Back to Journals » Pathology and Laboratory Medicine International » Volume 9
Comparative study of Widal test against stool culture for typhoid fever suspected cases in southern Ethiopia
Authors Ameya G, Atalel E, Kebede B , Yohannes B
Received 8 October 2016
Accepted for publication 15 December 2016
Published 31 January 2017 Volume 2017:9 Pages 1—7
DOI https://doi.org/10.2147/PLMI.S124155
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Paul Zhang
Gemechu Ameya,1 Edemew Atalel,2 Berhanu Kebede,3 Bethel Yohannes4
1Department of Medical Laboratory Science, College of Medicine and Health Sciences, Arba Minch University, Arba Minch, 2Department of Medical Laboratory Science, Durbetie Primary Hospital, Amahara, 3Department of Medical Laboratory Science, Dalifage Primary Hospital, Afar, 4Department of Medical Laboratory Science, Hayilemariyam Hospital, Adama, Ethiopia
Introduction: Infection caused by Salmonella enterica subsp. serotype Typhi remains an important public health problem in developing countries. Culture is an effective diagnostic method of confirming this infection. Diagnosis in developing countries is mostly done by Widal test, which is nonreliable. The aim of this study was to compare the Widal test against stool culture in typhoid-suspected cases and to evaluate the agreement between test methods.
Methodology: A cross-sectional study design was conducted on typhoid-suspected cases in southern Ethiopia. Collected data were entered into Epi-Info version 3.5.1 and exported to SPSS version 20.0 for further analysis. Kappa test was used to assess the agreement between the tests. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to compare the Widal test against stool culture.
Results: A total of 95 patients participated in the study, of whom 49 (51.6%) were females and 46 (48.4%) were males. The age range of the suspected cases were between 10 and 62 years, with mean age of 27.9 years. Of the examined cases, 65 (68.4%) were positive for slide agglutination Widal test, whereas only 19 (20.0%) were positive for S. enterica serotype Typhi by stool culture. The sensitivity, specificity, PPV, and NPV of slide agglutination test against stool culture were 84.2%, 35.5%, 24.6%, and 90.0%, respectively. Slide agglutination test has a poor agreement with the stool culture (kappa = 0.103), but tube titration test has a fair agreement with the stool culture (kappa = 0.325).
Conclusion: Widal test has a low specificity and PPV but has better sensitivity and NPV than a stool culture. It also has a poor agreement with the stool culture. Therefore, physicians should not be totally dependent on the Widal test in endemic areas and in areas where it is the only diagnostic method for typhoid fever.
Keywords: typhoid fever, Widal slide agglutination test, Widal tube titration test, stool culture
Background
Typhoid fever is a systemic disease caused by Salmonella enterica serotype Typhi and is a major cause of morbidity and mortality worldwide.1 It emerged as an important infectious disease in the early 19th century. Humans are the only natural host and reservoir for typhoid fever agent. Infection occurs in all age groups, and it is transmitted by ingestion of food or water contaminated with feces.2 The highest incidence occurs where water supplies serving large populations are contaminated with feces.3 The illness begins with mounting fever, headache, vagueness, abdominal pain, and constipation, which may be followed by the appearance of rashes during the third week; the patient reaches a state of prolonged apathy, toxemia, delirium, disorientation, or coma, followed by diarrhea which if left untreated can lead to complications affecting various organs of the body.4
Culture of the causative organism is considered an effective diagnostic procedure in suspected typhoid fever.5 Cultures can be obtained from various body fluids including blood, bone marrow, urine, stool, and rose spots. Blood, urine, and stool culture are the better diagnostic methods, but they are expensive techniques and some bacterial culture facilities are often unavailable and also it requires laboratory equipment and technical training.6 Stools collected from acute patients may be positive when blood culture is negative. It is also important for the monitoring of carriage of S. enterica serotype Typhi after apparent clinical cure.6,7
It is difficult to estimate the real impact of typhoid fever because of its clinical picture, which is confused with that of many other febrile infections. Additionally, the disease is underestimated because there are no bacteriology laboratories in most areas of developing countries. These factors are believed to result in many cases being undiagnosed.7 There are few established surveillance systems for typhoid in the developing world, because of lack of effective diagnostic methods.8 The disease remains a serious public health problem in developing countries, and its diagnosis on clinical grounds is difficult.9 Laboratory diagnosis of salmonella infection requires isolation and identification of S. enterica serotype Typhi in many areas where the disease is endemic.1,10 However, in most developing countries including Ethiopia, the situation is different as the Widal test seems to be the only laboratory means employed in the diagnosis of typhoid fever among suspected patients.10
Widal test is very easy and cheap to perform and requires minimal training and equipment. It is an agglutination test, which measures agglutinating antibodies against the lipopolysaccharide O and protein flagella H antigens of S. enterica serotype Typhi.11,12 Sharing of these antigens by other Salmonella serotypes and other members of Enterobacteriaceae makes the role of the Widal test even more controversial in diagnosing typhoid fever.13 This is harmful in terms of specificity and sensitivity as compared to culture. Most of the studies compared Widal test against blood culture, which needs advanced microbiology equipment and trained manpower. Stool culture is relatively easy and cost-effective compared to blood culture, and it has not been compared with Widal test so far in our region.
The value of the Widal test in the diagnosis of typhoid fever cases has long been discussed.14 The test has been used for over a century in developing countries, but its specificity has been debated. Sensitivity and specificity of Widal test vary from place to place.15–18 Hence, evaluating the result of this test is necessary for correct interpretation of the result.19 Therefore, the aim of this study was to compare Widal test, which is the main diagnostic method in developing countries, against stool culture.
Methods
Study design and settings
This cross-sectional study design was conducted to compare Widal test against stool culture in typhoid fever-suspected patients in Arba Minch Hospital. Arba Minch town is located 505 km south of Addis Ababa at an elevation of 1285 m above sea level. Arba Minch Hospital is the only hospital in the town in Southern Nations, Nationalities, and Peoples region. It was built in 1969 to serve ~500,000 people, but now it serves >2 million people based on figures published by the Central Statistical Agency in 2007.20
Population
All patients who visited Arba Minch Hospital laboratory from March to May 2016 were considered as controls. The study population was selected by systematic random sampling technique in patients with clinical symptoms of typhoid fever and visiting the laboratory for a Widal test in Arba Minch Hospital during the study period. Patients who had received antibiotic treatment within 3 months for their symptoms before coming to the hospital were excluded from the study.
Widal slide agglutination and tube titration test
For slide agglutination and tube titration Widal test, 4–5 mL of blood was collected by vein puncture in a nonanticoagulated tube. Then the collected venous blood was centrifuged, and two drops of serum were placed on the test card for both O and H antigens. Then a drop of S. enterica serotype Typhi O and H commercial antigens was added on a drop of serum on the card and rotated for 2 minutes on an orbital shaker. In each test batch, positive and negative controls were carried out using commercially provided reagent. Positive Widal test is determined by the formation of visible agglutination reaction from the mixture of S. enterica serotype Typhi antigen with the patient’s serum antibody on the testing card. Lastly, the result was reported as reactive and nonreactive.2
In tube agglutination test (titration), ten small tubes were arranged for both H and O antigens, and 0.9 mL of saline was added in the first tube and 0.5 mL of saline was added in the remaining nine tubes. Next, 0.1 mL of fresh cell-free serum was added to the first tube and mixed. Then 0.5 mL of 1:10 diluted serum in first tube was transferred to tube numbers 2–9, which contain 0.5 mL of saline, and twofold serial dilution was performed. From tube number 9, after mixing 0.5 mL of diluted serum was discarded and 1:20, 1:40, 1:80, 1:160, and so on serum serial dilution was obtained. Tube number 10 was used as negative control, hence it contained only saline. Then antigens were added and mixed well with diluted serum, and the tubes were incubated at 37°C for 2–4 h. A white light shining vertically above the tube was used to examine the presence of clumps. Positive titration value is determined by the formation of visible clumps at 1:80 and higher dilutions for O antigen and at 1:160 and higher dilutions for H antigen in the tube from the mixture of each antigen with the patient’s serum.2,21
Stool sample collection and culturing
Approximately 3–4 g of stool was collected by a sterile wide-mouthed plastic container from those who had clinical symptoms of typhoid fever and visited the laboratory for Widal test. Then a piece of stool was taken by using wire loop and streaked on xylose lysine deoxycholate (Oxoid Ltd, UK, code CM 0469) and MacConkey agar (Oxoid) and incubated at 37°C for 24 h. Then S. enterica serotype Typhi was identified by biochemical tests used to identify Enterobacteriaceae according to the method described in Clinical and Laboratory Standards Institute.21,22 Sterility of the prepared culture media was checked by incubating 5% representative of the batch culture at 35–37°C overnight and observing for bacterial growth. The positive control of American Type Culture Collection Salmonella species was inoculated parallel with the sample.
Data processing and analysis
Collected data were entered into Epi-Info version 3.5.1 and exported to SPSS version 20.0 for further analysis. Kappa test was used to assess the agreement between the tests. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to compare Widal test against stool culture.
Ethical approval and consent to participate
Institutional ethical clearance was obtained from the research and ethics review board of Arba Minch University, College of Medicine and Health Sciences. Formal letter of cooperation was also written to obtain permission from the medical director of Arba Minch Hospital before data collection. The purpose and importance of the study were explained, and written consent was secured from each participant. Confidentiality was maintained at all levels of the study. Participant involvement in the study was voluntary, and those who were unwilling and wanted to quit their participation at any stage were informed they could do so without any restriction.
Results
In stool culture, of the 95 patients, 19 (20%) [95% CI: (12, 28)] patients tested positive. Among these, 11 (57.9%) were females, while 8 (42.1%) were males. A relatively higher proportion of stool culture-positive S. enterica serotype Typhi was observed in age groups between 21 and 30 years, which accounts for ~42% (8/19) of positive cases. Of the 19 culture-positive suspected cases, 16 were positive with slide agglutination test, while 15 were positive with tube titration tests.
Of the 95 patients, 49 (51.6%) were females and 46 (48.4%) were males. The mean age of patients was 27.9 years. The age range of the participants was from 10 to 62 years. Among the typhoid fever-suspected patients, 65 (68.4%) were positive for Widal slide agglutination test, and of these Widal slide reactive patients, 53 (81.5%) were reactive for both O and H antigens. All patients with Widal slide test reactive were exhibited agglutination for O antigen with 30 (46.2%) males and 35 (53.8%) females. At the same time, H antigen was reactive in 53 (55.8%) patients, with 25 (47.2%) males and 28 (52.8%) females (Figure 1).
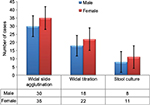  | Figure 1 Widal slide agglutination, tube titration, and stool culture positive result by sex in the typhoid fever suspected patients. |
Widal tube agglutination test (titration) was also performed for those patients whose slide agglutination test result indicated reactive. Patients who were positive by either O antigen (≥1:80 titration value) or H antigen (≥1:160 titration value) by this test totaled 40 (42.1%). Among these, 19 (20.0%) patients were positive by both O and H antigen titration test. The results of the titration test showed that 37 (38.9%) patients were positive for O antigen and 19 (20%) for H antigen. There was no clump or positive titer value in 1:1280 and higher diluted serum for both H and O antigens (Figure 1).
Among 65 positive Widal slide agglutination tests, only 16 were positive for stool culture, and the three more positive stool cultures were negative with Widal slide agglutination test (Table 1). The overall sensitivity, specificity, PPV, and NPV of slide agglutination test were also 84.2%, 35.5%, 24.6%, and 90.0%, respectively, as compared to stool culture. This is similar with slide agglutination test of O antigen. Similarly, sensitivity, specificity, PPV, and NPV were 78.9%, 50%, 28.3%, and 90.5%, respectively, for H antigen (Table 2). The agreement between Widal slide agglutination test and stool culture was analyzed by kappa test. However, there was poor agreement between slide agglutination test and stool culture (kappa = 0.103).
  | Table 1 Comparison of Widal slide agglutination test with stool culture on typhoid fever-suspected cases |
We also compared Widal titration test with stool culture. Similar to slide agglutination test, among 65 positive Widal titration tests, only 15 were positive for stool culture, while the 4 positive stool cultures were negative with titration test (Table 3). The overall sensitivity, specificity, PPV, and NPV of tube titration test were also 78.9%, 67.1%, 37.5%, and 92.7%, respectively, as compared with stool culture in typhoid fever-suspected cases. There was some variation between both the antigens. The sensitivity, specificity, PPV, and NPV of tube titration test in O antigen were 57.9%, 65.8%, 29.7%, and 86.2%, respectively, and that in H antigen were 47.7%, 86.6%, 47.4%, and 86.6%, respectively (Table 2). Regarding agreement test, there was a fair agreement between Widal tube agglutination test (titration) and stool culture (kappa = 0.325).
  | Table 3 Comparison of Widal tube titration test with the stool culture on typhoid fever-suspected cases |
Discussion
S. enterica subsp. serotype Typhi is the causative agent of typhoid fever. Typhoid fever is more common in developing countries where there is limited safe water and health care system. Currently, different diagnostic techniques are applied in endemic areas, but isolation of the ethological agent from blood, urine, or stool is the most reliable means of confirming an infection. In developing countries like Ethiopia, Widal slide agglutination test is the common serological test used to diagnose typhoid fever. In this study, H agglutinations were less sensitive and more specific and yielded more PPVs than O agglutinins, but NPV is almost similar. Study showed that the level of H agglutinins is unhelpful in the diagnosis of typhoid, maintaining that the H agglutinin titer remains elevated for a longer period than the O agglutinin titer after an episode of typhoid fever and may also rise as a nonspecific response to other infections.14 Other studies, however, have indicated that the H agglutinin titer is as useful as or more useful than O agglutinin titer.23 The aim of this study was to compare Widal slide agglutination and tube titration test against stool culture for S. enterica subsp. serotype Typhi.
In the current study, the magnitude of typhoid fever was 68.4% [95% CI: (59, 77.8)], 42.1% [95% CI: (32.2, 52.1)], and 20% [95% CI: (12, 28)] by Widal slide agglutination, Widal titration, and stool culture, respectively. This indicates that there was a great variation among the tests. This high positive result in Widal slide agglutination test may be associated with cross-reacting antibodies from serum of febrile patients other than typhoid fever. Low proportion of Widal test was observed in studies conducted in Ethiopia (32.6%)24 and Nigeria (55%),25 as compared with the current finding. This difference might be due to difference in sociodemographic status, immune status, and antibody that cross-reacts with S. enterica serotype Typhi during infection. Three cases among 19 cultures confirmed that typhoid fever cases had a negative slide agglutination test. The false-negative Widal test results were due to either early infection of the disease before production of detectable antibody or the patient was a typhoid fever carrier.26
Sensitivity of Widal slide agglutination and Widal tube titration test was 84.2% [95% CI: (76.9, 91.5)] and 78.9% [95% CI: (70.7, 87.1)], respectively, by either H or O antigen. This is the ability of Widal slide agglutination and tube titration test to detect true-positive results against stool culture. Similar finding was observed in a study conducted in Tanzania (75%).15 Lower sensitivity of Widal test was observed in a study conducted in Vietnam (64%).17 In typhoid fever-suspected Indian children, sensitivity of Widal test was 64.6%,27 and in Iranian children, it was 75.86%.18 This difference may be observed due to difference in immune statistics between adults and children.
In this study, the ability of Widal slide agglutination and tube titration test to detect true-negative result against stool culture was 35.5% [95% CI: (25.9, 45.1)] and 67.1% [95% CI: (57.7, 76.5)], respectively. This has a major impact on provision of effective patient management and reduces trust of patients on health service. Other studies conducted in Nigeria on adults,28 in Malaysia,16 and in hospitals of Vietnam also showed that Widal test had low specificity.17 Unlike the current study, the specificity of the study conducted in Tanzania (98%),15 and Iran18 was found to be higher than the current study. This may be due to geographical variations. The Widal agglutination varies with geographical location based on the endemicity of enteric fever, prevalence of nontyphoid salmonella infection, and other infections which cross-react with salmonella antigen.29
PPV of slide agglutination test was very low (24.6%). It is the most important measure of a clinical diagnostic method since it represents the proportion of patients with positive test results that are correctly diagnosed.30 On the other hand, the NPV value of slide agglutination test was 90.0%. This indicates that a negative Widal test result has a good predictive value for the absence of the disease, but a positive result would have a low predictive value for the presence of typhoid fever.15
The assessment of agreement between two different diagnostic methods can also indicate how the test methods are close to each other. Statistically, there was a fair agreement (kappa = 0.338) between Widal slide agglutination and Widal titer of anti O detection, and poor agreement (kappa = 0.173) between slide and titration method of anti-H detection. Similar finding was observed in a study conducted in Jimma.31 But the current finding disagrees with a study conducted in Addis Ababa, which indicated moderate agreement (kappa = 0.406) between slide and titration methods of anti-O screening, while there was fair agreement (kappa = 0.311) for anti-H screening of both the methods. The agreement between Widal slide agglutination test and stool culture was poor (kappa = 0.103). This indicates that the result of Widal test in diagnosis of typhoid fever less likely agrees with stool culture.
In our study, stool culture had good specificity, but there are a number of factors that should be considered while using stool culture for diagnosis of typhoid fever-suspected cases. Stool culture is mostly positive after 3 weeks of infection with S. enterica serotype Typhi, while Widal test can detect earlier.32 In early infection, the stool culture is positive only in 30%–40% of cases.33 This is the most important reason why we prefer Widal test over stool culture for early detection of the disease infection. The other factor that may affect the stool culture is its proneness to contamination that may result in misdiagnosis.
Furthermore, in developing countries like Ethiopia, cost and trained manpower are always the major concern, and stool culture is less available in routine diagnosis. Alternatively, when we see the cost-effectiveness and simplicity of Widal tests, it is still preferred over stool culture. Widal test on acute and convalescent paired sera is frequently used in resource-limited regions to diagnose and treat patients with suspected typhoid fever.34 Although Widal test has poor sensitivity and specificity, it is not absolutely discouraged in resource-limited countries.
Conclusion
In the current study, Widal test has a low specificity and PPV, but it has high sensitivity and good NPV with stool culture. Using slide agglutination test as the only laboratory test for the diagnosis of typhoid fever will result in misdiagnosis. Slide agglutination test has fair agreement with the tube titration test, whereas it has poor agreement with the stool culture and tube titration test has fair agreement with the stool culture. By considering this finding, physicians should not totally depend on Widal test in the diagnosis of typhoid fever in endemic areas and where it is the only diagnostic method. If there is no access for culture, it is better to use tube agglutination (titration) test than slide agglutination test.
Acknowledgments
The authors are grateful to Arba Minch General Hospital for permission for data collection and the hospital laboratory staff for their assistance during data collection. The authors also wish to thank assistant professor Tsegaye Tsalla for his constructive advice in the study.
Author contributions
GA: participated in the design of the study, monitored data collection, analyzed the data, and drafted the manuscript. EA, BK, and BY: conducted data collections, participated in the design of the study, and participated in drafting of the manuscript. All the authors have read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Bhanu S, Vandana S, Archana S. Comparative study of the diagnostic procedures in salmonella infection, causative agent of typhoid fever – an overview study. IRJP. 2001;2(9):127–129. | ||
Andualem G, Abebe T, Kebede N, Gebre-Selassie S, Mihret A, Alemayehu H. A comparative study of Widal test with blood culture in the diagnosis of typhoid fever in febrile patients. BMC Res Notes. 2014;7:653. | ||
Coleman W, Buxton BH. The bacteriology of the blood in typhoid fever. Am J Med Sci. 1907;133:896–903. | ||
Gerald KT. Inflammatory diarrhea in infectious diseases. In: Fauci AS, Braunwald E, Kasper DL, et al. editors. Harrison’s Manual of medicine. 17th ed. New York: McGraw Hill; 2009:456–457. | ||
Wain J, Diep TS, Be Bay PV, et al. Specimens and culture media for the laboratory diagnosis of typhoid fever. J Infect Dev Ctries. 2008;2(6):469–474. | ||
World Health Organization. Management procedures of food handling personnel. World Health Organization Technical Report Series 785. WHO, Geneva; 1989. | ||
Vandepitte J, Verhaegen J, Engback E, Rohner P, Piot P, Heuck CC. Basic Laboratory Procedures in Clinical Bacteriology. 2nd ed. Geneva: WHO; 2003. | ||
Molla B, Kleer J, Sinell HJ. Antibiotic resistance pattern of food borne Salmonella isolates in Addis Ababa (Ethiopia). Berl Munch Tierarztl Wochenschr. 1999;112(2):41–43. | ||
Ochiai RL, Acosta CJ, Danovaro-Holliday MC, et al. A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ. 2008;86(4):260–268. | ||
Olopoenia LA, King AL. Widal agglutination test – 100 years later: still plagued by controversy. Postgrad Med J. 2000;76:80–84. | ||
Grunbaum AS. Preliminary note on the use of the agglutinative action of human serum for the diagnosis of enteric fever. Lancet. 1896;148(3812):806–807. | ||
Parry CM, Hoa NTT, Diep TS, et al. Value of a single-tube Widal test in diagnosis of typhoid fever in Vietnam. J Clin Microbial. 1999;37:2882–2886. | ||
Saha SK, Ruhulamin M, Hanif M, Islam M, Khan WA. Interpretation of the Widal test in the diagnosis of typhoid fever in Bangladeshi children. Ann Trop Paediatr. 1996;16(1):75–78. | ||
Schroeder SA. Interpretation of serological tests for typhoid fever. JAMA. 1968;206(4):839–840. | ||
Ley B, George Mtove G, Thriemer K, et al. Evaluation of the Widal tube agglutination test for the diagnosis of typhoid fever among children admitted to a rural hospital in Tanzania and a comparison with previous studies. BMC Infect Dis. 2010;10:180. | ||
Lim PL, Tam FC, Cheong YM, Jegathesan M. Onestep 2-minute test to detect typhoid-specific antibodies based on particle separation in tubes. J Clin Microbiol. 1998;36(8):2271–2278. | ||
Olsen SJ, Pruckler J, Bibb W, et al. Evaluation of rapid diagnostic tests for typhoid fever. J Clin Microbiol. 2004;42(5):1885–1889. | ||
Noorbakhsh S, Rimaz S, Rahbarimanesh AA, Mamishi S. Interpretation of the Widal test in infected children. Iranian J Publ Health. 2003;32(1):35–37. | ||
Wain J, Hosoglu S. The laboratory diagnosis of enteric fever. J Infect Dev Ctries. 2008;2(6):421–425. | ||
Federal Democratic Republic of Ethiopia, Population census commission and UNFPA Summary and Statistical report of the 2007 Population and Housing Census. Addis Ababa, Ethiopia; 2008. | ||
Cheesbrough M. District Laboratory Practice in Tropical Countries. Part-2, 2nd ed. Cambridge, UK: Cambridge University Press; 2006. | ||
Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Approved Standard. 18th ed. USA: Wayne Press; 2009. | ||
Brodie J. Antibodies and the burden of typhoid outbreak of 1964. J Hyg (Lond). 1977;79(2):161–180. | ||
Andualem G, Abebe T, Kebede N, Gebre-Selassie S, Mihret A, Alemayehu H. A comparative study of Widal test with blood culture in the diagnosis of typhoid fever in febrile patients. BMC Res Notes. 2014;7:653. | ||
Itah AY, Uweh EE. Bacteria isolation from blood, stool and urine of typhoid patient in a developing in a developing country. Southeast Asian J Trop Med Public Health. 2005;36(3):673–677. | ||
Olopoenia LA, King AL. Widal agglutination test – 100 years later: still plagued by controversy. Postgrad Med J. 2000;76:80–84. | ||
Beig FK, Ahmad F, Ekram M, Shukla I. Typhidot M and Diazo test vis-à-vis blood culture and Widal test in the early diagnosis of typhoid fever in children in a resource poor setting. Braz J Infect Dis. 2010;14(6):589–593. | ||
Ramyil MS, Ihuoma OJ, Ogundeko TO, et al. Comparative study on the use of Widal test and stool culture in the laboratory diagnosis of Salmonella infection in adult and children in Jos Metropolis, Plateau State, Nigeria. IJSR. 2013;2(12):435–441. | ||
Acharya T. Widal test: principle, procedure, results, interpretation, significance and limitation. Microbe online, 2016. Available from: https://microbeonline.com/widal-test-principle-procedure-results/. Accessed May 7, 2016. | ||
Altman DG, Bland JM: Diagnostic tests 2: predictive values. BMJ. 1994;309(6947):102. | ||
Mamo Y, Belachew T, Abebe W, Gebre-Selassie S, Jira C. Pattern of Widal agglutination reaction in apparently healthy population of Jimma town, southwest Ethiopia. Ethiop Med J. 2007;45(1):69–77. | ||
Agbulu CO, Nkiruka CM, Yaji ME. Comparison of Widal test and stool culture technique in diagnosing typhoid fever in Okpokwu Local Government Area, Benue State, Nigeria. Acad J Microbiol Res. 2016;4(1):001–004. | ||
Edelman R, Levine MM. Summary of an international workshop on typhoid fever. Rev Infect Dis. 1986;8:329. | ||
House D, Chinh NT, Diep TS, et al. Use of paired serum samples for serodiagnosis of typhoid fever. J Clin Microbiol. 2005;43:4889–4890. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

