res. 2020;21(1):1–15. doi:10.1186/s12931-020-01540-8
13. Ismaila AS, Haeussler K, Czira A, et al. Comparative efficacy of umeclidinium/vilanterol versus other bronchodilators for the treatment of chronic obstructive pulmonary disease: a network meta-analysis. Adv Ther. 2022;1–51.
14. Hsieh M-J, Chen N-H, Cheng S-L, et al. Comparing Clinical Outcomes of Tiotropium/Olodaterol, Umeclidinium/Vilanterol, and Indacaterol/Glycopyrronium Fixed-Dose Combination Therapy in Patients with Chronic Obstructive Pulmonary Disease in Taiwan: a Multicenter Cohort Study. Int J Chron Obstruct Pulmon Dis. 2022;17:967. doi:10.2147/COPD.S353799
15. Requena G, Banks V, Czira A, et al. Characterization of Patients with Chronic Obstructive Pulmonary Disease Initiating Single-Inhaler Long-Acting Muscarinic Antagonist/Long-Acting β2-Agonist Dual Therapy in a Primary Care Setting in England. Int J Chron Obstruct Pulmon Dis. 2022;17:1781. doi:10.2147/COPD.S365480
16. Requena G, Wolf A, Williams R, et al. Feasibility of using Clinical Practice Research Datalink data to identify patients with chronic obstructive pulmonary disease to enrol into real‐world trials. Pharmacoepidemiol Drug Saf. 2021;30(4):472–481. doi:10.1002/pds.5188
17. Wolf A, Dedman D, Campbell J, et al. Data resource profile: clinical practice research Datalink (CPRD) aurum. Int J Epidemiol. 2019;48(6):1740–1740g. doi:10.1093/ije/dyz034
18. Quint JK, Müllerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ open. 2014;4(7):e005540. doi:10.1136/bmjopen-2014-005540
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






